Abstract
Effects of plasma heat source characteristics on nitrogen absorption in gas tungsten arc (GTA) weld metal was investigated using two-dimensional spectroscopic measurements and numerical analyses. In the condition of 1 % nitrogen-mixed shielding gas, nitrogen content of weld metal in a helium arc or an argon-hydrogen arc decreased compared to that of an argon arc. These changes in nitrogen content had a strong correlation with arc voltages for the respective shielding gases, so it was considered that nitrogen absorption in weld metal was affected by arc plasma heat source characteristics. The spectroscopic measurements of these arcs showed that the plasma temperature near the molten pool surface in the helium arc or the argon-hydrogen arc indicated around 6,500 K, while the plasma temperature in the argon arc achieved more than 10,000 K. Additionally, from numerical analyses of GTA dealing with metal vapor, it was found that the plasma temperature in the helium arc was reduced by the metal vapor generated from the molten pool. As a result, the reduction of nitrogen disassociation caused by decreasing plasma temperature was considered as the significant factor in lowering nitrogen absorption in the helium arc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gas-shielding arc welding such as gas tungsten arc (GTA) welding and gas metal arc (GMA) welding has been broadly used in the industry as a welding method of high efficiency and high quality. Shielding gas is used for shielding the arc plasma and molten metal from the atmosphere and has a significant role in obtaining high-quality weld metal.
Nitrogen is known as an impurity gas for welding, and a lot of studies on the nitrogen absorption of weld metal have been conducted [1–3]. In general, the nitrogen content of weld metal in arc welding does not follow Sievert’s law, but significantly increases with merely several percent of nitrogen mixed into the shielding gas [1], as shown in Fig. 1. In the case of Ar + 1 % N2, the nitrogen content of weld metal reaches the nitrogen equilibrium solubility [2] which is defined for a molten iron of 2,000 K in 100 % nitrogen molecular of 1 atmospheric pressure. It is considered in the arc welding process that nitrogen molecules in the shielding gas are dissociated and turn into nitrogen atoms in a plasma region, and thus, nitrogen absorption into molten metal is accelerated.
On the other hand, some reports have also been submitted for the influences of shielding gas components on the nitrogen content of the weld metal. The report by Kobayashi et al. [4] on GMA welding demonstrates that an atmosphere made of Ar + N2 or He + N2 causes nitrogen content in the weld metal to reach the nitrogen equilibrium solubility at a nitrogen mixture ratio of several percent. Nevertheless, the mixture of H2 + N2 or CO2 + N2 is likely to cause relatively lower nitrogen content in the weld metal under the condition of lower nitrogen mixture ratios. This inclination is the same as that in GTA welding, [5] as it is regarded that an atmosphere of H2 + N2 has less nitrogen content in the weld metal relative to that of Ar + N2 at the same nitrogen mixture ratio.
As described above, it is assumed that the shielding gas constitutes an activating gas of arc plasma and causes a major impact on the nitrogen-absorbing phenomenon of the molten metal through the plasma heat source characteristics. Accordingly, the object of this paper is to clarify the effects of plasma heat source characteristics on the nitrogen content of weld metal in GTA welding. In Section 2, the nitrogen content of weld metal in various types of 1 % nitrogen-mixed shielding gases was measured, and in Section 3, the spectroscopic measurement of plasma temperature and molten pool temperature, which affect nitrogen dissociation phenomenon, was carried out. In addition, in Section 4, the effects of plasma heat source characteristics on the nitrogen-absorbing phenomenon of molten metal were considered through numerical analysis.
