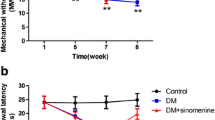
Abstract
Background
It has been reported that neuropathic pain can be overcome by targeting the NR2B subunit of N-methyl-D-aspartate receptors (NR2B). This study aimed to investigate the effects of minocycline on phosphorylated and total expression of NR2B in the spinal cord of rats with diabetic neuropathic pain.
Methods
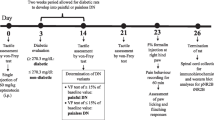
A total of 32 Sprague–Dawley male rats were randomly assigned into four groups (n = 8); control healthy, control diabetic (PDN), and PDN rats that received 80 µg or 160 µg intrathecal minocycline respectively. The rats were induced to develop diabetes and allowed to develop into the early phase of PDN for two weeks. Hot-plate and formalin tests were conducted. Intrathecal treatment of minocycline or normal saline was conducted for 7 days. The rats were sacrificed to obtain the lumbar enlargement region of the spinal cord (L4-L5) for immunohistochemistry and western blot analyses to determine the expression of phosphorylated (pNR2B) and total NR2B (NR2B).
Results
PDN rats showed enhanced flinching (phase 1: p < 0.001, early phase 2: p < 0.001, and late phase 2: p < 0.05) and licking responses (phase 1: p < 0.001 and early phase 2: p < 0.05). PDN rats were also associated with higher spinal expressions of pNR2B and NR2B (p < 0.001) but no significant effect on thermal hyperalgesia. Minocycline inhibited formalin-induced flinching and licking responses (phase 1: p < 0.001, early phase 2: p < 0.001, and late phase 2: p < 0.05) in PDN rats with lowered spinal expressions of pNR2B (p < 0.01) and NR2B (p < 0.001) in a dose-dependent manner.
Conclusion
Minocycline alleviates nociceptive responses in PDN rats, possibly via suppression of NR2B activation. Therefore, minocycline could be one of the potential therapeutic antinociceptive drugs for the management of neuropathic pain.






Similar content being viewed by others
Data Availability
All the data are shown in the manuscript.
References
Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B 1 receptor in early diabetic pain neuropathy. J Neuroinflammation. 2010;7:36. https://doi.org/10.1186/1742-2094-7-36.
Sima AA. Diabetic neuropathy in type 1 and type 2 diabetes and the effects of C-peptide. J Neurol Sci. 2004;220:133–6. https://doi.org/10.1016/j.jns.2004.03.014.
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6:432. https://doi.org/10.4239/wjd.v6.i3.432.
Li C-D, Zhao J-Y, Chen J-L, Lu J-H, Zhang M-B, Huang Q, Cao Y-N, Jia G-L, Tao Y-X, Li J, Cao H. Mechanism of the JAK2/STAT3-CAV-1-NR2B signaling pathway in painful diabetic neuropathy. Endocrine. 2019;64:55–66. https://doi.org/10.1007/s12020-019-01880-6.
Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, Sandkühler J. Selective activation of microglia facilitates synaptic strength. J Neurosci. 2015;35:4552–70. https://doi.org/10.1523/JNEUROSCI.2061-14.2015.
Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Charpentier TL, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loefflier J-P, Kawellars A, Verney C, Mantz J, Gressens P. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–49. https://doi.org/10.1002/ana.23626.
Ji R-R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain®. 2013; 154 Suppl:S10–28. https://doi.org/10.1016/j.pain.2013.06.022.
Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–55. https://doi.org/10.1592/phco.28.5.646.
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyota T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006;29:1538–44. https://doi.org/10.2337/dc05-2370.
Ziegler D, Hanefeld M, Ruhnau K, Mei H, Lobisch M, Schűtte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant α-lipoic acid. Diabetologia. 1995;38:1425–33. https://doi.org/10.1007/BF00400603.
Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, Vinik A, Giuliani M, Stevens C, Barbano R, Dyck PJ. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: a randomized controlled trial. J Am Med Assoc. 2000;284:2215–21. https://doi.org/10.1001/jama.284.17.2215.
Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials Br Med J. 2002;324. https://doi.org/10.1136/bmj.324.7339.705.
Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC) α/βII. J Bio Chem. 2007;282:15208–16. https://doi.org/10.1074/jbc.M611907200.
Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. https://doi.org/10.1016/j.ejphar.2011.04.014.
Moini-Zanjani T, Ostad S-N, Labibi F, Ameli H, Mosaffa N, Sabetkasaei M. Minocycline effects on IL-6 concentration in macrophage and microglial cells in a rat model of neuropathic pain. Iran Biomed J. 2016;20:273–79. https://doi.org/10.22045/ibj.2016.04
Ismail CAN, Suppian R, Ab Aziz CB, Long I. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J Diabetes Metab Disord. 2019;18:181–90. https://doi.org/10.1007/s40200-019-00411-4.
