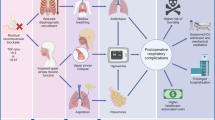
Abstract
Purpose of Review
We summarize the current status of neuromuscular blockade monitoring and reversal strategies in children, and the gaps that exist in our knowledge and approach compared to adult practice.
Recent Findings
Children experience residual neuromuscular blockade and are at risk of subsequent complications, though this is less well studied compared to adults. Objective (quantitative) monitoring of neuromuscular blockade in children has been challenging due to patient size and surgical positioning. Recent dosing indications for sugammadex in children and advances in objective monitoring may provide measures to improve patient safety in daily pediatric anesthesia practice.
Summary
As evidence-based practice guidelines are formed, investigations supporting the safe use, monitoring, and reversal of neuromuscular blockade in children are needed. Objective monitoring for appropriate management and reversal of neuromuscular blockade in children should be encouraged, even when using sugammadex.
Search Strategy
A PubMed search was completed using key terms “pediatric anesthesia,” “residual neuromuscular blockade,” “neuromuscular monitoring,” “quantitative monitoring,” and “sugammadex.” The search was conducted in July 2022 and was restricted to English literature. The search included meta-analyses, randomized controlled trials, clinical trials, observational studies, case reports, and reviews within the past 20 years.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Murphy GS, Brull SJ. Quantitative neuromuscular monitoring and postoperative outcomes: A narrative review. Anesthesiology. 2022;136(2):345–61. https://doi.org/10.1097/ALN.0000000000004044.
Saager L, Maiese EM, Bash LD, Meyer TA, Minkowitz H, Groudine S, et al. Incidence, risk factors, and consequences of residual neuromuscular block in the United States: the prospective, observational, multicenter RECITE-US study. J Clin Anesth. 2019;55:33–41. https://doi.org/10.1016/j.jclinane.2018.12.042.
Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110–9. https://doi.org/10.1213/ANE.0b013e3181c07428.
Kotake Y, Ochiai R, Suzuki T, Ogawa S, Takagi S, Ozaki M, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117(2):345–51. https://doi.org/10.1213/ANE.0b013e3182999672.
Naguib M, Brull SJ, Kopman AF, Hunter JM, Fulesdi B, Arkes HR, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127(1):71–80. https://doi.org/10.1213/ANE.0000000000002670.
Nemes R, Renew JR. Clinical practice guideline for the management of neuromuscular blockade: what are the recommendations in the USA and other countries? Curr Anesthesiol Rep. 2020;10(2):90–8. https://doi.org/10.1007/s40140-020-00389-3.
Blobner M, Hollmann MW, Luedi MM, Johnson KB. Pro-con debate: do we need quantitative neuromuscular monitoring in the era of sugammadex? Anesth Analg. 2022;135(1):39–48. https://doi.org/10.1213/ANE.0000000000005925.
•• Faulk DJ, Austin TM, Thomas JJ, Strupp K, Macrae AW, Yaster M. A survey of the society for pediatric anesthesia on the use, monitoring, and antagonism of neuromuscular blockade. Anesth Analg. 2021;132(6):1518–26. https://doi.org/10.1213/ANE.0000000000005386Survey of pediatric anesthesia providers in the U.S. showing decreased use of train-of-four monitoring in recent graduates who tend to use sugammadex.
•• Klucka J, Kosinova M, Krikava I, Stoudek R, Toukalkova M, Stourac P. Residual neuromuscular block in paediatric anaesthesia. Br J Anaesth. 2019;122(1):e1–2. https://doi.org/10.1016/j.bja.2018.10.001Prospective observational cohort study showing high rates of residual neuromuscular blockade in PACU as measured by acceleromyography.
Vested M, Tarpgaard M, Eriksen K, Rasmussen LS. Incidence of residual neuromuscular blockade in children below 3 years after a single bolus of cisatracurium 0.1 mg/kg: a quality assurance study. Acta Anaesthesiol Scand. 2020;64(2):168–72. https://doi.org/10.1111/aas.13495.
•• Ledowski T, O’Dea B, Meyerkort L, Hegarty M, von Ungern-Sternberg BS. Postoperative residual neuromuscular paralysis at an Australian tertiary children’s hospital. Anesthesiol Res Pract. 2015;2015: 410248. https://doi.org/10.1155/2015/410248Audit of Australian tertiary pediatric center showing high rates of residual neuromuscular blockade even after a reversal agent was given.