2 Nitrogen content of weld metal
The extra-low carbon steel was used for the experiments. The chemical compositions of the steel plate are shown in Table 1. These are a component system close to pure iron containing almost no alloy elements. A bead on plate welding was applied to the steel plate having a size of 50 mm in width, 100 mm in length, and 6 mm in thickness. A direct-current power source of an inverter type was used, and welding was performed under straight polarity using tungsten electrodes that have a diameter of 3.2 mm and a tip angle of 60 °. The welding current, arc length, and traveling speed were fixed at 250 A, 5 and 300 mm/min, respectively. Argon, helium, a mixture gas of Ar + He, and a mixture gas of Ar + H2 in each, of which 1 % nitrogen was mixed were used for the shielding gas.
The example of weld bead appearances, where Ar + 1 % N2, He + 1 % N2, and Ar + 7 % H2 + 1 % N2 were respectively used as a shielding gas, is shown in Fig. 2. In the use of He + 1 % N2 or Ar + 7 % H2 + 1 % N2, blowholes were not generated in the weld bead, but were remarkably generated in the use of Ar + 1 % N2.
Figure 3 shows the nitrogen content of the weld metal that was welded with various mixture ratios of hydrogen or helium in Ar + 1 % N2 shielding gas. As surmised from the weld bead appearances, a rise in the mixture ratio of helium or hydrogen gave the result that the nitrogen content of the weld metal decreased. In particular, when hydrogen was mixed, the weld metal nitrogen content largely diminished in the increase of the hydrogen mixture ratio, and it went down to almost half than in Ar + 1 % N2 gas at the mixture condition of Ar + 7 % H2 + 1 % N2. Meanwhile, when helium mixture ratio was raised, the nitrogen content of weld metal saw little change with a maintained value around 0.04 %, however, the nitrogen content sharply declined in a helium mixture ratio of more than 80 %.
This inclination is similar to that of arc voltage in Ar + He mixture gas and Ar + H2 mixture gas. In other words, it is known that almost same arc voltages as in argon are produced at a helium mixture ratio of approximately 80 % or less, however, higher arc voltages can be obtained even at a slight hydrogen mixing ratio in Ar + H2 gas [6].
Therefore, the relationship between arc voltage and weld metal nitrogen content was compared among the respective shielding gases. As shown in Fig. 4, it emerges that an increase in arc voltage decreases the weld metal nitrogen content regardless of the shielding gas types. In addition, this result allows us to surmise that there is an extremely close correlation between plasma heat source characteristics and weld metal nitrogen content.
Meanwhile, it is assumed that an increase in arc voltage, i.e., increasing the plasma temperature, raises the dissociation ratio of nitrogen in the plasma [7]. Thus, this appears to bring inconsistency with the idea that an increase in arc voltage causes the lowering nitrogen content of weld metal. However, it is known that arc plasma having high energy density such as helium arcs or Ar + H2 mixture arcs generates a large amount of metal vapor from the molten pool [8]. The generated metal vapor affects the heat source characteristics of plasma; and according to the numerical analysis of helium arc by Yamamoto et al. [9], for instance, it is reported that the generation of metal vapor lowers the plasma temperature in the vicinity of the molten pool.
Hereupon, the relationship between dissociated nitrogen status and plasma temperature is shown in Fig. 5. The dissociation ratio of nitrogen was treated as the ratio of the summation of nitrogen atoms and nitrogen ions to the number of whole particles. Nitrogen starts dissociating at around a plasma temperature of 4,000 K and becomes almost completely dissociated in about 10,000 K.
Generally, the maximum temperature of arc plasma is considered to be around 20,000 K, and the nitrogen is under almost full dissociation. However, it is assumed that the plasma temperature drastically diminishes in the vicinity of the molten pool, because the temperature of the molten pool surface is considered to be approximately 2,000–3,000 K. Moreover, the plasma temperature in the vicinity of the molten pool further diminishes due to the generation of metal vapor. Therefore, it is regarded that the quantification of the plasma temperature under the generation of metal vapor enables us to comprehend the nitrogen dissociating characteristics and the nitrogen-absorbing phenomenon in the molten pool.