Ismail CAN, Suppian R, Ab Aziz CB, Long I. Minocycline and Ifenprodil Prevent Development of Painful Diabetic Neuropathy in Streptozotocin-induced Diabetic Rat Model. Int J Life Sci. 2018;6:29–40.
Huang L-E, Guo S-H, Thitiseranee L, Yang Y, Zhou Y-F, Yao Y-X. N-methyl-D-aspartate receptor subtype 2B antagonist, Ro 25–6981, attenuates neuropathic pain by inhibiting postsynaptic density 95 expression. Sci Rep. 2018;8:7848. https://doi.org/10.1038/s41598-018-26209-7.
Xu Y, Zhang K, Miao J, Zhao P, Lv M, Li J, Fu Z, Luo X, Zhu P. The spinal NR2BR/ERK2 pathway as a target for the central sensitization of collagen-induced arthritis pain. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0201021.
Daulhac L, Mallet C, Courteix C, Etienne M, Duroux W, Privat A-M, Sechalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–54. https://doi.org/10.1124/mol.106.025478.
Lu R, Schmidtko A. Direct intrathecal drug delivery in mice for detecting in vivo effects of cGMP on pain processing. In: Guanylate Cyclase and Cyclic GMP: Methods and Protocol. Springer; 2013. pp 215–221.
Zhou R, Xu T, Liu X, Chen Y, Kong D, Tian H, Yue M, Huang D, Zeng J. Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain. J Pain Res. 2018;11:615. https://doi.org/10.2147/JPR.S154437.
Wheeler-Aceto H, Cowan A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology. 1991;104:35–44. https://doi.org/10.1007/BF02244551.
Babaei BF, Zare S, Heydari R, Farokhi F. Effects of melatonin and vitamin E on peripheral neuropathic pain in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2010;2:1–8.
Long I, Suppian R, Ismail Z. The effects of pre-emptive administration of ketamine and norbni on pain behavior, c-fos, and prodynorphin protein expression in the rat spinal cord after formalin-induced pain is modulated by the dream protein. Korean J Pain. 2013;26:255–64. https://doi.org/10.3344/kjp.2013.26.3.255.
Molander C, Xu Q, Grant G. The cytoarthritic organization of the spinal cord in the rat. I. The lower throacic and lumboscaral cord. J Comp Neurol. 1984;230:133–41.
Juárez-Rojop IE, Granados-Soto V, Díaz-Zagoya JC, Glores-Murrieta FJ, Torres-López JE. Involvement of cholecystokinin in peripheral nociceptive sensitization during diabetes in rats as revealed by the formalin response. Pain. 2006;122:118–25. https://doi.org/10.1016/j.pain.2006.01.018.
Freshwater JD, Svensson CI, Malmberg AB, Calcutt NA. Elevated spinal cyclooxygenase and prostaglandin release during hyperalgesia in diabetic rats. Diabetes. 2002;51:2249–55. https://doi.org/10.2337/diabetes.51.7.2249.
Malmberg AB, Yaksh TL. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J Neurosci. 1995;15:2768–76. https://doi.org/10.1523/JNEUROSCI.15-04-02768.1995.
Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre-and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992;77:757–63. https://doi.org/10.1097/00000542-199210000-00021.
Kim D, Kim MA, Cho I-H, Kim MS, Lee S, Jo E-K, Choi S-Y, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. https://doi.org/10.1074/jbc.M607277200.
Bastos LF, Godin AM, Zhang Y, Jarussophon S, Ferreira BC, Machado RR, Maier SF, Konishi Y, de FreitasRP, Fiebich BL, Watkins LR, Coelho MM, Moraes MFD. A minocycline derivative reduces nerve injury-induced allodynia, LPS-induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci Lett. 2013;543:157–62. https://doi.org/10.1016/j.neulet.2013.03.014.
Anjaneyulu M, Chopra K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:1001–5. https://doi.org/10.1016/S0278-5846(03)00160-X.
Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–61. https://doi.org/10.1016/j.ejphar.2006.03.006.
Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin-diabetic rats. Pain. 1998;76:151–7. https://doi.org/10.1016/S0304-3959(98)00037-2.
DeLeo JA, Coombs DW, Willenbring S, Colburn RW, Fromm C, Wagner R, Twitchcll BB. Characterization of a neuropathic pain model: sciatic cryoneurolysis in the rat. Pain. 1994;56:9–16. https://doi.org/10.1016/0304-3959(94)90145-7.
