Abstract
Purpose
Given that the relationship between vitamin D status and metabolic diseases such as obesity and type 2 diabetes (T2D) remains unclear, this review will focus on the genetic associations, which are less prone to confounding, between vitamin D-related single nucleotide polymorphisms (SNPs) and metabolic diseases.
Methods
A literature search of relevant articles was performed on PubMed up to December 2019. Those articles that had examined the association of vitamin D-related SNPs with obesity and/or T2D were included. Two reviewers independently evaluated the eligibility for the inclusion criteria and extracted the data. In total, 73 articles were included in this review.
Results
There is a lack of research focusing on the association of vitamin D synthesis-related genes with obesity and T2D; however, the limited available research, although inconsistent, is suggestive of a protective effect on T2D risk. While there are several studies that investigated the vitamin D metabolism-related SNPs, the research focusing on vitamin D activation, catabolism and transport genes is limited. Studies on CYP27B1, CYP24A1 and GC genes demonstrated a lack of association with obesity and T2D in Europeans; however, significant associations with T2D were found in South Asians. VDR gene SNPs have been extensively researched; in particular, the focus has been mainly on BsmI (rs1544410), TaqI (rs731236), ApaI (rs7975232) and FokI (rs2228570) SNPs. Even though the association between VDR SNPs and metabolic diseases remain inconsistent, some positive associations showing potential effects on obesity and T2D in specific ethnic groups were identified.
Conclusions
Overall, this literature review suggests that ethnic-specific genetic associations are involved. Further research utilizing large studies is necessary to better understand these ethnic-specific genetic associations between vitamin D deficiency and metabolic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D is a fat-soluble vitamin and a secosteroid prohormone that plays a crucial role in bone mineralization through the absorption and regulation of calcium and phosphate levels [1]. The vitamin D endocrine system regulates calcium homoeostasis and a range of physiological functions such as cell growth, proliferation, differentiation, immune function, inflammation, and apoptosis [2]. A broad spectrum of diseases has been related to vitamin D deficiency and research, to date, suggests that vitamin D deficiency is a marker of ill health with effective connection to all-cause mortality, obesity, diabetes, cardiovascular risk, hypertension, dyslipidaemia, multiple sclerosis, Alzheimer, and some types of cancer [3,4,5,6,7]. However, causality is yet to be proven for any disease that is associated with vitamin D deficiency.
Vitamin D3 (cholecalciferol) is the natural form of vitamin D and the body can synthesise it in the skin in response to the sunlight exposure. Ultraviolet-B (UVB) (290–315 nm wavelength) skin irradiation initiates the photochemical conversion of 7DHC (provitamin D3) to previtamin D3 by breaking the 9,10 carbon-carbon bond, which is then quickly thermally isomerized to vitamin D3. Diet is another source of vitamin D3, which can be obtained from animal foods such as oily fish, egg yolk, liver, butter, fortified milk and cheese. Vitamin D2 (ergocalciferol) originates from conversion of a plant sterol, ergosterol, and is solely obtained from the diet which includes plant-sourced foods such as yeast and mushrooms [8, 9]. Vitamin D3 has a superior bioavailability than vitamin D2; nonetheless, they both go through the same metabolic pathway to produce the active hormonal forms [4, 10]. Vitamin D is biologically inert and has to undergo hydroxylation twice before it can perform its physiological functions. Vitamin D binding protein (DBP/GC) is the key transport protein, which binds over 85% of the circulating 25(OH)D and vitamin D metabolites, and it transports these metabolites to target cells. In the liver, vitamin D (cholecalciferol and ergocalciferol) is converted by the enzyme 25-hydroxylase (CYP2R1) into 25-hydroxyvitamin D [25(OH)D], also known as calcidiol, which is the primary circulating form of vitamin D. Subsequently, the kidney, acting as an endocrine gland, converts 25(OH)D by the action of the enzyme 1α-hydroxylase (CYB27B1) to the active hormonal form 1α, 25-dihydroxyvitamin D [1,25(OH)2D], also known as calcitriol, which then binds to VDR and regulates calcium homeostasis and bone metabolism (Fig. 1). The VDR, a member of the nuclear receptor family, is a receptor specific to vitamin D through which vitamin D exerts its function, and it has been discovered in a multitude of cell membranes of tissues that have no musculoskeletal function; this implies the involvement of vitamin D in various extra-skeletal biological functions [3, 4, 6].
Vitamin D synthesis and metabolism. Upon exposure to Ultraviolet-B (290–315 nm wavelength) skin irradiation, 7-dehydrocholesterol produces pre-vitamin D3, by breaking the C (9–10) bond at the B ring, which then undergoes a thermally induced rearrangement to form vitamin D3. Vitamin D can also enter the body from dietary sources in two forms: vitamin D3 (cholecalciferol) from fish, eggs, fortified milk and supplements and vitamin D2 (ergocalciferol) from mushrooms and yeast. Once transported to the liver, vitamin D is hydroxylated to 25(OH)D (calcidiol) by 25-hydroxylase enzymes (CYP2R1 & CYP27A1). In the kidneys, 25(OH)D is further hydroxylated by two enzymes to activate or inactivate vitamin D. For activation, the 1α-hydroxylase enzyme (CYP27B1) converts 25(OH)D to 1α,25-dihydroxyvitamin D (calcitriol), which is transported by vitamin D binding protein (DBP/GC). Finally, Calcitriol binds to vitamin D receptor (VDR) to perform its biological function. For inactivation, the 24-hydroxylase enzyme (CYP24A1) catabolizes 25(OH)D to 24,25-dihydroxy vitamin D. Control of metabolism of vitamin D is exerted primarily by biliary excretion
Heritability of vitamin D deficiency has been reported by twin and family studies to range between 20–85% [11]. Although there is a great variation in the estimation of the heritability results, they do show that genetic factors play a role in circulating serum 25(OH)D levels. Candidate gene studies have reported several single nucleotide polymorphisms (SNPs) related to serum 25(OH)D levels mainly with genes that are involved in synthesis and metabolism of vitamin D such as DHCR7, CYP2R1, CYP27B1, CYP24A1, DBP/GC, VDR [12]. Genome wide association studies (GWAS) have confirmed the association between genetic polymorphisms in the genes such as GC, DHCR7, CYP2R1 and CYP24A1 and 25(OH)D concentrations [11].
There exists a plethora of studies that have reported association of genetic variants with low vitamin D levels and a wide spectrum of associated diseases [13,14,15,16]. This article aims to evaluate the results of the associations between vitamin D-related genetic variants and metabolic diseases such as obesity and type 2 diabetes (T2D). Understanding the possible underlying genetic factors of vitamin D metabolism will lead to an increased understanding of the biological mechanisms underlying vitamin D deficiency and its effects on metabolic diseases.
Methods and materials
Study identification
To review published research articles relevant to the topic, a literature search of PubMed (National Library of Medicine) https://www.ncbi.nlm.nih.gov/pubmed/ was performed up to December 2019. The following key terms were used to search for research articles: “vitamin D genetics and diabetes” (n = 543), “vitamin D genetics and obesity” (n = 202), “vitamin D gene polymorphisms and diabetes” (n = 308), “vitamin D gene polymorphisms and obesity” (n = 85), “Genetic variants of vitamin D and diabetes” (n = 79), “Genetic variants of vitamin D and obesity” (n = 31), “vitamin D SNPs and diabetes” (n = 150), “vitamin D SNPs and obesity” (n = 57). As a result of all the search combinations, a total of 1,455 articles were obtained. Citations from relevant papers and review papers were examined to identify additional relevant articles for inclusion.