Fortier LP, McKeen D, Turner K, de Medicis E, Warriner B, Jones PM, et al. The RECITE study: a Canadian prospective, multicenter study of the incidence and severity of residual neuromuscular blockade. Anesth Analg. 2015;121(2):366–72. https://doi.org/10.1213/ANE.0000000000000757.
Nemes R, Fulesdi B, Pongracz A, Asztalos L, Szabo-Maak Z, Lengyel S, et al. Impact of reversal strategies on the incidence of postoperative residual paralysis after rocuronium relaxation without neuromuscular monitoring: a partially randomised placebo controlled trial. Eur J Anaesthesiol. 2017;34(9):609–16. https://doi.org/10.1097/EJA.0000000000000585.
Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, et al. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology. 2008;109(3):389–98. https://doi.org/10.1097/ALN.0b013e318182af3b.
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Vender JS, et al. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology. 2011;115(5):946–54. https://doi.org/10.1097/ALN.0b013e3182342840.
Meretoja OA. Neuromuscular blocking agents in paediatric patients: influence of age on the response. Anaesth Intensive Care. 1990;18(4):440–8. https://doi.org/10.1177/0310057x9001800403.
Driessen JJ, Robertson EN, Van Egmond J, Booij LH. The time-course of action and recovery of rocuronium 0.3 mg x kg(-1) in infants and children during halothane anaesthesia measured with acceleromyography. Paediatr Anaesth. 2000;10(5):493–7. https://doi.org/10.1046/j.1460-9592.2000.00543.x.
Rapp HJ, Altenmueller CA, Waschke C. Neuromuscular recovery following rocuronium bromide single dose in infants. Paediatr Anaesth. 2004;14(4):329–35. https://doi.org/10.1046/j.1460-9592.2003.01216.x.
Taivainen T, Meretoja OA, Erkola O, Rautoma P, Juvakoski M. Rocuronium in infants, children and adults during balanced anaesthesia. Paediatr Anaesth. 1996;6(4):271–5. https://doi.org/10.1111/j.1460-9592.1996.tb00449.x.
Meakin GH. Neuromuscular blocking drugs in infants and children. Continuing Educ Anaesth Critical Care Pain. 2007;7(5):143–7. https://doi.org/10.1093/bjaceaccp/mkm032.
Meretoja OA. Neuromuscular block and current treatment strategies for its reversal in children. Paediatr Anaesth. 2010;20(7):591–604. https://doi.org/10.1111/j.1460-9592.2010.03335.x.
Gilbertson LE, Fiedorek MC, Fiedorek CS, Trinh TA, Lam H, Austin TM. Prolonged neuromuscular block after rocuronium administration in laparoscopic pyloromyotomy patients: a retrospective bayesian regression analysis. Paediatr Anaesth. 2021;31(3):290–7. https://doi.org/10.1111/pan.14118.
Soffer OD, Kim A, Underwood E, Hansen A, Cornelissen L, Berde C. Neurophysiological assessment of prolonged recovery from neuromuscular blockade in the neonatal intensive care unit. Front Pediatr. 2020;8:580. https://doi.org/10.3389/fped.2020.00580.
Meretoja OA, Taivainen T, Jalkanen L, Wirtavuori K. Synergism between atracurium and vecuronium in infants and children during nitrous oxide-oxygen-alfentanil anaesthesia. Br J Anaesth. 1994;73(5):605–7. https://doi.org/10.1093/bja/73.5.605.
von Ungern-Sternberg BS, Hammer J, Schibler A, Frei FJ, Erb TO. Decrease of functional residual capacity and ventilation homogeneity after neuromuscular blockade in anesthetized young infants and preschool children. Anesthesiology. 2006;105(4):670–5. https://doi.org/10.1097/00000542-200610000-00010.
Trachsel D, Svendsen J, Erb TO, von Ungern-Sternberg BS. Effects of anaesthesia on paediatric lung function. Br J Anaesth. 2016;117(2):151–63. https://doi.org/10.1093/bja/aew173.
Isono S, Tanaka A, Ishikawa T, Nishino T. Developmental changes in collapsibility of the passive pharynx during infancy. Am J Respir Crit Care Med. 2000;162(3 Pt 1):832–6. https://doi.org/10.1164/ajrccm.162.3.9911089.
Adewale L. Anatomy and assessment of the pediatric airway. Paediatr Anaesth. 2009;19(Suppl 1):1–8. https://doi.org/10.1111/j.1460-9592.2009.03012.x.
