Abstract
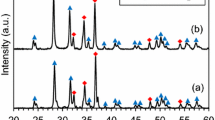
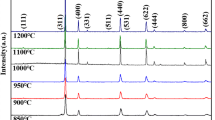
In this research, Zr-doped Gd2Ti2O7 pyrochlores, with the composition of Gd2(Ti1-xZr x )2O7, were firstly synthesized by self-propagating high-temperature synthesis plus quick pressing (SHS/QP) using CuO as the oxidant and Ti as the reductant. To improve the radiation resistance of titanate–pyrochlore, up to 35 at% Zr was incorporated to substitute the Ti site of Gd2Ti2O7 pyrochlore (Gd2(Ti0.75Zr0.35)2O7). XRD and SEM microstructural characterizations showed the formation of a composite ceramic with the major pyrochlore phase and the minor Cu phase. The generated temperature of samples decreased from 1702 to 1011°C with increasing Zr content. The effects of sintering temperature and pressure time on phase composition and microstructure were systematically studied. Besides, the influence of thermal transmission on the whole combustion process was also explored. The pyrochlore-based waste form possessed high bulk density of 6.25 g/cm3 and Vickers hardness of 10.81 GPa. The MCC-1 leaching test showed the normalized elemental leaching rates (42 d) of Cu, Gd, and Zr are 1.27×10-2, 1.33×10-3, and 8.44×10-7 g·m-2·d-1, respectively.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Jing JQ, Luan HW. On the history of world nuclear development and the road for China nuclear development. Northeast Electric Power Technology 2008, 29: 48–52. (in Chinese)
Ye Q-Z. Studies on the development strategy of China's nuclear power. Power System and Clean Energy 2010, 26: 3–6. (in Chinese)
International Atomic Energy Agency. Design and Operation of High Level Waste Vitrification and Storage Facility. Technical Reports Series No. 339, 1992.
Ojovan MI, Lee WE. An Introduction to Nuclear Waste Immobilization. Elsevier, 2005.
Barinova TV, Podbolotov KB, Borovinskaya IP, et al. Self-propagating high-temperature synthesis of ceramic matrices for immobilization of actinide-containing wastes. Radiochemistry 2014, 56: 554–559.
Kong L, Zhang Y, Karatchevtseva I. Preparation of Y2Ti2O7 pyrochlore glass-ceramics as potential waste forms for actinides: The effects of processing conditions. J Nucl Mater 2017, 494: 29–36.
Weber WJ, Ewing RC, Catlow CRA, et al. Radiation effects in crystalline ceramics for the immobilization of high-level nuclear waste and plutonium. J Mater Res 1998, 13: 1434–1484.
Zhang K, Wen G, Zhang H, et al. Self-propagating high-temperature synthesis of Y2Ti2O7 pyrochlore and its aqueous durability. J Nucl Mater 2015, 465: 1–5.
Peng L, Zhang K, Yin D, et al. Self-propagating synthesis, mechanical property and aqueous durability of Gd2Ti2O7 pyrochlore. Ceram Int 2016, 42: 18907–18913.
Fan L, Shu X, Lu X, et al. Phase structure and aqueous stability of TRPO waste incorporation into Gd2Zr2O7 pyrochlore. Ceram Int 2015, 41: 11741–11747.
Zhang FX, Wang JW, Lian J, et al. Phase stability and pressure dependence of defect formation in Gd2Ti2O7 and Gd2Zr2O7 pyrochlores. Phys Rev Lett 2008, 100: 045503.
Jafar M, Sengupta P, Achary SN, et al. Phase evolution and microstructural studies in CaZrTi2O7 (zirconolite)-Sm2Ti2O7 (pyrochlore) system. J Eur Ceram Soc 2014, 34: 4373–4381.
Ewing RC, Weber WJ, Lian J. Nuclear waste disposal—pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and "minor" actinides. J Appl Phys 2004, 95: 5949–5971.
Wan C, Qu Z, Du A, et al. Influence of B site substituent Ti on the structure and thermophysical properties of A2B2O7-type pyrochlore Gd2Zr2O7. Acta Mater 2009, 57: 4782–4789.
Fan L, Shu X, Ding Y, et al. Fabrication and phase transition of Gd2Zr2O7 ceramics immobilized various simulated radionuclides. J Nucl Mater 2015, 456: 467–470.
Glagovskii EM, Yudintsev SV, Kuprin AV, et al. Crystalline host phases for actinides, obtained by self-propagating high-temperature synthesis. Radiochemistry 2001, 43: 632–638.
Kulkami NK, Sampath S, Venugopal V. Preparation and characterization of Pu-pyrochlore: (La1_xPux)2Zr2O7 (x = 0–1). J Nucl Mater 2000, 281: 248–250.
Zhang KQ, Liu CG, Li FZ, et al. Study on the crystal structure of (Gd2-xCe x )Ti2O7 (0 ≤ x ≤ 0.8) pyrochlore. Adv Appl Ceram 2016, 115: 411–416.
