Abstract
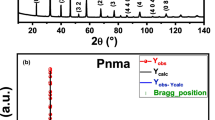
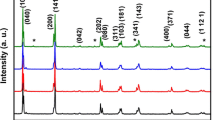
Nanocrystalline and bulk Li2TiO3 having monoclinic structure were prepared by mechanical alloying as well as conventional ceramic route. Complex impedance analysis in the frequency range of 100 Hz-1 MHz over a wide range of temperature (50–500 °C) indicates the presence of grain boundary effect along with the bulk contribution. The frequency-dependent conductivity plots exhibit power law dependence, suggesting three types of conduction in the material: low-frequency (100 Hz-1 kHz) conductivity showing long-range translational motion of electrons (frequency independent), mid-frequency (1–10 kHz) conductivity showing short-range hopping of charge carriers and high-frequency (10 kHz-1 MHz) conductivity showing conduction due to localized orientation of hopping mechanism. The electrical conductivity measurement of nanocrystalline and bulk Li2TiO3 with temperature shows the negative temperature coefficient of resistance (NTCR) behavior. The activation energy (0.77 eV for nano sample and 0.88 eV for bulk sample) study shows the conduction mechanism in both samples. The low activation energies of the samples suggest the presence of singly ionized oxygen vacancies in the conduction process.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Ohno H, Konishi S, Nagasaki T, et al. Correlation behavior of lithium and tritium in some solid breeder materials. J Nucl Mater 1985, 133–134: 181–185.
Roux N, Tanaka S, Johnson C, et al. Ceramic breeder material development. J Nucl Mater 1998, 41: 31–38.
Roux N, Avon J, Floreancing A, et al. Low-temperature tritium releasing ceramics as potential materials for the ITER breeding blanket. J Nucl Mater 1996, 233–237: 1431–1435.
Hofmann P, Dienst W. Compatibility studies of metallic materials with lithium-based oxides. J Nucl Mater 1988, 155–157: 485–490.
Rasneur B, Charpin J. Chemical properties of lithium ceramics: Reactivity with water and water vapour. J Nucl Mater 1988, 155–157: 461–465.
Vittal Rao TV, Bamankar YR, Mukerjee SK, et al. Preparation and characterization of Li2TiO3 pebbles by internal gelation sol-gel process. J Nucl Mater 2012, 426: 102–108.
Wu X, Wen Z, Han J, et al. Fabrication of Li2TiO3 pebbles by water based sol-gel method. Fusion Eng Des 2008, 83: 112–116.
Wu X, Wen Z, Lin B, et al. Sol-gel synthesis and sintering of nano-size Li2TiO3 powder. Mater Lett 2008, 62: 837–839.
Deptuła A, Łada W, Olczak T, et al. Preparation of lithium titanate by sol-gel method. Nukleonika 2001, 46: 95–100.
Deptuła A, Brykała M, Łada W, et al. Preparation of spherical particles of Li2TiO3 (with diameters below 100 μm) by sol-gel process. Fusion Eng Des 2009, 84: 681–684.
Lulewicz D, Roux N. Fabrication of Li2TiO3 pebbles by the extrusion-spheronisation-sintering process. J Nucl Mater 2002, 307–311: 803–806.
Mandal D, Sathiyamoorthy D, Govardhana Rao V. Preparation and characterization of lithium-titanate pebbles by solid-state reaction extrusion spheronization techniques for fusion reactor. Fusion Eng Des 2012, 87: 7–12.
Tsuchiya K, Kawamura H, Takayama T, et al. Control of particle size and density of Li2TiO3 pebbles fabricated by indirect wet process. J Nucl Mater 2005, 345: 239–244.
Jung C-H. Sintering characterization of Li2TiO3 ceramic breeder powders prepared by the solution combustion synthesis process. J Nucl Mater 2005, 341: 148–152.
Jung C-H, Lee SJ, Waltraud M, et al. A polymer solution technique for the synthesis of nano-sized Li2TiO3 ceramic breeder powders. J Nucl Mater 2008, 373: 194–198.
Sinclair DC, West AR. Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J Appl Phys 1989, 66: 3850.
Lanfredi S, Rodrigues ACM. Impedance spectroscopy study of the electrical conductivity and dielectric constant of polycrystalline LiNbO3. J Appl Phys 1999, 86: 2215.
Barsoukov E, Macdonald JR. Impedance Spectroscopy, 2nd edn. Hoboken, NJ: John Wiley & Sons, 2005.
Barranco AP, Piñar FC, Martínez OP, et al. AC behaviour and conductive mechanisms of 2.5 mol% La2O3 doped PbZr0.53Ti0.47O3 ferroelectric ceramics. J Eur Ceram Soc 1999, 19: 2677–2683.
Bharadwaj SSN, Victor P, Venkateswarulu P, et al. AC transport studies of La-modified antiferroelectric lead zirconate thin films. Phys Rev B 2002, 65: 174106.
Cao W, Gerhardt R. Calculation of various relaxation times and conductivity for a single dielectric relaxation process. Solid State Ionics 1990, 42: 213–221.
Gerhardt R. Impedance and dielectric spectroscopy revisited: Distinguishing localized relaxation from long-range conductivity. J Phys Chem Solids 1994, 55: 1491–1506.
Argall F, Jonscher AK. Dielectric properties of thin films of aluminium oxide and silicon oxide. Thin Solid Films 1968, 2: 185–210.
Fehr Th, Schmidbauer E. Electrical conductivity of Li2TiO3 ceramics. Solid State Ionics 2007, 178: 35–41.
León C, Rivera A, Várez A, et al. Origin of constant loss in ionic conductors. Phys Rev Lett 2001, 86: 1279–1282.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dash, U., Sahoo, S., Chaudhuri, P. et al. Electrical properties of bulk and nano Li2TiO3 ceramics: A comparative study. J Adv Ceram 3, 89–97 (2014). https://doi.org/10.1007/s40145-014-0094-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-014-0094-0