Abstract
Purpose of Review
This review describes case reports for patients with kratom-associated adverse events in order to assist clinicians with patient management. A stepwise approach is proposed for assessing active kratom users as well as considerations for the management of toxicities or withdrawal.
Recent Findings
Multiple in vitro and in vivo studies illustrate the pharmacologic and toxicologic effects of kratom extract. No randomized controlled trials in humans exist that assess the safety and efficacy of the substance. Cross-sectional surveys from active users and reports from poison control centers have shown acute and chronic physiological and psychological adverse events.
Summary
Reports of adverse effects associated with kratom use have demonstrated hypothyroidism, hypogonadism, hepatitis, acute respiratory distress syndrome, posterior reversible encephalopathy syndrome, seizure, and coma. Overdose toxidrome leads to respiratory failure, cardiac arrest, and fatalities. Adult and neonatal withdrawal symptoms have also occurred. Clinicians should be aware of the risks and benefits of kratom use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We completed a review of Mitragyna speciosa (MS), which is a psychoactive plant that belongs to the Rubiaceae family, or coffee family (Fig. 1) [1, 2]. Human consumption of the plant leaves was first documented in the late nineteenth century when native’s used it for ceremonial and medicinal purposes in the tropical and subtropical regions of southeast Asia and Africa [2, 3]. Traditionally, MS was used to control ailments such as fever, diarrhea, cough, fatigue, and decreased libido, in addition to treat conditions such as enteritis, parasitic and herpes zoster infections, diabetes, hypertension, and depression [2,3,4,5,6,7,8]. Natives consumed MS by various routes: chewed raw, boiled in water as a beverage, or inhaled as a vapor [6,7,8]. Today, MS is known as kratom, first named by natives in Thailand [8]. Kratom use has surpassed its region of origin and reached consumers around the world, mainly for its proclaimed psychoactive properties [9]. In 2016, The American Kratom Association (AKA) estimated that there are 3–5 million active and regular kratom users in the United States (USA). [10] Despite longstanding and increasing kratom use, controlled trials evaluating efficacy and safety have not been performed [11••]. Surveys of active kratom users reported dual and dose-dependent stimulant-like properties and opioid-like euphoric effects: as decreased pain (85%), increased energy (84%), and less depressive mood (80%) [12••]. These effects are exploited medicinally for analgesia, withdrawal from illicit drug or prescription opioid medicine, or background mental/emotional disorder [13]. The positive experiences from kratom such as euphoria, relaxation, sociability, and productivity have been attractive for recreational use in substance use disorder and in cultural festivities [14, 15].
Mitragyna Speciosa tree, leaf, and capsules. a Tree. b Leaf. c Capsules. Adopted from Drug Enforcement Agency (DEA) [1]
Pharmacology and Preclinical Studies
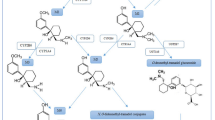
Kratom contains about 25 pharmacologically active alkaloids, of which mitragynine (MG) and 7-hydroxymitragynine (7-HMG) are the most prevalent [16]. MG and 7-HMG are dose-dependent agonists of the μ-, κ-, and δ-opioid receptors in the central nervous system (CNS) as well as non-opioid receptors [6, 7, 16, 17••, 18, 19]. Their non-selectivity likely results in the beneficial multimodal effects of kratom especially for analgesia and mood elevation [20]. In vitro studies have exhibited cytotoxic effects on human neuronal, hepatic, and cardiac myocyte cell lines and have shown neuromuscular blocking effects [21,22,23,24]. Kratom was also reported to inhibit CYP 450 hepatic enzyme, particularly CYP 3A4, 2D6, and 1A2 [29, 30]. In toxicological in vivo animal studies, test subjects exhibited adverse effects such as hepatitis, nephrotoxicity, reduced sperm production, addiction and tolerance, long-term cognitive deficits, generalized convulsions, and fatalities with high doses [18, 19, 25,26,27,28].