Study selection
Any study that was published in PubMed and written in English was included. Only genetic association studies examining the association of vitamin D-related SNPs with diabetes and/or obesity were included. Studies were excluded if they were (1) animal studies; (2) studies in pregnant women; (3) studies on humans identified with disease other than metabolic diseases; (4) randomized controlled trials; (5) gene-vitamin D interaction studies, (6) haplotype studies, (7) studies with outcome as serum 25(OH)D, bone disease, metabolic syndrome, type 1 diabetes, diabetic complications or any other disease except for obesity and T2D.
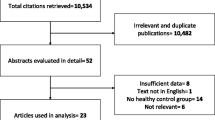
The article titles were reviewed to eliminate duplication and relevant papers were chosen (n = 112). Abstracts of the chosen articles were read to further determine their relevance to our topic. After reading the full text of these papers, 73 articles were considered relevant and were included to extract the data for this review (Fig. 2).
Data extraction
The studies were identified by a single investigator (BA), and the following data were double-extracted independently by two reviewers (VK and AS): first author, publication year, location or ethnicity of participants, sample size, mean age, study design, SNP position, name and reference SNP (rs) ID, genotype and allele distribution for vitamin D. Corresponding authors were contacted to provide any additional information where needed.
This review will look at the genes that function upstream and influence 25(OH)D synthesis (DHCR7, CYP2R1) and the genes that function downstream and play a role in 25(OH)D metabolism (CYP24A1, CYP27B1, GC/DBP, VDR) (Fig. 1). The following sections will focus on the SNPs in the vitamin D pathway-related genes and their associations with obesity traits and T2D.
Vitamin D synthesis genes
Despite several studies that have examined the association between vitamin D deficiency and metabolic diseases [13,14,15,16], the literature is remarkably scarce in studies investigating the association of genes involved in the synthesis of 25(OH)D with metabolic diseases such as obesity and T2D.
7-Dehydrocholesterol Reductase (DHCR7)
7-Dehydrocholesterol reductase enzyme is encoded by the DHCR7 gene and is located on chromosome 11q13.4. The main function of DHCR7 is to convert 7DHC to cholesterol [12]. 7DHC is a substrate of the enzyme DHCR7 and is the precursor of vitamin D (specifically vitamin D3). The conversion of 7DHC to vitamin D3 in the skin is facilitated by exposure to UVB light from the sun which causes the cleavage of the C (9–10) bond in 7DHC to form vitamin D3 [17]. The production of cholesterol from 7DHC in the skin reduces the availability of 7DHC for vitamin D synthesis, which limits vitamin D production [18].
Cytochrome P450 Family 2 Subfamily R member 1 / 25-Hydroxylase (CYP2R1)
The CYP2R1 gene is located on chromosome 11q15.2. CYP2R1 encodes 25-hydroxylase enzyme in the liver, which is the main enzyme responsible for the conversion of vitamin D3 and vitamin D2 to the main circulating form of vitamin D [25(OH)D] [19].
Obesity
Two cross-sectional studies investigated SNPs in the vitamin D synthesis-related genes; DHCR7 SNP was examined in a small study (n = 323) of African ethnicity and CYP2R1 SNP was studied in nearly 7,000 Chinese women [20, 21]. Nominal significant associations were reported with obesity traits in the Chinese study for CYP2R1 rs10832313 polymorphism; however, this did not remain significant after correction for multiple testing [20] and no significant associations were reported for DHCR7 SNP rs12785878 in the African population [21]. There are two notable large studies by Vimaleswaran et al. that examined SNPs from the DHCR7 and CYP2R1 vitamin D synthesis-related genes in relation to obesity using data from multiple Caucasian cohorts [22, 23]. One study was a bi-directional Mendelian Randomization study (n = 42,024) which showed that 10% genetically higher BMI was associated with 4.2% lower concentrations of 25(OH)D but no significant effect of vitamin D allelic scores on obesity was reported [22]. The other study was a genetic association analysis that analyzed 5,224 participants from the 1958 British birth cohort (1958BC) and 123,865 individuals from the GIANT (Genetic Investigation of Anthropometric Traits) consortium. None of the vitamin D synthesis SNPs was significantly associated with obesity traits [23]. Although the number of studies that investigated the association between vitamin D synthesis genes and obesity were small, the lack of association found in the two very large meta-analysis studies [22, 23] does suggest that the vitamin D synthesis-related gene polymorphisms may not be a contributing factor to the development of obesity. However, further studies with large number of samples are required to confirm the role of these SNPs in obesity and its related traits.
Type 2 diabetes
Studies using vitamin D synthesis-related gene polymorphisms suggest an association with low serum 25(OH)D concentrations and increased diabetes risk [24]. A recent prospective observational study in Italians (n = 2,163) demonstrated an association between DHCR7 (rs12785878) and 25(OH)D concentrations (P = 1 × 10− 4) in T2D patients [25]. Furthermore, in a recent large Mendelian Randomization meta-analysis of 10 studies from European and Chinese populations (n = 58,312 cases and 370,000 controls), the allelic score of two SNPs from the vitamin D synthesis-related genes, DHCR7 (rs12785878) and CYP2R1 (rs10741657), were shown to be significantly associated with lower risk of T2D (P = .01), where a 25 nmol/l higher 25(OH)D concentration was associated with a 14% lower risk of diabetes [26]. A Mendelian Randomization analysis in 96,423 Danish individuals examined four genetic polymorphisms in the DHCR7 and CYP2R1 genes in relation to T2D [24], where the DHCR7 allele score (rs11234027 + rs7944926) showed a significant association with increased risk of T2D (P for trend = 0.04); but there were no significant associations between CYP2R1 SNPs or allele scores and risk of diabetes [24]. In the Chinese Han population (n = 794), the ‘G’ allele carriers of the CYP2R1 SNPs, rs10766197 and rs1993116, had 1.64 and 1.76 times increased risk of developing T2D compared with ‘AA’ homozygotes, respectively (P = .024 and P = .048, respectively) [27]. However, the studies in 53,088 Germans and 4,877 Norwegians failed to show an association of SNPs in the DHCR7 (rs12785878, rs3829251, rs3794060) and CYP2R1 (rs10741657) genes with T2D [28, 29]. Although there is inconsistency in the results across various studies, a recent large Mendelian Randomization meta-analysis (n = 428,312) [26] has provided evidence that genetically instrumented higher 25(OH)D has a protective effect against diabetes risk. However, more studies are needed to confirm this finding and understand the functional significance of vitamin D synthesis-related genes in T2D.
Vitamin D metabolism genes
Several studies have examined the association of vitamin D metabolism-related SNPs with metabolic diseases; however, majority of the studies have been restricted to the VDR SNPs and only a few studies have investigated the association of genes involved in activation, catabolism and transport of 25(OH)D with metabolic diseases. Hence, VDR–related SNPs are discussed in a separate section.
I. Activation, Catabolism, and Transport Genes
Cytochrome P450 Family 27 Subfamily B member 1 / 1α-Hydroxylase (CYP27B1)
The CYP27B1 gene is located on chromosome 12q14.1. The activating enzyme 1α-hydroxylase is encoded by the CYP27B1 gene in the kidney where 25(OH)D is converted to the active 1α,25(OH)2D which binds to the VDR to perform its biological functions [30, 31].
Cytochrome P450 Family 24 Subfamily A member 1 / 24-Hydroxylase (CYP24A1)
The CYP24A1 gene, which is located on chromosome 20q13.2, codes for the vitamin D inactivating enzyme 24-hydroxlase. This enzyme controls the levels of vitamin D in blood serum by breaking down the active form to biliary excretory products and by reducing intestinal absorption of calcium and phosphate. Mutations in the gene have been shown to result in hypercalcemia and nephrolithiasis [32, 33].
Vitamin D Binding Protein (DBP) / Group-Specific Component (GC).