3 Spectroscopic measurement of plasma temperature and molten pool temperature
3.1 Temperature measurement method
The plasma temperature and surface temperature of the molten pool were measured for an argon arc, a helium arc, and an Ar + 7 % H2 arc. The temperature was identified by spectroscopic measurements of the stationary arcs that were ignited on a pure iron plate of 10-mm thickness. The welding current and the arc length were fixed at 150 A and 3 mm, respectively.
Figure 6 [9] shows helium arcs on a water-cooled copper plate and on an iron plate. Only helium plasma in a red color can be observed on the water-cooled copper. Meanwhile, on the iron plate, the iron plasma with blue color radiation can be observed in the vicinity of the base metal, while helium plasma appears below the tungsten electrode. Thus, spectroscopic measurement is required to be carried out in consideration of the iron vapor.
Comparison of helium arcs with metal vapor and without metal vapor[7]
Accordingly, in the present experiment, plasma temperature measurement was attempted by capturing simultaneous three-wavelength spectroscopic images. Therefore, the methods employed were: the Fowler–Milne method for the plasma region, which mainly consisted of shielding gas component; and the two-line relative intensity method for the region with iron vapor in the vicinity of the molten pool. The plasma in the vicinity of the molten pool consists of the shielding gas and the iron vapor so that the temperature of the iron vapor was measured by the two-line relative intensity method, which is not under the influence of iron vapor concentration ratio.
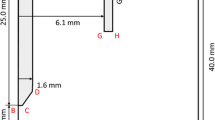
The spectroscopic measurement system in this works is schematically shown in Fig. 7. The plasma image was divided into 3 before Czerny–Turner type monochromator having a wavelength resolution of 0.4 nm, and each of those divided images was captured by high-speed cameras. The captured images were converted to temperature distributions by using the Fowler–Milne method and the two-line relative intensity method after Abel inversion.
The wavelengths used for the measurement are tabulated in Table 2. Temperature conversion with an argon of 696.5 nm spectral line for argon arc and Ar + 7 % He arc, and a helium 587.6 nm spectral line for helium arc was conducted using the Fowler–Milne method. In addition, irrespective of the shielding gas component, the two-line relative intensity method was applied to the temperature conversion with iron spectrums of 537.1 and 538.3 nm in the region of metal vapor generation. The plasma temperature distribution was obtained by superimposing the temperature distribution of the iron vapor on the temperature distribution of the individual shielding gas.
On the other hand, two-color pyrometry was employed for the temperature measurement of the molten pool surface. A schematic diagram of the measurement method is shown in Fig. 8. The divided two images of a molten pool were passed through an interference filter and those spectral images were recorded by the high-speed video camera. Furthermore, the central wavelength of the interference filter was set at 950 and 980 nm.
3.2 Temperature measurement results
The distributions of the plasma temperature and the molten pool surface temperature are shown in Fig. 9 concerning the case where the shielding gas is argon, helium, and Ar + 7 % H2, respectively. The plasma temperature was measured under a stationary condition of 4 s after the arc ignition, and the molten pool’s temperature was immediately measured after 2 ms from the arc’s dissipation.
In the case of argon arc, the plasma temperature below the tungsten electrode indicates around 18,000 K, and this fact is consistent with conventional knowledge. In addition, it is shown that no iron vapor is observed and that the plasma temperature near the molten pool leads to a high temperature of 12,000 K. Meanwhile, the helium arc allows the plasma temperature near the molten pool to lower due to the generated iron vapor. The plasma temperature reduces to nearly 6,500 K in the central part of the arc near the molten pool, while the plasma having an elevated temperature surpassing 18,000 K is produced below the tungsten electrode. Moreover, the Ar + 7 % H2 arc also exhibited a similar inclination, and the plasma temperature in the central part of an arc just above the molten pool reduced to around 6,500 K.