Emerging Use and Regulatory Concerns
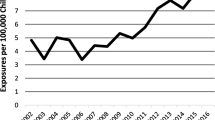
Increasing kratom use has resulted in increased reports of adverse events. Attributing these reports solely to kratom is complicated by the absence of standardized product manufacturing and voluntary adverse event reporting. Between 2011 and 2017, calls to US Poison Control Centers (PCC) show that kratom poisoning and fatality cases have increased 58-fold in 6 years. One-third of these cases resulted in healthcare facility admission [31••]. Cross-sectional surveys highlighted opioid-like adverse events such as intoxication, addiction, and kratom-associated withdrawal symptoms [11••, 33,34,35,36,37,38]. Many western countries and a number of US states have banned kratom possession and/or sales. Despite these control efforts, availability online has eased the accessibility to kratom products. In the USA, many kratom products used are without good manufacturing practices outlined by the Food and Drug Administration (FDA) or subject to quality assessments. In February 2018, the FDA issued warnings regarding 44 fatalities associated with kratom [32]. In 2019, two warnings were issued regarding selected kratom product impurities; a warning in February for potential heavy metal poisoning following laboratory discovery of elevated lead and nickel levels and another warning in March for nationwide recall for potential Salmonella contamination following a series of Salmonella infection outbreaks [39, 40]. A 2017 survey showed that about 40% of active kratom users consult their healthcare professionals (physician, nurse, or pharmacist) about use indicating that there is a public need for evaluating risks and benefits [11••]. This review is intended to help healthcare professionals assess the adverse events associated with kratom use. In addition, we propose a stepwise algorithm for assessing patients actively using kratom and discuss considerations for management of kratom-associated adverse events and toxicities.
Literature Case Review
We performed a systematic literature review using MEDLINE, Embase, and Google Scholar. Main search terms were “Mitragyna speciosa” and “kratom” combined with appropriate Boolean phrase. We included only case reports and case series written in English that were published from January 2008 to March 2019. Botanical, pharmacokinetic, analytical, or preclinical studies, and surveys were excluded. Overall, there were 41 cases on kratom-associated adverse events or toxicities (Table 1). Kratom-associated adverse events were as follows: kratom-associated withdrawal symptoms (KAWS) in adults [41,42,43,44,45,46,47,48,49,50,51,52], kratom-associated neonatal abstinence syndrome (KANAS) [48, 52,53,54,55,56], hypothyroidism [43], hypogonadism [57], kratom-induced hepatoxicity (KIH) [58,59,60,61,62,63,64,65], CNS effects causing seizure and coma or posterior reversible encephalopathy syndrome (PRES) [39, 66, 67], acute respiratory distress syndrome (ARDS) [68, 69], overdose toxidrome [70, 71], and fatalities [72,73,74,75,76,77,78,79,80]. There were six case series of aggregated patient’s data [48, 49, 51, 73, 77, 80]. There were also mixed presentations: KAWS and seizure [41], hypothyroidism [43], or KANAS [48].
Kratom-Associated Withdrawal in Adults [41,42,43,44,45,46,47,48,49,50,51,52]
Tolerance generally occurred after 3 months [42], and a few patients described escalating their doses four to ten times their initial dose within as early as a few weeks [44, 50] to achieve the same effect. Perceived experiences may also change with the course of intake. Generally, initial experience is beneficial; some patients describe a sense of euphoria, productivity, relaxation, and pain control. Addiction has led to intravenous administration of kratom extract with reported occurrences of thrombophlebitis and infection requiring antibiotics [47]. Duration of kratom addiction ranges from 1 to 3 years, and the doses used by addicted patients ranged from 14 to 42 g per day. KAW was described in several adult populations: those with chronic pain, substance use disorder, pregnant women, and recently delivered mothers. Most patients presented to a healthcare professional with self-identified symptoms of withdrawal and expressed their desire to obtain a supervised kratom detoxification or induction/maintenance regimen because of a failed attempt to self-abstain. Many patients often encounter KAW following use as an opioid alternative for pain control or reduction of chronic opioid use. Patients have also described withdrawal after recreational intravenous use along with other illicit substances. Symptoms of KAW generally develop within 6–12 h after last reported use. Symptoms have included rhinorrhea, restlessness, anxiety, irritability, sleep disturbance, sweating, chills, craving, pain, pruritis, goose bumps, abdominal cramps, and diarrhea. Though similar to opioid withdrawal, KAW has been reported to be less severe. Change in patient’s psychiatric functioning has not been reported except in patient with a diagnosed schizoaffective disorder [45, 51]. Substance-seeking behavior has been observed, and some patients may hide doses after claiming abstinence. Case reports have highlighted the benefits of using pharmacological interventions for the purpose of long-term abstinence from kratom use via maintenance therapies [41, 49, 50, 52] or acute control of KAW [42,43,44, 46, 48, 51] via detoxifying regimens.
For induction/maintenance, only partial μ-opioid receptor agonist regimens have been used, such as buprenorphine plus naloxone [41, 49, 50, 52], buprenorphine/norbuprenorphine [49], or buprenorphine [52]. These regimens showed benefits beyond control of KAW such as reducing dose requirements of pain medication [49], or prevention of KANAS [52]. Symptom-triggered dose-escalation was used for immediate effects, often escalating the buprenorphine dose by 2–4 mg as needed. To monitor adherence to therapy and abstinence from kratom, blood and urine testing for buprenorphine, MG, and 7-HMG was performed.