The DBP/GC gene is located on chromosome 4q13.3. The GC gene encodes for vitamin D binding protein (DBP), which is a glycoprotein secreted by the liver, that binds to vitamin D and its metabolites from the gut and skin and transports them to target tissues and organs and, hence, factors affecting DBP levels can also affect vitamin D concentrations. Nearly 85% of serum 25(OH)D is bound to DBP and the remainder 15% binds to albumin. Approximately 0.4% of total 1α,25(OH)2D3 and 0.03% of total 25(OH)D3 exist in the unbound, free form in the serum of healthy individuals (excluding pregnant women) [34, 35].
Obesity
To date, there has been only one study in Europeans (n = 5,224) [23] that has investigated the association of CYP27B1 SNPs, rs1048691 and rs10877012, with obesity and this study did not find any significant association of these SNPs with obesity-related outcomes. The same study in 5,224 Europeans also failed to show an association of 22 CYP24A1 SNPs with obesity [23]. This is in line with another study in up to 700 Chinese women [20], which also did not show a significant association of the CYP24A1 SNP rs2248359 with obesity traits after correction for multiple testing. For the GC gene, while a study in 5,224 participants from the 1958 British Birth Cohort failed to show an association of the 13 SNPs in the GC gene with obesity outcomes [23], studies in Caucasian nuclear families [36] (n = 1,837), Bahraini population [37] (n = 406) and African population [21] (n = 323) showed significant associations of SNPs in the GC gene with obesity-related outcomes. Based on the Quantitative Transmission Disequilibrium Test in the Caucasian nuclear family study, it was shown that the GC SNP rs17467825 increased percent fat mass (PFM) by 1.42 times and the haplotype ‘GAA’ in the GC gene increased PFM by 1.19 times [36]. In the African population, the ‘TT’ genotype of the GC SNP rs2298849 was associated with 1.76 times increased risk of overweight [21]. Furthermore, the vitamin D metabolism-related genes were also analysed as a risk score in a Mendelian Randomization study in 42,024 Caucasians and there was no significant causal effect of the vitamin D metabolism-risk score on obesity [22].
Only a few studies have focused on the genes involved in activation, catabolism and transport of 25OHD of which there has been only one study on activation gene CYP27B1 in relation to obesity [23]. Three studies have examined the catabolism gene CYP24A1 SNPs; however, none of them showed a significant association with obesity traits [20, 22, 23]. Five studies included SNPs from the transport gene GC [21,22,23, 36, 37], where only three studies showed significant associations with obesity [21, 36, 37]. Given that majority of the studies failed to find an association of these SNPs with obesity outcomes, it is quite unlikely for the SNPs in the CYP27B1, CYP24A1 and GC to have a significant functional role in obesity-related metabolic pathways.
Type 2 diabetes
There have been only two studies [28, 38] that examined SNPs from vitamin D activating CYP27B1 gene in relation to T2D and both the studies, the prospective case-cohort study in 53,088 Germans [28] and a cross-sectional study in 522 individuals from a Polish population [38], failed to show an association of CYP27B1 SNPs, rs10877012 and rs184712, with T2D, respectively. Five studies have explored the association of SNPs in vitamin D catabolism CYP24A1 gene with T2D [26, 28, 29, 39, 40]. Two Chinese case-control family-based studies (n = 1,560 & n = 1,556) examined CYP24A1 SNPs rs2248359 and rs4809957 [39, 40]; while the study in 1,556 individuals showed no association of the SNP rs4809957 with T2D [40], the study in 1,560 individuals demonstrated an association of the SNP rs2248359 with T2D in women (P = .036) but not in men (P = .816) [39]. It was shown that the ‘T’ allele of the SNP rs2248359 was transmitted 1.39 times more in offspring of T2D participant compared to non-T2D participant (P = .035) suggesting that ‘T’ allele might be a risk factor for T2D.
Despite large sample size, three studies, one in Germans (n = 53,088) [28], the other in Norwegians (n = 4,877) [29], and a study in Chinese (n = 5,566) [26] failed to find an association between CYP24A1 SNP rs6013897 and T2D. In the vitamin D transport gene, GC, significant associations were reported in Asians [41,42,43] but not in European populations such as Germans, Polish and Norwegians [26, 28, 29, 44]. However, a recent prospective observational study in Europeans recruited from Italian outpatient clinics did provide an evidence of association between GC (rs4588) and 25(OH)D concentrations (P = 1 × 10− 6) in T2D patients [25]. The GC SNPs, rs7041 (codon 416) and rs4588 (codon 420), showed a significant association with T2D in a Bangladeshi population (n = 211) [42]. The participants with Glu/Glu at codon 416 had 2.87 times increased risk of T2D and the participants with Lys/Lys genotype at codon 420 had 8.9 times increased risk of T2D. Furthermore, the combined allele score of these two SNPs, rs7041 and rs4588, was significantly associated with T2D in a case-control study in a Pakistani population (n = 330) [41]. In a meta-analysis of studies of Caucasians and Asians, there was no association in the overall analysis of SNPs at codon 416 and codon 420 with the risk of T2D, however, after stratification based on ethnicity, a significant association was found in Asians at codon 420, where the allele ‘Lys’ had a 1.49 times increased risk of T2D. In addition, a 1.36 times increased T2D risk was observed for those with ‘Asp/Asp’ genotype at codon 416 compared to those with ‘Glu/Asp’ and ‘Glu/Glu’ genotypes [43].
To date, most of the large studies failed to demonstrate a significant association of the SNPs in the CYP27B1 and CYP24A1 genes with T2D suggesting that these genes are unlikely to play a potential role in the pathogenesis of T2D. However, based on the published studies, genetic variants in GC gene may have an impact on T2D among Asians but not in Europeans, which could be due to the existence of genetic heterogeneity across the two ethnicities. The possible mechanism of action of GC in T2D could be mediated through the regulation of plasma calcium levels, which is known to regulate insulin synthesis and secretion, and through a direct action on pancreatic beta-cell function [45].
II. The Vitamin D Receptor (VDR)
The VDR gene is located on chromosome 12q13.11. Vitamin D receptor, encoded by VDR gene, is a member of the nuclear receptor of transcription factors. The secosteroid 1α,25(OH)2D3, a natural ligand to VDR, enters the target cell and binds to its receptor. The 1α,25(OH)2D3-VDR complex heterodimerizes with the retinoid X receptor (RXR) and binds to the vitamin D response element (VDRE), a sequence of DNA nucleotides in the promoter region of the vitamin D regulated genes. The VDR/RXR-VDRE complex attracts coactivators and gene transcription is initiated to produce mRNA, which is then translated to the corresponding protein [46,47,48]. The VDR gene is predominantly expressed in kidneys, bones and the intestinal tract for bone homeostasis but further expression has been discovered in almost all human tissues and organs including adipose tissue and cells involved in the regulation of glucose metabolism, such as muscle and pancreatic cells [49]. Several functional VDR SNPs are known: BsmI, ApaI, Tru9I in intron 8, TaqI in exon 9 and FokI in exon 2. These genetic variants are named after their restriction enzyme sites [49]. Another VDR SNP is Cdx2, which is found in the promoter region [3]. Some of the SNPs in the VDR are restriction fragment length polymorphisms such as BsmI, ApaI and TaqI. VDR SNPs are closely linked to a microsatellite poly A repeat of variable length in the 3´UTR region which is thought to affect VDR translation and may affect mRNA stability [3, 50]. VDR SNPs such as BsmI, ApaI and TaqI and FokI are the most commonly studied genetic variants in association with non-skeletal outcomes [51]. Genetic variants in the VDR gene have also shown to contribute to the genetic susceptibility of T2D by modulating insulin secretion and affecting cellular insulin sensitivity [45]. Allelic differences in the VDR gene have also shown to be possible contributors to obesity through modulating adipocyte function and affecting adipocyte inflammation [45, 51, 52].