Sickafus KE, Minervini L, Grimes RW, et al. Radiation tolerance of complex oxides. Science 2000, 289: 748–751.
Begg BD, Hess NJ, Weber WJ, R. et al. Heavy-ion irradiation effects on structures and acid dissolution of pyrochlores. J Nucl Mater 2001, 288: 208–216.
Finch RJ, Bullen DB. Scientific Basis for Nuclear Waste Management XXVI: Symposium Held December 2–5, 2002, Boston, Massachusetts, USA. Warrendale, PA, USA: Materials Research Society, 2003.
Tang J, Chen X, Pan S, et al. Synthesis of Gd2Zr2O7 pyrochlore with cubic structure at high pressure and high temperature conditions. Atomic Energy Science and Technology 2010, 44: 394–399. (in Chinese)
Lu X, Ding Y, Dan H, et al. Rapid synthesis of single phase Gd2Zr2O7 pyrochlore waste forms by microwave sintering. Ceram Int 2014, 40: 13191–13194.
Xu Q, Pan W. Wang J, et al. Preparation and characterization of Gd2Zr2O7 ceramic by spark plasma sintering. Key Eng Mater 2005, 280–283: 1507–1510.
Muthuraman M, Dhas NA, Patil KC. Combustion synthesis of oxide materials for nuclear waste immobilization. Bull Mater Sci 1994, 17: 977–987.
Merzhanov AG. History and recent developments in SHS. Ceram Int 1995, 21: 371–379.
Yudintsev SV, Ioudintseva TS, Mokhov AV, et al. Study of pyrochlore and garnet-based matrices for actinide waste produced by a self-propagating high-temperature synthesis. MRS Proceedings 2003, 807, DOI: 10.1557/PR0C-807-273.
Yudintsev SV, Stefanovskii SV, Kir'Yanova OI, et al. Radiation resistance of fused titanium ceramic for actinide immobilization. Atomic Energy 2001, 90: 487–494.
Laverov NP, Yudintsev SV, Stefanovsky SV, et al. Radiation stability of actiniae matrices. Dokl Earth Sci 2001, 377: 175–177.
Zhang K, Yin D, Peng L, et al. Self-propagating synthesis of Nd2O3-incorporated zirconolite/Mo composites and their aqueous durability. J Nucl Mater 2017, 491: 177–182.
Laverov N, Yudintsev S, Lapina M, et al. Phases formation rate at synthesis of actinide waste forms. MRS Proceedings 2002,757, DOI: 10.1557/PR0C-757-II3.20.
Wong-Ng W, McMurdie HF, Hubbard CR, et al. JCPDS-ICDD research associateship (cooperative program with NBS/NIST). J Res Natl Inst Stand Technol 2001, 106: 1013–1028.
Smith KL, Lumpkin GR, Blackford MG, et al. The durability of synroc. J Nucl Mater 1992, 190: 287–294.
ASTM C1220-98. Standard test method for static leaching of monolithic waste forms for disposal of radioactive waste. ASTM International, West Conshohocken, PA, USA, 1998.
Helean K, Begg B, Navrotsky A, et al. Enthalpies of formation of Gd2(Ti2-xZr x )O7 pyrochlores. MRS Proceedings 2000, 663, DOI: 10.1557/PR0C-663-691.
Wen G, Zhang K, Zhang H, et al. Immobilization and aqueous durability of Nd2O3 and CeO2 incorporation into rutile Ti02. Ceram Int 2015, 41: 6869–6875.
McGlinn P, Advocat T, Leturcq G, et al. Leaching behaviour of zirconolite in 0.001M citric acid at 90 °C under various flow regimes. MRS Proceedings 2006, 932, DOI: 10.1557/PR0C-932-61.1.
Yokoi H, Arita Y, Matsui T, et al. EXAFS study of (La1–x:M x )2Zr2O7 (M = Nd and Ce). J Nucl Mater 1996, 238: 163–168.
Kingery WD, Bowen HK, Uhlmann DR. Introduction to Ceramics, 2nd edn. New York: John Wiley and Sons Incorporated, 1976.
Acknowledgements
We sincerely appreciate the projects supported by the National Natural Science Foundation of China (Nos. 51672228 and 51202203), the Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials (No. 11zxfk26), the Young Outstanding Scientist Fund of Southwest University of Science and Technology (No. 13zx9108), and the Postgraduate Innovation Fund Project by Southwest University of Science and Technology (No. 16ycx010).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access The articles published in this journal are distributed under terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Peng, L., Zhang, K., He, Z. et al. Self-propagating high-temperature synthesis of ZrO2 incorporated Gd2Ti2O7 pyrochlore. J Adv Ceram 7, 41–49 (2018). https://doi.org/10.1007/s40145-017-0254-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-017-0254-0