Referring to Fig. 5, the nitrogen dissociation ratios in plasma temperature of 10,000 and 6,500 K can be estimated to be 99 and 40 %, respectively. Therefore, it is surmised that the helium arc as well as the Ar + 7 % H2 arc subdues the nitrogen absorption of the molten pool where the plasma temperature in the vicinity of the molten pool diminishes.
On the other hand, it is possible to confirm that the molten pool’s temperature with helium arc and Ar + 7 % H2 arc is high relative to that with argon. According to the previous study [10], a helium arc and an Ar + H2 arc have higher current densities caused by the plasma-pinch effect compared with a pure argon arc. Additionally, the temperature of the molten pool surface is increased caused by the higher current density arc. In this experiment, the temperature of molten pool for helium and Ar + 7 % H2 rise to approximately 2,800 K, while that for Ar is 2,300 K. It is known that the temperature surpasses approximately 2,400 K, the saturated iron pressure sharply increases. Hence, this fact allows us to understand that the metal vapor generated from the molten pool increases with the helium arc as well as with the Ar + 7 % H2 arc.
4 Numerical analysis of plasma characteristics
4.1 Numerical analysis model
The numerical analysis of the argon arc and helium arc was carried out. The base metal was assumed to be pure iron, with the welding current and the arc length set at 150 A and 5 mm, respectively. The dominant equations were mass continuity equation, energy conservation equation, momentum conservation equation, current continuity equation, and these equations were solved by using the SIMPLE method.
The iron vapor was considered to be supplied from the base metal surface, and the generation term of the mass continuity equation as expressed in Eq. (1) was introduced.
Where, ρ, v r , v z , and S denote the density of gas, the respective gas flow rates in the radial direction and in the axial direction, and the mass of the iron vapor generated per unit second, respectively. The evaporating rate of the iron was sought from the Langmuir equation as presented in Eq. (2). P, T, and M are the saturated vapor pressure of iron, the surface temperature of the molten pool, and the molecular weight of iron, respectively. Hereupon, it is reported[11] that the evaporation rate of the iron in the helium arc with 200 A is in the order of 0.05–0.1 mg/s. Accordingly, α was fixed at 0.025 so that the evaporation rate of the iron may fall upon roughly 0.1 mg/s in this numerical analysis of the helium arc.
The mixing phenomenon of the iron vapor with shielding gas was expressed by the diffusion equation [9] as shown in Eq. (3). The content of the iron vapor and shielding gas is determined by the convection term on the left side and the diffusion term on the right side. Hereupon, C and D are the mass fraction of the iron vapor and diffusion coefficient of the iron vapor in the shielding gas, respectively.
The numerical analysis was achieved by incorporating the Eqs. (1), (2), and (3) into the existing two-dimensional GTA model [9]. In addition, it was postulated that the atmospheric gas was the single gas of argon or helium. It was also assumed that a small ratio of nitrogen such as 1 % would have little effect on plasma characteristics. In other words, the temperatures of an argon arc and a helium arc containing iron vapor were simulated, and subsequently, the nitrogen dissociation statuses were evaluated from the calculated plasma temperature.
4.2 Results of the numerical analysis
Figure 10 shows the iron vapor concentration distribution, the plasma temperature distribution, and the dissociation ratio of nitrogen which was surmised from the plasma temperature, in the argon arc and helium arc.
Concerning the generation of the iron vapor, it emerges that the concentration of the iron vapor 0.1 mm above the molten pool is approximately 0.7 % in the argon arc, while it goes up to 8 % in the helium arc, with a large extension upward the molten pool. As for the temperature distribution of plasma, the obtained results allowed us to roughly reproduce the experimental results. In other words, the helium arc has a diminishing plasma temperature in contrast to the argon arc in the vicinity of the molten pool.