Obesity
BsmI SNP rs1544410
Thirteen studies have examined the association between the VDR BsmI SNP and obesity-related traits (Table 1), of which nine have reported a significant association in the Arab, Brazilian, Polish, French, Swedish, and Vietnamese populations (n = 140–891) [53,54,55,56,57,58,59,60,61]. In the Arab population, associations have been consistent in four studies (n = 198 – n = 891) [53,54,55, 60] suggesting that the presence of BsmI risk allele could be a risk factor for obesity in this ethnic group. Nevertheless, the sample sizes were relatively small; hence, larger studies are required in this population to confirm the risk of BsmI polymorphism on obesity.
ApaI SNP rs7975232
Twelve Studies have investigated the association between the VDR ApaI polymorphism and obesity traits (Table 1) and four of them have reported significant associations [56, 62, 68, 69] in the Chinese, Vietnamese and Czech populations (n = 140–882). Two studies in Asian postmenopausal women (n = 140; n = 260) reported significant associations with the ApaI variant (P = .036; P = .049, respectively) [56, 68], where the study in the postmenopausal Vietnamese women found that the ApaI risk allele ‘a’ had a 3 times increased risk of overweight and obesity [56]. Hence, ApaI risk allele may be an important factor predisposing individuals to adult onset obesity among Asian postmenopausal women; however, further large studies are warranted in men and women to validate the role of the ApaI variant in this group.
TaqI SNP rs731236
Fourteen studies have examined the association between the VDR TaqI polymorphism and obesity traits (Table 1), of which six have shown significant association in several populations including Saudi, Czech, Greek, French, and Chinese (n = 184–891) [54, 55, 61, 62, 65, 70]. In a case-control study in the Chinese Han population (n = 529), the TaqI polymorphism showed a strong association with obesity (P < .001) [65] where the ‘t’ allele was 2.67 times more prevalent in the obese group compared to the control group and the ‘tt’ genotype showed a 3.79 times increased risk of obesity. For the European and Arab populations, results have been largely inconsistent. Of the five studies in European population (n = 184–123,865), three reported an association between obesity traits and the TaqI polymorphism [61, 62, 70], while other studies failed to report a significant association (n = 701–123,865) [23, 63]. Similarly, in the Arab population, two studies reported an association of the TaqI polymorphism with obesity in Saudi individuals (n = 891; n = 300) [54, 55], where the minor ‘t’ allele was significantly more frequent in the obese group compared to the control group (P = .009; P = .041, respectively). But, three other studies in Arabs from Saudi, Bahrain, and UAE (n = 198–570) reported no significant association [37, 53, 60]. Although the results are inconsistent and conflicting, given that the majority of large studies failed to find an association, it is unlikely for the TaqI polymorphism to have a significant impact on obesity in Europeans and Arabs. However, due to existence of genetic heterogeneity, the polymorphism may have an effect on obesity in other ethnic groups such as Chinese population.
FokI SNP rs2228570
Twelve Studies have investigated the association between the VDR FokI polymorphism and obesity traits (Table 1) and only three of these have been consistent in reporting a significant association in Caucasian men (n = 302) and Czech (n = 517) and Chinese (n = 882) populations [62, 69, 71]. Studies in other ethnic groups such as Europeans, Asians, and Arabs (n = 140–1,773) failed to find an association of the FokI variant with obesity traits [53, 56, 58, 60, 61, 63, 65, 66, 72]. The overall evidence from these genetic epidemiological studies failed to support a consistent association of this polymorphism with obesity traits.
Cdx2 SNP rs11568820
Three Studies have investigated the association between the VDR Cdx2 SNP and obesity traits (Table 1). Two of these studies reported significant associations between Cdx2 SNP and obesity and its related traits [66, 72]. A significant association of Cdx2 SNP with waist circumference (WC) and abdominal height (AH) (P = .03; P = .05, respectively) was shown in a cross-sectional study in American Caucasian women (n = 1,773) [72]; however, the association did not remain significant after Bonferroni correction. On the other hand, a family-based study of 400 nuclear Chinese families (n = 1,215) [66] reported significant associations of Cdx2 SNP with body mass index (BMI) (P = .046), fat mass (FM) (P = .004) and PFM (P = .02). Furthermore, the analysis in 415 sons showed that those with ‘AA’ genotype had 5.4% higher BMI, 18.8% higher FM and 14.8% higher PFM compared to those with ‘AG’ genotype. Nonetheless, data from two large cohorts, the 1958 British Birth Cohort (n = 5,224) and the GIANT Consortium (n = 123,865), failed to find an association between the Cdx2 polymorphism and obesity-related traits [23]. Even though the results are inconsistent regarding the effect of the VDR Cdx2 polymorphism on obesity traits, majority of the large studies in Caucasians have failed to find significant associations and hence this polymorphism is unlikely to have an impact among the Caucasian population. However, such large studies in other ethnic groups are required to confirm the role of this polymorphism in obesity.
Other VDR SNPs
Four studies have investigated other VDR SNPs; of which, two have shown significant association [62, 72], while the other two failed to report a significant association with obesity traits [23, 69]. A study in American Caucasian women (n = 1,773) showed a significant association of five VDR SNPs (rs739837, rs2239179, rs3819545, rs3782905, and rs4760648) with obesity outcomes [72]. Another VDR SNP, EcoRV rs4516035 showed a significant association with sum of skin fold thickness (SSFT) (P = .02), where there was 7.7 times decrease in SSFT among those with the ‘GG’ genotype compared to those with ‘AA’ genotype in 882 Czech individuals [62]. Given that the studies have been conducted in small number of samples, large studies are required to further elucidate the role of these SNPs in obesity.
Type 2 Diabetes
BsmI SNP rs1544410
Twenty six studies have examined the association between the VDR BsmI polymorphism and T2D (Table 2), of which only seven have demonstrated a significant association in Arab, Indian, Chinese and German populations (n = 80–627) [74,75,76,77,78,79,80]. The remaining fifteen studies failed to show a significant association in populations of similar ethnicities (n = 57–4,563) [53, 61, 81,82,83,84,85,86,87,88,89,90,91,92,93]. The meta-analysis studies have also shown inconsistent findings, where, of the four meta-analyses in Asian and Caucasian populations (n = 2,608–6,274) [94,95,96,97], two of the studies (n = 4,578; n = 6,274) showed a marginal association between BsmI SNP and risk of T2D (P = .033; P = .038, respectively) [95, 96]. Despite several studies have been carried out in multiple ethnic groups, the association between the VDR BsmI variant and T2D is still questionable. It is possible that the effect of gene-lifestyle interactions might mask the genetic effect in some of the populations and hence studies focusing on gene-diet and gene-physical activity interactions are required to confirm this association.
ApaI SNP rs7975232
None of the fifteen studies have demonstrated a significant association between ApaI polymorphism and risk of T2D (n = 171–4,563) (Table 2) including the two meta-analyses that investigated the association in up to 3,871 individuals [94, 95]. However, there was one study that reported a borderline association (P = .058) in a Caucasian US population (n = 1,545) [87]. Furthermore, a study in 171 Bangladeshi participants had shown a significant association (P = .006) between ApaI SNP and insulin secretion index (ISI) [84]. Based on these studies, it can be concluded that it is unlikely that the VDR ApaI SNP has a significant role in the development of T2D.