Videira RL, Vieira JE. What rules of thumb do clinicians use to decide whether to antagonize nondepolarizing neuromuscular blocking drugs? Anesth Analg. 2011;113(5):1192–6. https://doi.org/10.1213/ANE.0b013e31822c986e.
Viby-Mogensen J, Jensen NH, Engbaek J, Ording H, Skovgaard LT, Chraemmer-Jorgensen B. Tactile and visual evaluation of the response to train-of-four nerve stimulation. Anesthesiology. 1985;63(4):440–3. https://doi.org/10.1097/00000542-198510000-00015.
Thilen SR, Hansen BE, Ramaiah R, Kent CD, Treggiari MM, Bhananker SM. Intraoperative neuromuscular monitoring site and residual paralysis. Anesthesiology. 2012;117(5):964–72. https://doi.org/10.1097/ALN.0b013e31826f8fdd.
Larsen PB, Gatke MR, Fredensborg BB, Berg H, Engbaek J, Viby-Mogensen J. Acceleromyography of the orbicularis oculi muscle II: comparing the orbicularis oculi and adductor pollicis muscles. Acta Anaesthesiol Scand. 2002;46(9):1131–6. https://doi.org/10.1034/j.1399-6576.2002.460912.x.
Khandkar C, Liang S, Phillips S, Lee CY, Stewart PA. Comparison of kinemyography and electromyography during spontaneous recovery from non-depolarising neuromuscular blockade. Anaesth Intensive Care. 2016;44(6):745–51. https://doi.org/10.1177/0310057X1604400618.
Stewart PA, Freelander N, Liang S, Heller G, Phillips S. Comparison of electromyography and kinemyography during recovery from non-depolarising neuromuscular blockade. Anaesth Intensive Care. 2014;42(3):378–84. https://doi.org/10.1177/0310057X1404200316.
Salminen J, van Gils M, Paloheimo M, Yli-Hankala A. Comparison of train-of-four ratios measured with Datex-Ohmeda’s M-NMT MechanoSensor and M-NMT ElectroSensor. J Clin Monit Comput. 2016;30(3):295–300. https://doi.org/10.1007/s10877-015-9717-4.
Gaffar EA, Fattah SA, Atef HM, Omera MA, Abdel-Aziz MA. Kinemyography (KMG) versus electromyography (EMG) neuromuscular monitoring in pediatric patients receiving cisatracurium during general anesthesia. Egyptian Journal of Anaesthesia. 2013;29(3):247–53. https://doi.org/10.1016/j.egja.2013.02.006.
Abdel-Ghaffar M, Ismail S, Omera M, Atef H, Abdel-Aziz M. Kinemyography (KMG) versus electromyography (EMG) neuromuscular monitoring in pediatric patients receiving Rocuronium during general anesthesia: 3AP1–4. Eur J Anaesthesiol. 2013;30:38 (00003643-201306001-00117).
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Deshur M, et al. Comparison of the TOFscan and the TOF-Watch SX during recovery of neuromuscular function. Anesthesiology. 2018;129(5):880–8. https://doi.org/10.1097/ALN.0000000000002400.
Yhim HB, Jang YE, Lee JH, Kim EH, Kim JT, Kim HS. Comparison of the TOFscan and the TOF-Watch SX during pediatric neuromuscular function recovery: a prospective observational study. Perioper Med (Lond). 2021;10(1):45. https://doi.org/10.1186/s13741-021-00215-2.
Blobner M, Hunter JM, Meistelman C, Hoeft A, Hollmann MW, Kirmeier E, et al. Use of a train-of-four ratio of 0.95 versus 0.9 for tracheal extubation: an exploratory analysis of POPULAR data. Br J Anaesth. 2020;124(1):63–72. https://doi.org/10.1016/j.bja.2019.08.023.
Driessen JJ, Robertson EN, Booij LH. Acceleromyography in neonates and small infants: baseline calibration and recovery of the responses after neuromuscular blockade with rocuronium. Eur J Anaesthesiol. 2005;22(1):11–5. https://doi.org/10.1017/s0265021505000037.
•• Unterbuchner C, Werkmann M, Ziegleder R, Kraus S, Seyfried T, Graf B, et al. Shortening of the twitch stabilization period by tetanic stimulation in acceleromyography in infants, children and young adults (STSTS-Study): a prospective randomised, controlled trial. J Clin Monit Comput. 2020;34(6):1343–9. https://doi.org/10.1007/s10877-019-00435-4A prospective randomized controlled trial highlighting the challenges of using acceleromyography in young patients.