It is known that a helium arc has lower electrical conductivities and higher thermal conductivities. The lower electrical conductivities increase the Joule heating and the higher thermal conductivities contract the plasma region with the thermal pinch effects. Therefore, the helium arc has an extended high-temperature region in the vicinity of the tungsten electrode. On the other hand, it can be identified that the helium arc has a diminishing plasma temperature in the vicinity of the molten pool. While the plasma temperature of the argon arc reached 11,000 K, that of the helium arc went down to 5,900 K. In addition, the center of the molten pool surface had a temperature of 2,450 K with argon and that of 2,870 K with helium.
The dissociation ratio of the nitrogen in the plasma was calculated from these temperature distributions. The argon arc has almost fully dissociated nitrogen also in the vicinity of the molten pool. However, the dissociation ratio reduced to 44 % at a position 0.1 mm away from the surface of the molten pool in the helium arc. In addition, the distribution in the radial direction also reveals that the region with low dissociation ratios expands in the helium arc.
It is assumed that the iron vapor greatly relates to a decrease in the plasma temperature of the helium arc as well as the concomitant nitrogen’s disassociation ratio. Accordingly, it is regarded that the iron vapor generated from the molten pool increases the electrical conductivity, thus, the Joule heating near to the molten pool decreases, and the plasma temperature also diminishes. In addition, as mentioned in Section 3, the generation of iron vapor is identified also in the Ar + 7 % H2 arc; it is thus considered that the plasma temperature in the vicinity of the molten pool decreases according to the same mechanism as in the helium arc.
By the abovementioned experimental measurements and numerical analyses, it appears that the helium or Ar + H2 arc creates a higher arc plasma temperature near the molten pool leading to the generation iron vapor that would subsequently reduce the plasma temperature of the arc away from the pool, followed by inhibition in disassociation of nitrogen in the plasma environment and reduction of nitrogen absorption in weld metal. In contrast, the plasma temperature near the molten pool with an argon arc is not sufficient to produce metal vapor and ironically that aids to the greater disassociation of nitrogen and absorption of the same in weld metal.
Hereupon, focused on the nitrogen absorption phenomenon of the molten pool, a decrease in the nitrogen absorption of the molten pool is caused by not only a reduction in the atomic nitrogen ratio owing to the dissociation inhibition of nitrogen but also a decrease in nitrogen partial pressure due to an increase in the iron vapor concentration. Therefore, the effects of these results were compared.
The iron vapor concentration of 0.1 mm above the molten pool is shown in Fig. 11. The iron vapor concentration in the helium arc is approximately 8 % at maximum. Even when it is postulated that the iron vapor concentration on the surface of the molten pool coincides with the saturated vapor pressure of the molten iron, the iron vapor pressure falls upon approximately 20 % at 2,800 K of the temperature at the molten pool; therefore, a reduction in nitrogen partial pressure owing to the iron vapor concentration is likely to have minor effects.
Figure 12 shows the dissociation ratio of nitrogen 0.1 mm above the molten pool. For both argon and helium, the dissociation ratio indicates the maximum value at the central part of the plasma. While the argon arc indicates a high dissociation ratio, such as 100 %, at the central part of the plasma and approximately 70 % even at 2 mm away from the central part, the helium arc dissociates nitrogen of approximately 40 % at the center and almost little nitrogen at 2 mm away from there. Figure 3 reveals that in contrast to the weld metal nitrogen content with Ar + 1 % N2 being approximately 0.04 %, that with He + 1 % N2 is halved to around 0.02 %. This result coincides with the numerical analysis results according to which the atomic nitrogen in the plasma of the helium arc reduces to 40 %. Accordingly, in the nitrogen absorption phenomenon of the weld metal, it is considered that the heat source characteristics of the plasma including metal vapor fulfills an extremely significant role.
5 Conclusions
In order to consider the effects of the plasma’s heat source characteristics affecting the nitrogen absorption phenomenon of GTA weld metal, the measurement of the weld metal nitrogen content under various shielding gases, the spectroscopic measurement of the plasma and the molten metal’s temperature, and the numerical analysis of nitrogen dissociation status were carried out. The obtained results are described as in the following:
-
1)
When welding is conducted using shielding gases with a mixture ratio of 1 % N2, the nitrogen content of the helium arc or Ar + 7 % H2 arc reduces to approximately half relative to the argon arc. The intense correlation between plasma heat source characteristics and weld metal nitrogen content was implied by the fact that the nitrogen content of the weld metal decreases according to the increase of the arc voltage.