TaqI SNP rs731236
Despite twenty three studies have been carried out to explore the association between VDR TaqI polymorphism and T2D (Table 2), only one small study has reported a significant association in the Indian population (n = 80) where ‘t’ allele had a 1.5 times increased risk of T2D [76]. Even the two large meta-analyses in Caucasians and Asians (n up to 3,826) have failed to show an association [94, 95]. Given that majority of the large studies failed to demonstrate a significant association, the VDR TaqI SNP might not be a strong candidate for T2D.
FokI SNP rs2228570
To date, eighteen studies have examined the association between the VDR FokI SNP and T2D (Table 2); five studies in the Saudi, Emirati, Egyptian and the Chilean populations (n = 100–4,077) [53, 78, 81, 83, 85] and three meta-analyses (n = 2,070–4,077) in Asians and Caucasians [94,95,96] have reported significant associations. Given that large meta-analysis studies have confirmed the association of the SNP with T2D, the VDR FokI SNP might have an important role to play in T2D among Asians and Caucasians. Future meta-analyses should focus on other ethnic groups to identify the existence of genetic heterogeneity in the association between VDR FokI SNP and T2D.
Cdx2 SNP rs11568820
Only two studies have examined the association between the VDR Cdx2 SNP and T2D (Table 2); one in the Italian population (n = 1,788) where individuals with ‘AA’ genotype of the SNP had 1.43 times increased risk of T2D compared to those with ‘GG + GA’ genotypes (P = .002) [105] and the other one in the Norwegian populations (n = 4,563), which failed to show an association [93]. More studies in different ethnic groups are required to understand the role of the VDR Cdx2 SNP in T2D.
BglI SNP rs739837
Of the four studies that examined the association between the VDR BglI polymorphism and T2D (Table 2), two large case-control studies in the Chinese Han population (n = 1,191 and n = 3,714) reported a significant association of the BglI polymorphism with increased risk of T2D [92, 104]. However, there was no association between the BglI variant and risk of T2D in a smaller study in the Chinese Han (n = 420) and Chinese Hui (n = 269) populations [79] as well as no association was observed in the Caucasian population (1958 British Birth Cohort, n = 5,160) [73]. This inconsistency can be attributed to the sample size and the existence of genetic heterogeneity between the Caucasian and Chinese ethnic groups; but additional large studies are warranted to confirm or refute these findings.
Other VDR SNPs
A few studies have investigated other SNPs in the VDR gene; three studies reported a significant association of the VDR SNPs, rs2239179, rs7968585, rs2189480 and rs3847987, with T2D risk [92, 93, 106]. The two VDR SNPs, rs2189480 and rs3847987, were reported to have significant association (P = < 0.003 and P = .032, respectively) with T2D in a Chinese Henan population (n = 574) [106]. The VDR SNP rs2239179 was found to be significantly associated with increased risk of T2D (P = .049) in Chinese Han men who were above 55 years (n = 1,191) [92] and the VDR SNP rs7968585 showed a significant association with T2D (P = .044) in the Norwegian population (n = 4,563) [93]. These SNPs might have a functional importance in the pathogenesis of T2D, which needs to be further investigated and evaluated by large-scale studies in different populations.
Conclusions
In summary, our review has pooled all available data related to the association of vitamin D pathway genes with metabolic diseases such as obesity and T2D and has identified 57 significant associations of vitamin D pathway genes with obesity and its related traits such as BMI, WC, FM, SSFT, and AH in SNPs from 5 genes in the vitamin D pathway. Of the 57 associations, only one was from the vitamin D synthesis-related genes (DHCR7, CYP2R1). The vast majority of the associations (56 associations) were identified in the vitamin D metabolism-related genes (CYP24A1, GC and VDR) and, in particular, the VDR gene SNPs showed 48 significant associations with obesity outcomes. In addition, our review has identified 35 significant associations in relation to T2D in SNPs from 7 genes in the vitamin D pathway. A similar pattern of association was seen where only five significant associations were reported for vitamin D synthesis-related genes (DHCR7 and CYP2R1) as compared to the vitamin D metabolism-related genes (CYP24A1, GC and VDR), where 30 significant associations were observed, and, in particular, 26 significant associations with T2D was seen for the VDR SNPs.
The vitamin D synthesis-related genes, DHCR7 and CYP2R1, have not been adequately investigated in relation to obesity and T2D. There is a gap in the research pertaining to the effect of vitamin D synthesis-related gene polymorphisms on obesity and T2D. For vitamin D metabolism-related genes, the literature is still lacking in several ethnic groups, and available results are inconsistent. Understanding how genetics influence serum 25(OH)D levels is important for identifying persons at risk of vitamin D deficiency and improving the understanding of the observed association between vitamin D deficiency and several diseases. Large well-designed genetic association studies considering gene-environment interactions in multiple ethnic populations are necessary to improve the understanding of the role of vitamin D-related polymorphisms in metabolic diseases. Furthermore, functional characterization of the vitamin D-related SNPs is highly warranted to facilitate the understanding of the pathogenetic mechanisms of obesity and diabetes, which will provide the platform for developing strategies to prevent and treat metabolic diseases.
Data Availability
The data generated from the literature search are included in the Tables 1 and 2.
Abbreviations
- T2D:
-
Type 2 diabetes
- SNP:
-
Single nucleotide polymorphism
- 25(OH)D:
-
25 hydroxyvitamin D
- 1,25(OH)2D:
-
1,25 dihydroxyvitamin D
- DHCR7 :
-
7-dehydrocholesterol reductase
- CYP2R1 :
-
25-hydroxylase.
- CYP27B1 :
-
1α-hydroxylase
- CYP24A1 :
-
24-hydroxylase
- GC :
-
Group-specific component
- DBP :
-
Vitamin D binding protein
- VDR :
-
Vitamin D receptor
- GWAS:
-
Genome wide association studies
- RXR:
-
Retinoid X receptor
- VDRE:
-
Vitamin D receptor elements
- BMI:
-
Body mass index
- PFM:
-
Percentage fat mass
- FM:
-
Fat mass
- WC:
-
Waist circumference
- AH:
-
Abdominal Height
- SSFT:
-
Sum of skin fold thickness
- TBF:
-
Total body fat
- VF:
-
Visceral fat
- BM:
-
Body mass
- WHR:
-
Waist hip ratio
- PBF:
-
Percent body fat
- PTF:
-
Percent total fat
- PVD:
-
Percent visceral deposit
- TSFT:
-
Triceps skinfold thickness
- BG:
-
Blood glucose
- ISI:
-
Insulin secretion index
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycated haemoglobin
- FSG:
-
Fasting serum glucose
References
Khundmiri SJ, Murray RD, Lederer E, PTH, Vitamin D. Compr Physiol. 2016;6:561–601.
Basit S. Vitamin D in health and disease: a literature review. Br J Biomed Sci. 2013;70:161–72.
Dusso AS, Brown AJ, Slatopolsky E, Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28.
Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13:404–17.
Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89.
Dastani Z, Li R, Richards B. Genetic regulation of vitamin D levels. Calcif Tissue Int. 2013;92:106–17.
Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91:115–24.
Holick MF High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006, 81, 353–373.
Wang TJ. Vitamin D and Cardiovascular Disease. Annu Rev Med. 2016;67:261–72.
Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(Suppl 2):491–9.
Jiang X, Kiel DP, Kraft P. The genetics of vitamin D. Bone. 2019;126:59–77.
Bahrami A, Sadeghnia HR, Tabatabaeizadeh SA, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, Ferns GA, Mobarhan MG, Avan A. Genetic and epigenetic factors influencing vitamin D status. J Cell Physiol. 2018;233:4033–43.
Erkus E, Aktas G, Kocak MZ, Duman TT, Atak BM, Savli H. Diabetic regulation of subjects with type 2 diabetes mellitus is associated with serum vitamin D levels. Rev Assoc Med Bras (1992). 2019;65:51–55.