Bowdle A, Bussey L, Michaelsen K, Jelacic S, Nair B, Togashi K, et al. A comparison of a prototype electromyograph vs. a mechanomyograph and an acceleromyograph for assessment of neuromuscular blockade. Anaesthesia. 2020;75(2):187–95. https://doi.org/10.1111/anae.14872.
Nemes R, Lengyel S, Nagy G, Hampton DR, Gray M, Renew JR, et al. Ipsilateral and simultaneous comparison of responses from acceleromyography- and electromyography-based neuromuscular monitors. Anesthesiology. 2021;135(4):597–611. https://doi.org/10.1097/ALN.0000000000003896.
Lee W. The latest trend in neuromuscular monitoring: return of the electromyography. Anesth Pain Med (Seoul). 2021;16(2):133–7. https://doi.org/10.17085/apm.21014.
Owusu-Bediako K KS, Rice-Weimer J, Tobias J. Quantitative train-of-four monitoring in pediatric patients using electromyography. ASA Annual Meeting Oct 09, 2021. 2021;ASA Abstract A4061(Virtual presentation). http://asaabstracts.com/strands/asaabstracts/abstract.htm?year=2021&index=8&absnum=5093.
McCluskey A, Meakin G, Hopkinson JM, Baker RD. A comparison of acceleromyography and mechanomyography for determination of the dose-response curve of rocuronium in children. Anaesthesia. 1997;52(4):345–9. https://doi.org/10.1111/j.1365-2044.1997.104-az0101.x.
Jung W, Hwang M, Won YJ, Lim BG, Kong MH, Lee IO. Comparison of clinical validation of acceleromyography and electromyography in children who were administered rocuronium during general anesthesia: a prospective double-blinded randomized study. Korean J Anesthesiol. 2016;69(1):21–6. https://doi.org/10.4097/kjae.2016.69.1.21.
Sauer M, Stahn A, Soltesz S, Noeldge-Schomburg G, Mencke T. The influence of residual neuromuscular block on the incidence of critical respiratory events. A randomised, prospective, placebo-controlled trial. Eur J Anaesthesiol. 2011;28(12):842–8. https://doi.org/10.1097/EJA.0b013e328345cd11.
Eikermann M, Groeben H, Husing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003;98(6):1333–7. https://doi.org/10.1097/00000542-200306000-00006.
Kumar GV, Nair AP, Murthy HS, Jalaja KR, Ramachandra K, Parameshwara G. Residual neuromuscular blockade affects postoperative pulmonary function. Anesthesiology. 2012;117(6):1234–44. https://doi.org/10.1097/ALN.0b013e3182715b80.
Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92(4):977–84. https://doi.org/10.1097/00000542-200004000-00014.
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg. 2013;117(1):133–41. https://doi.org/10.1213/ANE.0b013e3182742e75.
Butterly A, Bittner EA, George E, Sandberg WS, Eikermann M, Schmidt U. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010;105(3):304–9. https://doi.org/10.1093/bja/aeq157.
Grabitz SD, Rajaratnam N, Chhagani K, Thevathasan T, Teja BJ, Deng H, et al. The effects of postoperative residual neuromuscular blockade on hospital costs and intensive care unit admission: a population-based cohort study. Anesth Analg. 2019;128(6):1129–36. https://doi.org/10.1213/ANE.0000000000004028.
Kirmeier E, Eriksson LI, Lewald H, Jonsson Fagerlund M, Hoeft A, Hollmann M, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7(2):129–40. https://doi.org/10.1016/S2213-2600(18)30294-7.
Scheffenbichler FT, Rudolph MI, Friedrich S, Althoff FC, Xu X, Spicer AC, et al. Effects of high neuromuscular blocking agent dose on post-operative respiratory complications in infants and children. Acta Anaesthesiol Scand. 2020;64(2):156–67. https://doi.org/10.1111/aas.13478.
Ansermino JM, Sanderson PM, Bevan JC, Bevan DR. Acceleromyography improves detection of residual neuromuscular blockade in children. Can J Anaesth. 1996;43(6):589–94. https://doi.org/10.1007/bf03011772.
Martinez-Ubieto J, Ortega-Lucea S, Pascual-Bellosta A, Arazo-Iglesias I, Gil-Bona J, Jimenez-Bernardo T, et al. Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reversed with neostigmine versus sugammadex. Minerva Anestesiol. 2016;82(7):735–42.
