Abstract
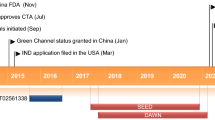
Despite advances in the management of type 2 diabetes mellitus (T2DM), one-third of patients with diabetes do not achieve the desired glycemic goal. Considering this inadequacy, many agents that activate glucokinase have been investigated over the last two decades but were withdrawn before submission for marketing permission. Dorzagliatin is the first glucokinase activator that has been granted approval for T2DM, only in China. As overstimulation of glucokinase is linked with pathophysiological disturbances such as fatty liver and cardiovascular issues and a loss of therapeutic efficacy with time. This review aims to highlight the benefits of glucokinase activators vis-à-vis the risks associated with chronic enzymatic activation. We discuss the multisystem disturbances expected with chronic activation of the enzyme, the lessons learned with glucokinase activators of the past, the major efficacy and safety findings with dorzagliatin and its pharmacological properties, and the status of other glucokinase activators in the pipeline. The approval of dorzagliatin in China was based on the SEED and the DAWN trials, the major pivotal phase III trials that enrolled patients with T2DM with a mean glycosylated hemoglobin of 8.3–8.4%, and a mean age of 53–54.5 years from multiple sites in China. Patients with uncontrolled diabetes, cardiac diseases, organ dysfunction, and a history of severe hypoglycemia were excluded. Both trials had a randomized double-blind placebo-controlled phase of 24 weeks followed by an open-label phase of 28 weeks with dorzagliatin. Drug-naïve patients with T2DM with a disease duration of 11.7 months were enrolled in the SEED trial while the DAWN trial involved patients with T2DM with a mean duration of 71.5 months and receiving background metformin therapy. Compared with placebo, the decline in glycosylated hemoglobin at 24 weeks was more with dorzagliatin with an estimated treatment difference of − 0.57% in the SEED trial and − 0.66% in the DAWN trial. The desired glycosylated hemoglobin (< 7%) was also attained at more than two times higher rates with dorzagliatin. The glycemic improvement was sustained in the SEED trial but decreased over 52 weeks in the DAWN trial. Hyperlipidemia was observed in 12–14% of patients taking dorzagliatin versus 9–11% of patients receiving a placebo. Additional adverse effects noticed over 52 weeks with dorzagliatin included an elevation in liver enzymes, hyperuricemia, hyperlacticacidemia, renal dysfunction, and cardiovascular disturbances. Considering the statistically significant improvement in glycosylated hemoglobin with dorzagliatin in patients with T2DM, the drug may be given a chance in treatment-naïve patients with a shorter disease history. However, with the waning therapeutic efficacy witnessed in patients with long-standing diabetes, which was also one of the potential concerns with previously tested molecules, extended studies involving patients with chronic and uncontrolled diabetes are needed to comment upon the long-term therapeutic performance of dorzagliatin. Likewise, evidence needs to be generated from other countries, patients with organ dysfunction, a history of severe hypoglycemia, cardiac diseases, and elderly patients before extending the use of dorzagliatin. Apart from monitoring lipid profiles, long-term safety studies of dorzagliatin should involve the assessment of serum uric acid, lactate, renal function, liver function, and cardiovascular parameters.

Similar content being viewed by others
References
Diabetes. World Health Organization. https://www.who.int/health-topics/diabetes. Accessed 21 Feb 2024.
International Diabetes Federation. Facts and figures. https://idf.org/about-diabetes/facts-figures/. Accessed 21 Feb 2024.
Ma C-X, Ma X-N, Guan C-H, Li Y-D, Mauricio D, Fu S-B. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74.
de Pablos-Velasco P, Parhofer KG, Bradley C, Eschwège E, Gönder-Frederick L, Maheux P, et al. Current level of glycemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80:47–56.
Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005–2016. JAMA Intern Med. 2019;179:1376–85.
Bar-Tana J. Type 2 diabetes: unmet need, unresolved pathogenesis, mTORC1-centric paradigm. Rev Endocr Metab Disord. 2020;21:613–29.
International Hypoglycemia Study Group. Hypoglycemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385–96.
Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis. 2017;60:422–34.
Middleton TL, Wong J, Molyneaux L, Brooks BA, Yue DK, Twigg SM, et al. Cardiac effects of sulfonylurea-related hypoglycemia. Diabetes Care. 2017;40:663–70.
US FDA. FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious. Accessed 21 Feb 2024.
Gorgojo-Martínez JJ, Mezquita-Raya P, Carretero-Gómez J, Castro A, Cebrián-Cuenca A, de Torres-Sánchez A, et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with Glp-1 receptor agonists: a multidisciplinary expert consensus. J Clin Med. 2022;12:145.
Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56.
Uitrakul S, Aksonnam K, Srivichai P, Wicheannarat S, Incomenoy S. The incidence and risk factors of urinary tract infection in patients with type 2 diabetes mellitus using SGLT2 inhibitors: a real-world observational study. Med Basel Switz. 2022;9:59.
Yang H, Choi E, Park E, Na E, Chung SY, Kim B, et al. Risk of genital and urinary tract infections associated with SGLT-2 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes mellitus: a retrospective cohort study in Korea. Pharmacol Res Perspect. 2022;10: e00910.
Toulis KA, Nirantharakumar K, Pourzitaki C, Barnett AH, Tahrani AA. Glucokinase activators for type 2 diabetes: challenges and future developments. Drugs. 2020;80:467–75.
Nakamura A, Terauchi Y. Present status of clinical deployment of glucokinase activators. J Diabetes Investig. 2015;6:124–32.
Ren Y, Li L, Wan L, Huang Y, Cao S. Glucokinase as an emerging anti-diabetes target and recent progress in the development of its agonists. J Enzym Inhib Med Chem. 2022;37:606–15.
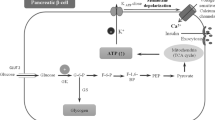
Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front Physiol. 2019;10:148.
Fournel A, Marlin A, Abot A, Pasquio C, Cirillo C, Cani PD, et al. Glucosensing in the gastrointestinal tract: impact on glucose metabolism. Am J Physiol Gastrointest Liver Physiol. 2016;310:G645–58.
Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors. 2010;10:9002–25.
Lamy CM, Sanno H, Labouèbe G, Picard A, Magnan C, Chatton J-Y, et al. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014;19:527–38.
Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–63.
Murphy R, Tura A, Clark PM, Holst JJ, Mari A, Hattersley AT. Glucokinase, the pancreatic glucose sensor, is not the gut glucose sensor. Diabetologia. 2009;52:154–9.
Nakamura A, Togashi Y, Orime K, Sato K, Shirakawa J, Ohsugi M, et al. Control of beta cell function and proliferation in mice stimulated by small-molecule glucokinase activator under various conditions. Diabetologia. 2012;55:1745–54.
Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, et al. Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Investig. 2007;117:246–57.
Hulín J, Škopková M, Valkovičová T, Mikulajová S, Rosoľanková M, Papcun P, et al. Clinical implications of the glucokinase impaired function: GCK MODY today. Physiol Res. 2020;69:995–1011.
Loh WJ, Dacay LM, Tan CSH, Ang SF, Yap F, Lim SC, et al. Glucokinase activating mutation causing hypoglycemia diagnosed late in adult who fasts for Ramadhan. Endocrinol Diabetes Metab Case Rep. 2021;2021:21–0043.
Challis BG, Harris J, Sleigh A, Isaac I, Orme SM, Seevaratnam N, et al. Familial adult onset hyperinsulinism due to an activating glucokinase mutation: implications for pharmacological glucokinase activation. Clin Endocrinol (Oxf). 2014;81:855–61.
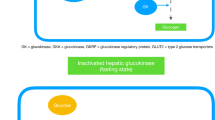
Haeusler RA, Camastra S, Astiarraga B, Nannipieri M, Anselmino M, Ferrannini E. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol Metab. 2015;4:222–6.
Caro JF, Triester S, Patel VK, Tapscott EB, Frazier NL, Dohm GL. Liver glucokinase: decreased activity in patients with type II diabetes. Horm Metab Res. 1995;27:19–22.
Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, et al. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–83.
Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, et al. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zucker diabetic fatty rats. Diabetes. 2009;58:78–86.
Shin J-S, Torres TP, Catlin RL, Donahue EP, Shiota M. A defect in glucose-induced dissociation of glucokinase from the regulatory protein in Zucker diabetic fatty rats in the early stage of diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1381–90.
Shiota M, Postic C, Fujimoto Y, Jetton TL, Dixon K, Pan D, et al. Glucokinase gene locus transgenic mice are resistant to the development of obesity-induced type 2 diabetes. Diabetes. 2001;50:622–9.
O’Doherty RM, Lehman DL, Télémaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes. 1999;48:2022–7.
Peter A, Stefan N, Cegan A, Walenta M, Wagner S, Königsrainer A, et al. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab. 2011;96:E1126–30.
Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:3112–21.
Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PRV, Orho-Melander M, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–8.
Rees MG, Wincovitch S, Schultz J, Waterstradt R, Beer NL, Baltrusch S, et al. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia. 2012;55:114–22.