Mathieu SV, Fischer K, Dawson-Hughes B, Freystaetter G, Beuschlein F, Schietzel S, Egli A; Bischoff-Ferrari H. A. Association between 25-Hydroxyvitamin D Status and Components of Body Composition and Glucose Metabolism in Older Men and Women. Nutrients. 2018;10.
Pantovic A, Zec M, Zekovic M, Obrenovic R, Stankovic S, Glibetic M. Vitamin D Is Inversely Related to Obesity: Cross-Sectional Study in a Small Cohort of Serbian Adults. J Am Coll Nutr. 2019;38:405–14.
Zheng JS, Imamura F, Sharp SJ, van der Schouw YT, Sluijs I, Gundersen TE, Ardanaz E, Boeing H, Bonet C, Gomez JH, et al. Association of Plasma Vitamin D Metabolites With Incident Type 2 Diabetes: EPIC-InterAct Case-Cohort Study. J Clin Endocrinol Metab. 2019;104:1293–303.
Kuan V, Martineau AR, Griffiths CJ, Hypponen E, Walton R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144–53.
Prabhu AV, Luu W, Li D, Sharpe LJ, Brown AJ. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog Lipid Res. 2016;64:138–51.
Abbas MA. Physiological functions of Vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165:369–81.
Dorjgochoo T, Shi J, Gao YT, Long J, Delahanty R, Xiang YB, Cai Q, Shu XO. Genetic variants in vitamin D metabolism-related genes and body mass index: analysis of genome-wide scan data of approximately 7000 Chinese women. Int J Obes (Lond). 2012;36:1252–5.
Foucan L, Velayoudom-Cephise FL, Larifla L, Armand C, Deloumeaux J, Fagour C, Plumasseau J, Portlis ML, Liu L, Bonnet F, et al. Polymorphisms in GC and NADSYN1 Genes are associated with vitamin D status and metabolic profile in Non-diabetic adults. BMC Endocr Disord. 2013;13:36–43.
Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383.
Vimaleswaran KS, Cavadino A, Berry DJ, Whittaker JC, Power C, Jarvelin MR, Hypponen E. Genetic association analysis of vitamin D pathway with obesity traits. Int J Obes (Lond). 2013;37:1399–406.
Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:298–306.
Bertoccini L, Bailetti D, Buzzetti R, Cavallo MG, Copetti M, Cossu E, D’Angelo P, De Cosmo S, Di Mauro L, Leonetti F, et al. Variability in genes regulating vitamin D metabolism is associated with vitamin D levels in type 2 diabetes. Oncotarget. 2018;9:34911–8.
Lu L, Bennett DA, Millwood IY, Parish S, McCarthy MI, Mahajan A, Lin X, Bragg F, Guo Y, Holmes MV, et al. Association of vitamin D with risk of type 2 diabetes: A Mendelian randomisation study in European and Chinese adults. PLoS Med. 2018;15:e1002566.
Wang Y, Yu F, Yu S, Zhang D, Wang J, Han H, Sun H, Xue Y, Ba Y, Wang C, et al. Triangular relationship between CYP2R1 gene polymorphism, serum 25(OH)D3 levels and T2DM in a Chinese rural population. Gene. 2018;678:172–6.
Buijsse B, Boeing H, Hirche F, Weikert C, Schulze MB, Gottschald M, Kühn T, Katzke VA, Teucher B, Dierkes J, et al. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: a prospective case-cohort study. Eur J Epidemiol. 2013;28:743–52.
Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njolstad I, Lochen ML, Figenschau Y, Berg JP, Svartberg J, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromso Study. PLoS One. 2012;7:e37295.
Jones G, Kottler ML, Schlingmann KP. Genetic Diseases of Vitamin D Metabolizing Enzymes. Endocrinol Metab Clin North Am. 2017;46:1095–117.
Landrier JF, Karkeni E, Marcotorchino J, Bonnet L, Tourniaire F. Vitamin D modulates adipose tissue biology: possible consequences for obesity? Proc Nutr Soc. 2016;75:38–46.
Molin A, Coudray N, Richard N, Kottler M-L, Deschenes G, Kesler-Roussey G, Tiulpakov A, Baudoin R, Jones G, Kaufmann M, et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. J Clin Endocrinol Metab. 2015;100:E1343–52.
Nesterova G, Malicdan MC, Yasuda K, Sakaki T, Vilboux T, Ciccone C, Horst R, Huang Y, Golas G, Introne W, et al. 1,25-(OH)2D-24 Hydroxylase (CYP24A1) Deficiency as a Cause of Nephrolithiasis. Clin J Am Soc Nephrol. 2013;8:649–57.
Bikle DD, Schwartz J, Vitamin DB, Protein. Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front Endocrinol. 2019;10:317–7.
Jassil NK, Sharma A, Bikle D, Wang X. Vitamin D binding protein and 25-hydroxyvitamin D levels: emerging clinical applications. Endocr Pract. 2017;23:605–13.
Jiang H, Xiong DH, Guo YF, Shen H, Xiao P, Yang F, Chen Y, Zhang F, Recker RR, Deng H. W. Association analysis of vitamin D-binding protein gene polymorphisms with variations of obesity-related traits in Caucasian nuclear families. Int J Obes (Lond). 2007;31:1319–24.
Almesri N, Das NS, Ali ME, Gumaa K, Giha HA. Independent associations of polymorphisms in vitamin D binding protein (GC) and vitamin D receptor (VDR) genes with obesity and plasma 25OHD3 levels demonstrate sex dimorphism. Appl Physiol Nutr Metab. 2016;41:345–53.
Malecki MT, Klupa T, Wolkow P, Bochenski J, Wanic K, Sieradzki J. Association study of the vitamin D: 1alpha-hydroxylase (CYP1alpha) gene and type 2 diabetes mellitus in a Polish population. Diabetes Metab. 2003;29:119–24.
Yu S, Li X, Wang Y, Mao Z, Wang C, Ba Y, Li W. Maternal transmission disequilibrium of rs2248359 in type 2 diabetes mellitus families and its association with vitamin D level in offspring. Sci Rep. 2018;8:1345–52.
Yu S, Li X, Wang Y, Mao Z, Wang C, Ba Y, Li W. Transmission disequilibrium of rs4809957 in type 2 diabetes mellitus families and its association with vitamin D deficiency: A family-based case-control study. J Diabetes Complications. 2018;32:406–10.
Iqbal K, Islam N, Azam I, Asghar A, Mehboobali N, Iqbal MP. Association of Vitamin D binding protein polymorphism with risk of type 2 diabetes mellitus in a Pakistani urban population: A case control study. J Pak Med Assoc. 2017;67:1658–63.
Rahman MM, Hosen MB, Faruk MO, Hasan MM, Kabir Y, Howlader M. Z. H. Association of vitamin D and vitamin D binding protein (DBP) gene polymorphism with susceptibility of type 2 diabetes mellitus in Bangladesh. Gene. 2017;636:42–7.
Wang G, Li Y, Li L, Yu F, Cui L, Ba Y, Li W, Wang C. Association of the vitamin D binding protein polymorphisms with the risk of type 2 diabetes mellitus: a meta-analysis. BMJ Open. 2014;4:e005617.
Malecki MT, Klupa T, Wanic K, Cyganek K, Frey J, Sieradzki J. Vitamin D binding protein gene and genetic susceptibility to type 2 diabetes mellitus in a Polish population. Diabetes Res Clin Pract. 2002;57:99–104.
Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–97.
Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79:1–9.
Nezbedova P, Brtko J. 1alpha,25-dihydroxyvitamin D3 inducible transcription factor and its role in the vitamin D action. Endocr Regul. 2004;38:29–38.