•• Voss T, Wang A, DeAngelis M, Speek M, Saldien V, Hammer GB, et al. Sugammadex for reversal of neuromuscular blockade in pediatric patients: results from a phase IV randomized study. Paediatr Anaesth. 2022;32(3):436–45. https://doi.org/10.1111/pan.14370A randomized double-blind phase IV trial to determine dosing guidelines for sugammadex use in patients aged 2-17 years.
Gaver RS, Brenn BR, Gartley A, Donahue BS. Retrospective analysis of the safety and efficacy of sugammadex versus neostigmine for the reversal of neuromuscular blockade in children. Anesth Analg. 2019;129(4):1124–9. https://doi.org/10.1213/ANE.0000000000004207.
Alonso A, de Boer HD, Booij L. Reversal of rocuronium-induced neuromuscular block by sugammadex in neonates: 10AP1–3. Eur J Anaesthesiol. 2014;31:163.
Carlos RV, Torres ML, de Boer HD. Rocuronium and sugammadex in a 3 days old neonate for draining an ovarian cyst. Neuromuscular management and review of the literature. Braz J Anesthesiol. 2016;66(4):430–2. https://doi.org/10.1016/j.bjane.2015.01.004.
Matsui M, Konishi J, Suzuki T, Sekijima C, Miyazawa N, Yamamoto S. Reversibility of rocuronium-induced deep neuromuscular block with sugammadex in infants and children-a randomized study. Biol Pharm Bull. 2019;42(10):1637–40. https://doi.org/10.1248/bpb.b19-00044.
Lang B, Han L, Zeng L, Zhang Q, Chen S, Huang L, et al. Efficacy and safety of sugammadex for neuromuscular blockade reversal in pediatric patients: an updated meta-analysis of randomized controlled trials with trial sequential analysis. BMC Pediatr. 2022;22(1):295. https://doi.org/10.1186/s12887-022-03288-0.
Kheterpal S, Vaughn MT, Dubovoy TZ, Shah NJ, Bash LD, Colquhoun DA, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132(6):1371–81. https://doi.org/10.1097/ALN.0000000000003256.
Beltran RJ, Mpody C, Nafiu OO, Tobias JD. Association of sugammadex or neostigmine with major postoperative pulmonary complications in children. Anesth Analg. 2022. https://doi.org/10.1213/ane.0000000000005872.
• Carollo DS, White WM. Postoperative recurarization in a pediatric patient after sugammadex reversal of rocuronium-induced neuromuscular blockade: a case report. A A Pract. 2019;13(6):204–5. https://doi.org/10.1213/XAA.0000000000001023Case report documenting recurarization after sugammadex use in an infant.
• Lorinc AN, Lawson KC, Niconchuk JA, Modes KB, Moore JD, Brenn BR. Residual weakness and recurarization after sugammadex administration in pediatric patients: a case series. A A Pract. 2020;14(7): e01225. https://doi.org/10.1213/XAA.0000000000001225Case series documenting residual neuromuscular blockade after sugammadex use in infants.
Eleveld DJ, Kuizenga K, Proost JH, Wierda JM. A temporary decrease in twitch response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg. 2007;104(3):582–4. https://doi.org/10.1213/01.ane.0000250617.79166.7f.
Woloszczuk-Gebicka B, Zawadzka-Glos L, Lenarczyk J, Sitkowska BD, Rzewnicka I. Two cases of the “cannot ventilate, cannot intubate” scenario in children in view of recent recommendations. Anaesthesiol Intensive Ther. 2014;46(2):88–91. https://doi.org/10.5603/AIT.2014.0017.
Wakimoto M, Burrier C, Tobias JD. Sugammadex for rapid intraoperative reversal of neuromuscular blockade in a neonate. J Med Cases. 2018;9(12):400–2. https://doi.org/10.14740/jmc3213.
Efune PN, Alex G, Mehta SD. Emergency sugammadex reversal in an 850-G premature infant: a case report. J Pediatr Pharmacol Ther. 2021;26(1):107–10. https://doi.org/10.5863/1551-6776-26.1.107.
Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of Anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. 2022;136(1):31–81. https://doi.org/10.1097/aln.0000000000004002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with humans or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Patient Safety in Anesthesia
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cha, Y.M., Faulk, D.J. Management of Neuromuscular Block in Pediatric Patients — Safety Implications. Curr Anesthesiol Rep 12, 439–450 (2022). https://doi.org/10.1007/s40140-022-00537-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-022-00537-x