Saxena R, Hivert M-F, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–8.
Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46:1662–8.
Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–6.
Katz L, Manamley N, Snyder WJ, Dodds M, Agafonova N, Sierra-Johnson J, et al. AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycemia in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:191–5.
Wilding JPH, Leonsson-Zachrisson M, Wessman C, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 in patients with type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2013;15:750–9.
Zheng S, Shao F, Ding Y, Fu Z, Fu Q, Ding S, et al. Safety, pharmacokinetics, and pharmacodynamics of globalagliatin, a glucokinase activator, in Chinese patients with type 2 diabetes mellitus: a randomized, phase Ib, 28-day ascending dose study. Clin Drug Investig. 2020;40:1155–66.
Amin NB, Aggarwal N, Pall D, Paragh G, Denney WS, Le V, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab. 2015;17:751–9.
Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab. 2010;95:5028–36.
Drabkin M, Yogev Y, Zeller L, Zarivach R, Zalk R, Halperin D, et al. Hyperuricemia and gout caused by missense mutation in d-lactate dehydrogenase. J Clin Invest. 2019;129:5163–8.
Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45:145–54.
Litwack G. Chapter 6—Insulin and sugars. In: Litwack G, editor. Hum Biochem. Boston: Academic Press; 2018. pp. 131–60. https://www.sciencedirect.com/science/article/pii/B9780123838643000065. Accessed 19 Aug 2023.
Wang Y, Viollet B, Terkeltaub R, Liu-Bryan R. AMP-activated protein kinase suppresses urate crystal-induced inflammation and transduces colchicine effects in macrophages. Ann Rheum Dis. 2016;75:286–94.
Yang W, Zhu D, Gan S, Dong X, Su J, Li W, et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28:974–81.
Zhu D, Li X, Ma J, Zeng J, Gan S, Dong X, et al. Dorzagliatin in drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28:965–73.
Wang K, Shi M, Yang A, Fan B, Tam CHT, Lau E, et al. GCKR and GCK polymorphisms are associated with increased risk of end-stage kidney disease in Chinese patients with type 2 diabetes: the Hong Kong Diabetes Register (1995–2019). Diabetes Res Clin Pract. 2022;193: 110118.
Lian J, Guo J, Chen Z, Jiang Q, Ye H, Huang X, et al. Positive association between GCKR rs780093 polymorphism and coronary heart disease in the aged Han Chinese. Dis Markers. 2013;35:863–8.
Zahedi AS, Akbarzadeh M, Sedaghati-Khayat B, Seyedhamzehzadeh A, Daneshpour MS. GCKR common functional polymorphisms are associated with metabolic syndrome and its components: a 10-year retrospective cohort study in Iranian adults. Diabetol Metab Syndr. 2021;13:20.
Zhu D, Gan S, Liu Y, Ma J, Dong X, Song W, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6:627–36.
Zhu X-X, Zhu D-L, Li X-Y, Li Y-L, Jin X-W, Hu T-X, et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycemic control and pancreatic β-cell function in patients with type 2 diabetes: a 28-day treatment study using biomarker-guided patient selection. Diabetes Obes Metab. 2018;20:2113–20.
Chow E, Wang K, Lim CKP, Tsoi STF, Fan B, Poon E, et al. Dorzagliatin, a dual-acting glucokinase activator, increases insulin secretion and glucose sensitivity in glucokinase maturity-onset diabetes of the young and recent-onset type 2 diabetes. Diabetes. 2023;72:299–308.
Chen L, Zhang J, Sun Y, Zhao Y, Liu X, Fang Z, et al. A phase I open-label clinical trial to study drug-drug interactions of dorzagliatin and sitagliptin in patients with type 2 diabetes and obesity. Nat Commun. 2023;14:1405.
Rosengren A, Jing X, Eliasson L, Renström E. Why treatment fails in type 2 diabetes. PLoS Med. 2008;5: e215.
Tysoe O. Sulfonylurea secondary failure mechanism identified. Nat Rev Endocrinol. 2023;19:189–189.
Xu H, Sheng L, Chen W, Yuan F, Yang M, Li H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: results from a first-in-human single ascending dose study. Drug Des Dev Ther. 2016;10:1619–26.
Miao J, Fu P, Ren S, Hu C, Wang Y, Jiao C, et al. Effect of renal impairment on the pharmacokinetics and safety of dorzagliatin, a novel dual-acting glucokinase activator. Clin Transl Sci. 2022;15:548–57.
Kiyosue A, Hayashi N, Komori H, Leonsson-Zachrisson M, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:923–30.