Bikle D, Vitamin D. Production, Metabolism, and Mechanisms of Action. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A et al, editors Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK278935/.
Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab. 2005;31:318–25.
Rahmadhani R, Zaharan NL, Mohamed Z, Moy FM, Jalaludin MY. The associations between VDR BsmI polymorphisms and risk of vitamin D deficiency, obesity and insulin resistance in adolescents residing in a tropical country. PLoS One. 2017;12:e0178695.
Ruiz-Ojeda FJ, Anguita-Ruiz A, Leis R, Aguilera CM. Genetic Factors and Molecular Mechanisms of Vitamin D and Obesity Relationship. Ann Nutr Metab. 2018;73:89–99.
Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–23.
Al-Daghri NM, Al-Attas OS, Alkharfy KM, Khan N, Mohammed AK, Vinodson B, Ansari MG, Alenad A, Alokail MS. Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene. 2014;542:129–33.
Al-Daghri NM, Guerini FR, Al-Attas OS, Alokail MS, Alkharfy KM, Draz HM, Agliardi C, Costa AS, Saulle I, Mohammed AK, et al. Vitamin D receptor gene polymorphisms are associated with obesity and inflammosome activity. PLoS One. 2014;9:e102141.
Al-Hazmi AS, Al-Mehmadi MM, Al-Bogami SM, Shami AA, Al-Askary AA, Alomery AM, Al-Shehri SS, Dahlawi H, Abdulrazag K, Ali T, et al. Vitamin D receptor gene polymorphisms as a risk factor for obesity in Saudi men. Electron Physician. 2017;9:5427–33.
Binh TQ, Nakahori Y, Hien VT, Khan NC, Lam NT, Mai le B, Yamamoto S Correlations between genetic variance and adiposity measures, and gene x gene interactions for obesity in postmenopausal Vietnamese women. J Genet. 2011;90:1–9.
Ferrarezi DA, Bellili-Munoz N, Nicolau C, Cheurfa N, Guazzelli IC, Frazzatto E, Velho G, Villares S. M. Allelic variations in the vitamin D receptor gene, insulin secretion and parents’ heights are independently associated with height in obese children and adolescents. Metabolism. 2012;61:1413–21.
Filus A, Trzmiel A, Kuliczkowska-Plaksej J, Tworowska U, Jedrzejuk D, Milewicz A, Medras M. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. Aging Male. 2008;11:134–9.
Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004;150:323–8.
Hasan HA, AbuOdeh RO, Muda W, Mohamed H, Samsudin AR. Association of Vitamin D receptor gene polymorphisms with metabolic syndrome and its components among adult Arabs from the United Arab Emirates. Diabetes Metab Syndr. 2017;11(Suppl 2):531–7.
Ye WZ, Reis AF, Dubois-Laforgue D, Bellanne-Chantelot C, Timsit J, Velho G. Vitamin D receptor gene polymorphisms are associated with obesity in type 2 diabetic subjects with early age of onset. Eur J Endocrinol. 2001;145:181–6.
Bienertova-Vasku J, Zlamal F, Pohorala A, Mikes O, Goldbergova-Pavkova M, Novak J, Splichal Z, Pikhart H. Allelic variants in vitamin D receptor gene are associated with adiposity measures in the central-European population. BMC Med Genet. 2017;18:90.
Correa-Rodriguez M, Carrillo-Avila JA, Schmidt-RioValle J, Gonzalez-Jimenez E, Vargas S, Martin J, Rueda-Medina B. Genetic association analysis of vitamin D receptor gene polymorphisms and obesity-related phenotypes. Gene. 2018;640:51–6.
Tworowska-Bardzinska U, Lwow F, Kubicka E, Laczmanski L, Jedzrzejuk D, Dunajska K, Milewicz A. The vitamin D receptor gene BsmI polymorphism is not associated with anthropometric and biochemical parameters describing metabolic syndrome in postmenopausal women. Gynecol Endocrinol. 2008;24:514–8.
Fan HR, Lin LQ, Ma H, Li Y, Sun CH. Association between vitamin D receptor gene polymorphism (TaqI) and obesity in Chinese population. J Genet. 2015;94:473–8.
Gu JM, Xiao WJ, He JW, Zhang H, Hu WW, Hu YQ, Li M, Liu YJ, Fu WZ, Yu JB, et al. Association between VDR and ESR1 gene polymorphisms with bone and obesity phenotypes in Chinese male nuclear families. Acta Pharmacol Sin. 2009;30:1634–42.
Rivera-Leon EA, Palmeros-Sanchez B, Llamas-Covarrubias IM, Fernandez S, Armendariz-Borunda J, Gonzalez-Hita M, Bastidas-Ramirez BE, Zepeda-Moreno A, Sanchez-Enriquez S. Vitamin-D receptor gene polymorphisms (TaqI and ApaI) and circulating osteocalcin in type 2 diabetic patients and healthy subjects. Endokrynol Pol. 2015;66:329–33.
Xu H, Xiong DH, Xu FH, Zhang YY, Lei SF, Deng HW. Association between VDR ApaI polymorphism and hip bone mineral density can be modified by body mass index: a study on postmenopausal Chinese women. Acta Biochim Biophys Sin (Shanghai). 2005;37:61–7.
Shen F, Wang Y, Sun H, Zhang D, Yu F, Yu S, Han H, Wang J, Ba Y, Wang C, et al. Vitamin D receptor gene polymorphisms are associated with triceps skin fold thickness and body fat percentage but not with body mass index or waist circumference in Han Chinese. Lipids Health Dis. 2019;18:97.
Vasilopoulos Y, Sarafidou T, Kotsa K, Papadimitriou M, Goutzelas Y, Stamatis C, Bagiatis V, Tsekmekidou X, Yovos JG. Mamuris Z. VDR TaqI is associated with obesity in the Greek population. Gene. 2012;512:237–9.
Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:10–5.
Ochs-Balcom HM, Chennamaneni R, Millen AE, Shields PG, Marian C, Trevisan M, Freudenheim JL. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am J Clin Nutr. 2011;93:5–10.
Vimaleswaran KS, Power C, Hypponen E. Interaction between vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D concentrations on metabolic and cardiovascular disease outcomes. Diabetes Metab. 2014;40:386–9.
Al-Daghri NM, Al-Attas O, Alokail MS, Alkharfy KM, Draz HM, Agliardi C, Mohammed AK, Guerini FR, Clerici M. Vitamin D receptor gene polymorphisms and HLA DRB1*04 cosegregation in Saudi type 2 diabetes patients. J Immunol. 2012;188:1325–32.
Malik R, Farooq R, Mehta P, Ishaq S, Din I, Shah P, Majid S. Association of Vitamin D Receptor Gene Polymorphism in Adults With Type 2 Diabetes in the Kashmir Valley. Can J Diabetes. 2018;42:251–6.
Mukhopadhyaya PN, Acharya A, Chavan Y, Purohit SS, Mutha A. Metagenomic study of single-nucleotide polymorphism within candidate genes associated with type 2 diabetes in an Indian population. Genet Mol Res. 2010;9:2060–8.
Ortlepp JR, Lauscher J, Hoffmann R, Hanrath P, Joost HG. The vitamin D receptor gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. Diabet Med. 2001;18:842–5.
Safar HA, Chehadeh SEH, Abdel-Wareth L, Haq A, Jelinek HF, ElGhazali G, Anouti FA. Vitamin D receptor gene polymorphisms among Emirati patients with type 2 diabetes mellitus. J Steroid Biochem Mol Biol. 2018;175:119–24.
Xu JR, Yang Y, Liu XM, Wang YJ. Association of VDR polymorphisms with type 2 diabetes mellitus in Chinese Han and Hui populations. Genet Mol Res. 2014;13:9588–98.