-
2)
The spectroscopic measurement of the plasma temperature and the molten pool’s temperature enabled us to clarify that in comparison with the argon arc, the helium arc or the Ar + 7 % H2 mixture arc has a diminishing plasma temperature in the vicinity of the molten pools, with temperature rising on the molten pool’s surface.
-
3)
Similarly, also in the numerical analysis, results were obtained such that in comparison with the argon arc, the plasma temperature in the vicinity of the molten pools diminishes in the helium arc. It was considered that a decline in the plasma temperature of the helium arc is caused by a rise in metal vapor due to an elevated molten pool temperature. In addition, when the dissociation ratio of the nitrogen was surmised from the plasma temperature, it emerged that the atomic nitrogen concentration in the vicinity of the molten pools in the helium arc reduced to approximately half as much as that of argon.
-
4)
It is assumed that a decline in the plasma temperature in the vicinity of the molten pools due to the generation of iron vapor, accompanied by a reduction in atomic nitrogen content, cause intense impact on the reduction mechanism of weld metal nitrogen content in a helium arc and an Ar + H2 arc.
References
Uda M, Ohno S (1978) Spattering phenomenon for iron-nitrogen system during arc melting. Trans Nat Res Inst Met 20(No.6):358–365
Katz JD, King TB (1989) The kinetics of nitrogen absorption and desorption from a plasma arc by molten iron. Metall Trans B23B:175–185
Kokawa H (2003) Nitrogen absorption and desorption by steel during arc and laser welding. Journal of the Japan Welding Society No.5:112–121, in Japanese
Takuro Kobayashi, Takeshi Kuwana and Yasushi Kikuchi (1971) Effect of welding atmosphere and welding polarity on the nitrogen content of weld metals, 40-3:221-231 (in Japanese)
Takeshi Kuwana and Hiroyuki Kokawa (1983) The nitrogen absorption of iron weld metal by gas tungsten arc welding, 1-3: 392-398 (in Japanese)
Tanaka M, Murphy AB, Tashiro S (2011) Control of heat source properties of thermal plasma through design of gas composition. J Plasma Fusion Res 87–5:522–527, in Japanese
Mundra K, Debroy T (1995) A general model for partitioning of gases between a metal and its plasma environment. Metallugical and Materials Transactions B 26B:149–157
Terasaki H et al (2002) Effects of metal vapor on plasma state in gas tungsten arcs. Quarterly J Japan Welding Soc 20–2:201–206
Yamamoto K, Tanaka M, Tashiro S, Nakata K, Yamazaki K, Yamamoto E, Suzuki K, Murphy AB (2008) Metal vapor behavior in thermal plasma of gas tungsten arcs during welding. Sci Technol Weld Joining 13:566–572
Tanaka M, Tashiro S, Satoh T, Murphy AB, Lowke JJ (2008) Influence of shielding gas composition on arc properties in TIG welding. Sci Technol Weld Joining 13:225–231
Sakuma N, Hiraoka K (1995) Heat transfer and vaporization in Ar-He or A-H mixed gas tungsten arcs. Preprints of the National Meeting of JWS 56:115, in Japanese
Author information
Authors and Affiliations
Corresponding author
Additional information
Doc. IIW-2376, recommended for publication by Study Group SG-212 "The Physics of Welding".
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kodama, S., Sugiura, K., Nakanishi, S. et al. Effect of plasma heat source characteristics on nitrogen absorption in gas tungsten arc weld metal. Weld World 57, 925–932 (2013). https://doi.org/10.1007/s40194-013-0058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-013-0058-y