Tesfaye S, Malik R, Ward J. Vascular factors in diabetic neuropathy. Diabetologia. 1994;37:847–54. https://doi.org/10.1007/BF00400938.
Besson J-M, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. https://doi.org/10.1152/physrev.1987.67.1.67.
Abu-Ghefreh AAA, Masocha W. Enhancement of antinociception by coadminstration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naïve mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet Disord. 2010;1:276. https://doi.org/10.1186/1471-2474-11-276.
Sung C-S, Cherng C-H, Wen Z-H, Chang W-K, Huang S-Y, Lin S-L, Chan K-H, Wong C-S. Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1β–induced thermal hyperalgesic rats. Glia. 2012;60:2004–17. https://doi.org/10.1002/glia.22415.
Dang J-K, Wu Y, Cao H, Meng B, Huang C-C, Chen G, Li J, Song X-J, Lian Q-Q. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med. 2014;15:637–46. https://doi.org/10.1111/pme.12387.
Morel V, Pickering G, Etienne M, Dupuis A, Privat AM, Chalus M, Eschalier A, Daulhac L. Low doses of dextromethorphan have a beneficial effect in the treatment of neuropathic pain. Fund Clin Pharmacol. 2014;28:671–80. https://doi.org/10.1111/fcp.12076.
Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;674987:1–10. https://doi.org/10.1155/2014/674987.
Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;2:445–54. https://doi.org/10.1111/j.1460-9568.2005.04340.x.
Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang S-H, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325. https://doi.org/10.1038/nm.1883.
Khan AM, Curràs MC, Dao J, Jamal FA, Turkowski CA, Goel RK, Giliard ER, Wolfsohn SD, Stanley BG. Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemical/behavioral evidence. Am J Physiol Regul Integr Comp Physiol. 1999;276:R880–91. https://doi.org/10.1152/ajpregu.1999.276.3.R880.
Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spect. 2004;17:183–90. https://doi.org/10.2337/diaspect.17.3.183.
Sun JS, Yang YJ, Zhang YZ, Huang W, Li ZS, Zhang Y. Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats. Mol Med Rep. 2015;12:2677–82. https://doi.org/10.3892/mmr.2015.3735.
Tang J, Li Z-H, Ge S-N, Wang W, Mei X-P, Wang W, Mei X-P, Wang W, Zhang T, Xu L-X, Li J-L. The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complementary Altern Med. 2012. https://doi.org/10.1155/2012/185167.
Salaffi F, Giacobazzi G, Di Carlo M. Chronic pain in inflammatory arthritis: mechanisms, metrology, and emerging targets—a focus on the JAK-STAT pathway. Pain Res Manag. 2018;1–14. https://doi.org/10.1155/2018/8564215.
Carreño FR, Walch JD, Dutta M, Nedungadi TP, Cunningham JT. BDNF-TrkB pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol. 2011;23:894. https://doi.org/10.1111/j.1365-2826.2011.02209.x.
Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–37. https://doi.org/10.1523/JNEUROSCI.3686-05.2006.
Acknowledgements
We would like to thank Universiti Sains Malaysia and Ministry of Higher Education, Malaysia for the funding provided; Research University grant numbered RUI 1001/PPSK/812139 and Fundamental Research Grant Scheme numbered 203/PPSK/6171189. We also thank the staffs of Physiology Laboratory, Animal Research and Service Center, Central Research Laboratory, Cranial and Maxillofacial and Molecular and Molecular Laboratory for the direct and indirect technical guides.
Funding
This study was funded under Research University (RUI) grant (1001/PPSK/812139) by Universiti Sains Malaysia and Fundamental Research Grant Scheme (FRGS) (203/PPSK/6171189) by Ministry of Higher Education, Malaysia.
Author information
Authors and Affiliations
Contributions
Ismail CAN is responsible for the whole manuscript including figures and table. Ghazali AK guides on statistical analyses of the experimental data. Suppian R and Ab Aziz CB are responsible for proofreading and improving the quality of the manuscript. Long I provides research funding and finalizes the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All the experimentation of the study was approved by the Animal Ethics Committee, Universiti Sains Malaysia [USM/Animal Ethics Approval/2014 (91) (560)].
Conflicts of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ismail, C.A.N., Ghazali, A.K., Suppian, R. et al. Minocycline alleviates nociceptive response through modulating the expression of NR2B subunit of NMDA receptor in spinal cord of rat model of painful diabetic neuropathy. J Diabetes Metab Disord 20, 793–803 (2021). https://doi.org/10.1007/s40200-021-00820-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00820-4