Ericsson H, Sjöberg F, Heijer M, Dorani H, Johansson P, Wollbratt M, et al. The glucokinase activator AZD6370 decreases fasting and postprandial glucose in type 2 diabetes mellitus patients with effects influenced by dosing regimen and food. Diabetes Res Clin Pract. 2012;98:436–44.
Denney WS, Denham DS, Riggs MR, Amin NB. Glycemic effect and safety of a systemic, partial glucokinase activator, PF-04937319, in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomized, crossover, active-controlled study. Clin Pharmacol Drug Dev. 2016;5:517–27.
Liu D, Du Y, Yao X, Wei Y, Zhu J, Cui C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the glucokinase activator PB-201 and its effects on the glucose excursion profile in drug-naïve Chinese patients with type 2 diabetes: a randomised controlled, crossover, single-centre phase 1 trial. EClinicalMedicine. 2021;42: 101185.
Zhi J, Zhai S. Effects of piragliatin, a glucokinase activator, on fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. J Clin Pharmacol. 2016;56:231–8.
Vella A, Freeman JLR, Dunn I, Keller K, Buse JB, Valcarce C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci Transl Med. 2019;11:eaau3441.
Gao Q, Zhang W, Li T, Yang G, Zhu W, Chen N, et al. The efficacy and safety of glucokinase activators for the treatment of type-2 diabetes mellitus. Medicine (Baltim). 2021;100: e27476.
A 12-week, phase 2, randomized, double-blind, placebo controlled, dose-ranging, parallel group study to evaluate the efficacy and safety of once daily Pf-04991532 and sitagliptin in adult patients with type 2 diabetes mellitus inadequately controlled on metformin. ClinicalTrials.gov; 2013 Jun. Report No.: NCT01336738. Pfizer. https://clinicaltrials.gov/study/NCT01336738. Accessed 21 Feb 2024.
Safety and tolerability of multiple ascending doses of LY2608204 in patients with type 2 diabetes mellitus. ClinicalTrials.gov; 2018 Oct. Report No.: NCT01247363. Eli Lilly and Company. https://clinicaltrials.gov/study/NCT01247363. Accessed 21 Feb 2024.
Tsumura Y, Tsushima Y, Tamura A, Hasebe M, Kanou M, Kato H, et al. TMG-123, a novel glucokinase activator, exerts durable effects on hyperglycemia without increasing triglyceride in diabetic animal models. PLoS ONE. 2017;12: e0172252.
Klein KR, Freeman JLR, Dunn I, Dvergsten C, Kirkman MS, Buse JB, et al. The SimpliciT1 study: a randomized, double-blind, placebo-controlled phase 1b/2 adaptive study of TTP399, a hepatoselective glucokinase activator, for adjunctive treatment of type 1 diabetes. Diabetes Care. 2021;44:960–8.
A study of LY2599506 in patients with type 2 diabetes. https://clinicaltrials.gov/study/NCT01024244. Accessed 7 Mar 2023.
A study of LY2599506 (Oral Agent Medication: Glucokinase Activator 1) in type 2 diabetes mellitus. https://clinicaltrials.gov/study/NCT01029795. Accessed 7 Mar 2023.
A study to compare two forms of LY2608204 in healthy people. https://clinicaltrials.gov/study/NCT01313286. Accessed 7 Mar 2023.
Acknowledgements
Upinder Kaur and Sankha Shubhra Chakrabarti thank the Institutions of Eminence Scheme at the Banaras Hindu University for research support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received to write this review.
Conflict of Interest
Upinder Kaur, Bhairav Kumar Pathak, Tharik Jalal Meerashahib, Dondapati Venkata Vamshi Krishna, and Sankha Shubhra Chakrabarti have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable as the review did not involve any human or animal experiments.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable. The article reviews data from other published papers, which are available in the public domain.
Code Availability
Not applicable.
Authors’ Contributions
UK: planned the study, supervised the data extraction, verified the extracted data, performed the literature search, and wrote the first draft of the paper. BKP: performed the literature search, assisted in writing the paper and the presentation of data, and verified the extracted data. TJM: assisted in writing the paper and the tabular presentation of data, and verified the extracted data. DVVK: contributed to the tabular presentation of data and verification of the extracted data. SSC: edited the final draft of the paper and performed the literature search.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, U., Pathak, B.K., Meerashahib, T.J. et al. Should Glucokinase be Given a Chance in Diabetes Therapeutics? A Clinical-Pharmacological Review of Dorzagliatin and Lessons Learned So Far. Clin Drug Investig 44, 223–250 (2024). https://doi.org/10.1007/s40261-024-01351-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01351-5