Zhang H, Wang J, Yi B, Zhao Y, Liu Y, Zhang K, Cai X, Sun J, Huang L. Liao Q. BsmI polymorphisms in vitamin D receptor gene are associated with diabetic nephropathy in type 2 diabetes in the Han Chinese population. Gene. 2012;495:183–8.
Angel B, Lera L, Marquez C, Albala C. The association of VDR polymorphisms and type 2 diabetes in older people living in community in Santiago de Chile. Nutr Diabetes. 2018;8:31.
Bid HK, Konwar R, Aggarwal CG, Gautam S, Saxena M, Nayak VL, Banerjee M, Vitamin D. receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci. 2009;63:187–94.
Gendy HIE, Sadik NA, Helmy MY, Rashed LA. Vitamin D receptor gene polymorphisms and 25(OH) vitamin D: Lack of association to glycemic control and metabolic parameters in type 2 diabetic Egyptian patients. J Clin Transl Endocrinol. 2019;15:25–9.
Hitman GA, Mannan N, McDermott MF, Aganna E, Ogunkolade BW, Hales CN, Boucher BJ. Vitamin D receptor gene polymorphisms influence insulin secretion in Bangladeshi Asians. Diabetes. 1998;47:688–90.
Mackawy AM, Badawi ME. Association of vitamin D and vitamin D receptor gene polymorphisms with chronic inflammation, insulin resistance and metabolic syndrome components in type 2 diabetic Egyptian patients. Meta Gene. 2014;2:540–56.
Malecki MT, Frey J, Moczulski D, Klupa T, Kozek E, Sieradzki J. Vitamin D receptor gene polymorphisms and association with type 2 diabetes mellitus in a Polish population. Exp Clin Endocrinol Diabetes. 2003;111:505–9.
Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism. 2002;51:356–9.
Sarma D, Chauhan VS, Saikia KK, Sarma P, Nath S. Prevalence Pattern of Key Polymorphisms in the Vitamin D Receptor gene among Patients of Type 2 Diabetes Mellitus in Northeast India. Indian J Endocrinol Metab. 2018;22:229–35.
Shah DB, D Doshi D, Singh K, Patel R. Investigation of the VDR Gene Polymorphism in Unrelated Gujarati Group with and without Diabetic Mellitus Type-2. Res J Pharm Biol Chem Sci. 2015;6:465–8.
Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takacs I, Lakatos P. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol. 2001;144:385–9.
Vedralova M, Kotrbova-Kozak A, Zeleznikova V, Zoubkova H, Rychlik I, Cerna M. Polymorphisms in the vitamin D receptor gene and parathyroid hormone gene in the development and progression of diabetes mellitus and its chronic complications, diabetic nephropathy and non-diabetic renal disease. Kidney Blood Press Res. 2012;36:1–9.
Yu F, Wang C, Wang L, Jiang H, Ba Y, Cui L, Wang Y, Yu S, Li W. Study and evaluation the impact of vitamin D receptor variants on the risk of type 2 diabetes mellitus in Han Chinese. J Diabetes. 2017;9:275–84.
Zostautiene I, Jorde R, Schirmer H, Mathiesen EB, Njolstad I, Lochen ML, Wilsgaard T, Joakimsen RM, Kamycheva E. Genetic Variations in the Vitamin D Receptor Predict Type 2 Diabetes and Myocardial Infarction in a Community-Based Population: The Tromso Study. PLoS One. 2015;10:e0145359.
Li L, Wu B, Liu JY, Yang LB. Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res. 2013;44:235–41.
Wang Q, Xi B, Reilly KH, Liu M, Fu M. Quantitative assessment of the associations between four polymorphisms (FokI, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep. 2012;39:9405–14.
Yu F, Cui LL, Li X, Wang CJ, Ba Y, Wang L, Li J, Li C, Dai LP, Li WJ. The genetic polymorphisms in vitamin D receptor and the risk of type 2 diabetes mellitus: an updated meta-analysis. Asia Pac J Clin Nutr. 2016;25:614–24.
Zhu B, Zhao HL, Ou C, Huang LS, Li PZ, Lao M. Association of vitamin D receptor BsmI gene polymorphism with the risk of type 2 diabetes mellitus. J Recept Signal Transduct Res. 2014;34:458–62.
Dilmec F, Uzer E, Akkafa F, Kose E, van Kuilenburg AB. Detection of VDR gene ApaI and TaqI polymorphisms in patients with type 2 diabetes mellitus using PCR-RFLP method in a Turkish population. J Diabetes Complications. 2010;24:186–91.
Boullu-Sanchis S, Lepretre F, Hedelin G, Donnet JP, Schaffer P, Froguel P, Pinget M. Type 2 diabetes mellitus: association study of five candidate genes in an Indian population of Guadeloupe, genetic contribution of FABP2 polymorphism. Diabetes Metab. 1999;25:150–6.
Nosratabadi R, Arababadi MK, Salehabad VA. Vitamin D Receptor Polymorphisms in Type 2 Diabetes in Southeastern Iranian Patients. Lab Medicine. 2011;42:32–4.
Maia J, da Silva AS, do Carmo RF, de Mendonca TF, Griz LH, Moura P, Bandeira F. The association between vitamin D receptor gene polymorphisms (TaqI and FokI), Type 2 diabetes, and micro-/macrovascular complications in postmenopausal women. Appl Clin Genet. 2016;9:131–6.
Vural HC, Maltas E. RT-qPCR assay on the vitamin D receptor gene in type 2 diabetes and hypertension patients in Turkey. Genet Mol Res. 2012;11:582–90.
Bertoccini L, Sentinelli F, Leonetti F, Bailetti D, Capoccia D, Cimini FA, Barchetta I, Incani M, Lenzi A, Cossu E, et al. The vitamin D receptor functional variant rs2228570 (C > T) does not associate with type 2 diabetes mellitus. Endocr Res. 2017;42:331–5.
Jia J, Ding H, Yang K, Mao L, Zhao H, Zhan Y, Shen C. Vitamin D Receptor Genetic Polymorphism Is Significantly Associated with Risk of Type 2 Diabetes Mellitus in Chinese Han Population. Arch Med Res. 2015;46:572–9.
Sentinelli F, Bertoccini L, Barchetta I, Capoccia D, Incani M, Pani MG, Loche S, Angelico F, Arca M, Morini S, et al. The vitamin D receptor (VDR) gene rs11568820 variant is associated with type 2 diabetes and impaired insulin secretion in Italian adult subjects, and associates with increased cardio-metabolic risk in children. Nutr Metab Cardiovasc Dis. 2016;26:407–13.
Han H, Zhao MX, Wang Y, Wang J, Ren BN, Ge HN, Wang T, Sun BB, Ba Y, Li WJ. Significant Polymorphisms of Vitamin D Receptor Gene (rs2189480 and rs3847987) Related to the Risk of Type 2 Diabetes in Henan Rural Area. Biomed Environ Sci. 2019;32:58–62.
Acknowledgements
The authors thank corresponding authors, Marco G. Baroni, Franca Guerini and Yan Wang, for their valuable time in providing the requested information and also acknowledge Shelini Surendran and Sooad Alsulami for their contributions to the literature search.
Author information
Authors and Affiliations
Contributions
VKS conceived and designed the review and interpreted the results. BA extracted and interpreted the data, and this was double checked by VKS and AS. BA and VKS were involved in drafting the manuscript. All authors provided critical feedback and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alathari, B.E., Sabta, A.A., Kalpana, C.A. et al. Vitamin D pathway-related gene polymorphisms and their association with metabolic diseases: A literature review. J Diabetes Metab Disord 19, 1701–1729 (2020). https://doi.org/10.1007/s40200-020-00561-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00561-w