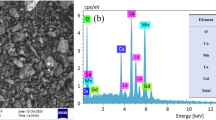
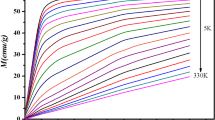
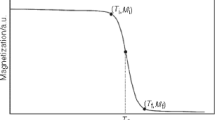
Abstract
In this work, a phenomenological model is applied to describe the magnetocaloric effect for the La0.75Ca0.25MnO3 system near a second-order phase transition from a ferromagnetic to a paramagnetic state. Based on this model, it can predict the values of the magnetocaloric properties from calculation of magnetization as a function of temperature under different external magnetic fields. The magnetic entropy change reaches a peak of about 5.39 J/(kg·K) at 257 K upon 4 T applied field variation. The ΔSM distribution is much more uniform than that of gadolinium, which is desirable for an Ericson-cycle magnetic refrigerator.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
de Oliveira A, von Ranke PJ. Theoretical aspects of themagnetocaloric effect. Phys Reports 2010, 489: 89.
Gschneidner KA, Pecharsky VK, Tsoko AO. Recent developments in magnetocaloric materials. Rep Prog Phys 2005, 68: 1479.
Hamad MA. Detecting giant electrocaloric effect in SrxBa1−xNb2O6 single crystals. Appl Phys Let 2012, 100: 192908.
Hamad MA. Magnetocaloric effect in polycrystalline Gd1−xCaxBaCo2O5.5. Mater Lett 2012, 82: 181–183.
Hamad MA. Investigations on electrocaloric properties of [111] oriented 0.955PbZn1/3Nb2/3O3-0.045PbTiO3 single crystals. Phase Transition 2012, DOI: 10.1080/01411594.2012.674527.
Hamad MA. Magnetocaloric effect in Ge0.95Mn0.05 films. J Supercond Nov Magn 2012, DOI: 10.1007/s10948-012-1762-3.
Hamad MA. Theoretical investigations on electrocaloric properties of relaxor ferroelectric 0.9PbMg1/3Nb2/3O3-0.1PbTiO3 thin film. J Comput Electron 2012, 11: 344–348
Hamad MA. Calculation of electrocaloric properties of ferroelectric SrBi2Ta2O9. Phase Transition 2012, 85: 159–168.
Hamad MA. Theoretical work on magnetocaloric effect in ceramic and sol-gel La0.67Ca0.33MnO3. J Therm Anal Calorim 2012, DOI: 10.1007/s10973-012-2505-1.
Debnath JC, Zeng R, Kim JH, et al. Improvement of refrigerant capacity of La0.7Ca0.3MnO3 material with a few percent Co doping. J Magn Magn Mater 2011, 323: 138–143.
Hamad MA. Magnetocaloric properties of La0.6Ca0.4MnO3. J Therm Anal Calorim 2012, DOI: 10.1007/s10973-012-2723-6.
Baldini M, Capogna L, Capone M, et al. Pressure induced magnetic phase separation in La0.75Ca0.25MnO3 manganite. J Phys: Condens Matter 2012, 24: 045601.
Schiffer P, Ramirez AP, Bao W, et al. Low temperature magnetoresistance and the magnetic phase diagram of La1−xCaxMnO3. Phys Rev Lett 1995, 75: 3336–3339.
Hamad MA. Prediction of energy loss of Ni0.58Zn0.42Fe2O4 nanocrystalline and Fe3O4 nanowire arrays. Jpn J Appl Phys 2010, 49: 085004.
Hamad MA. Calculations on nanocrystalline CoFe2O4 prepared by polymeric precursor method. J Supercond Nov Magn 2012, DOI: 10.1007/s10948-012-1783-y.
Hamad MA. Prediction of thermomagnetic properties of La0.67Ca0.33MnO3 and La0.67Sr0.33MnO3. Phase Transitions 2012, 85: 106–112.
Phan MH, SC Yu. Review of the magnetocaloric effect in manganite materials. J Magn Magn Mater 2007, 308: 325.
Földeaki M, Chahine R, Bose TK. Magnetic measurements: A powerful tool in magnetic refrigerator design. J Appl Phys 1995, 77: 3528–3537.
Terashita H, Garbe JJ, Neumeier JJ. Compositional dependence of the magnetocaloric effect in La1−xCaxMnO3 (0 ≤ x ≤ 0.52). Phys Rev B 2004, 70:094403.
Williams DV. Characterization of the structural and magnetic properties of Gd thin films. Ph.D. Thesis. Florida (USA): University of South Florida, 2010.
Goodenough JB. Theory of the role of covalence in the perovskite-type manganites [La, M(II)]MnO3. Phys Rev 1955, 100: 564.
Dan’kov SY, Tishin AM, Pecharsky VK, et al. Magnetic phase transitions and the magnetothermal properties of gadolinium. Phys Rev B 1998, 57: 3478–3490.
Pecharsky VK, Gschneidner KA. Magnetocaloric effect and magnetic refrigeration. J Magn Magn Mater 1999, 200: 44–56.
Bohigas X, Tejada J, del Barco E, et al. Tunable magnetocaloric effect in ceramic perovskites. Appl Phys Lett 1998, 73: 390.
Guo ZB, Du YW, Zhu JS, et al. Large magnetic entropy change in perovskite-type manganese oxides. Appl Phys Lett 1997, 78: 1142–1145.
Radaelli PG, Cox DE, Marezio M, et al. Simultaneous structural, magnetic, and electronic transitions in La1−xCaxMnO3 with x=0.25 and 0.50. Phys Rev Lett 1995, 75: 4488–4491.
Kim KH, Gu JY, Choi HS, et al. Frequency shifts of the internal phonon modes in La0.7Ca0.3MnO3. Phys Rev Lett 1996, 77: 1877–1880.
Tang T, Gu KM, Cao QQ, et al. Magnetocaloric properties of Ag-substituted perovskite-type manganites. J Magn Magn Mater 2000, 222: 110–114.
Phan MH, Tian SB, Yu SC, et al. Magnetic and magnetocaloric properties of La0.7Ca0.3−xBaxMnO3 compounds. J Magn Magn Mater 2003, 256: 306–310.
Sun Y, Tong W, Zhang YH. Large magnetic entropy change above 300 K in La0.67Sr0.33Mn0.9Cr0.1O3. J Magn Magn Mater 2001, 232: 205–208.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hamad, M.A. Theoretical work on magnetocaloric effect in La0.75Ca0.25MnO3. J Adv Ceram 1, 290–295 (2012). https://doi.org/10.1007/s40145-012-0027-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-012-0027-8