For detoxification regimens, shorter course therapies were used and their efficacy on kratom abstinence beyond the supervised course is not reported. Agents used included partial μ-opioid receptor agonists such as buprenorphine [43] or buprenorphine/naloxone [46], weak or full μ-opioid receptor agonists such as dihydrocodeine [42], morphine [48], or alpha-2 agonists such as oral clonidine [44, 51]. Antihistamines or pregabalin were added in some instances for symptom management.
Kratom-Associated Neonatal Abstinence Syndrome [48, 52,53,54,55,56]
In Smid and colleagues’ case, kratom use was stopped and kratom-associated neonatal abstinence syndrome (KANAS) was prevented when a buprenorphine regimen was started in week 22 of gestation. However, this same regimen failed to prevent KANAS in another pregnant woman when it was started at week 19 of gestation [52]. In one case report, kratom was used by the mother regularly for 2 years [48]. In all cases, kratom use overlapped with the entire course of pregnancy. KANAS occurred in 6–96 h after birth, with noted effects including reduced oral intake, excessive sucking, jitteriness, irritability, facial excoriations, hypertonia, sneezing, and excessive inconsolable high-pitched cry. The Finnegan score is a nursing assessment tool that grades the severity of the most common signs and symptoms of NAS, producing a final score with increasing severity.
It was used in three KANAS cases to grade the severity and guide therapy [54,55,56]. Their Finnegan score was calculated to be at the “Severe” category threshold (> 9 points) on presentation. Management of KANAS involved administration of full μ-opioid receptor agonists for detoxification. Almost all cases used morphine; only one case used intravenous methadone. Two cases reported using morphine as 10 mcg/kg/h either continuously or administered every 3 h [48, 54] and one case used 100 mcg/kg/day [55]. In all cases, the neonate responded to therapy on initiation. The Finnegan score was reported to drop by 7–9 points from baseline presentation indicating an improvement of KANAS. However, bradycardia and excessive sedation were associated with morphine use [54]. Weaning from morphine therapy was initiated by day 2 or 3. Oral morphine was used as a step-down approach once oral intake was tolerated. Though morphine has shown success in KANAS, Eldridge and colleagues’ case report has indicated possible rebound of KANAS following morphine discontinuation [54]. The cause was thought to be premature discontinuation 2 days after the start of morphine therapy. In this circumstance, clonidine 1 mcg/kg every 3 h subsequently showed improvement in NAS scores. This therapy was used for 1 day and discontinued thereafter. Total days of stay for patients ranged from 8 to 14 days but weaning off morphine can take up to 2 months due to risk of rebound KANAS [56]. In Davidson and colleagues’ case report, the neonate was monitored for 48 h after discontinuation of therapy [55].
Endocrine Effects: Hypothyroidism and Primary Hyperprolinemica [43, 57]
There were two cases that reported effects on the endocrine system. In LaBryer and colleagues’ case report, kratom administration was associated with elevated prolactin and reduced testosterone levels leading to hyperprolactinemia and hypogonadotropic hypogonadism without an alternative identifiable cause [57]. The clinical significance of this change was manifested by decreased energy and libido. Cessation of administration for 2 months lead to normalization of these hormones without a need for intervention. Notably, thyroid-stimulating hormone (TSH) levels were normal in this case. Sheleg and Collins presented a case with KAWS which included signs and symptoms of hypothyroidism confirmed by elevated TSH [43]. This developed after 7 months of consuming kratom tincture daily. The patient received an opioid detoxification regimen using buprenorphine for KAWS and levothyroxine for hypothyroidism. Fifteen months later, levothyroxine dose was reduced to 50 mcg per day after improvement in thyroid function. Conversely, a cross-sectional study of 19 long-term kratom consumers (76 to 94 mg of MG per day for more than 2 years) neither found the substance impairing to the levels of tetraiodothyronine, testosterone, or gonadotrophins nor did it show a dose-dependent effect of kratom on these hormones [81].
Kratom-Induced Hepatoxicity [58,59,60,61,62,63,64,65]
Kratom-induced hepatotoxicity (KIH) has been reported after 2 to 4 weeks of use and rechallenge has led to recurrence [59, 65]. The reported dose resulting in KIH was 14 to 21 g per day. In one case report, there was rapid dose-escalation (3 to 6 times the starting dose in 2 weeks) [58]. In all cases, patients presented to the emergency department for symptoms associated with hepatotoxicity including at least one of the following symptoms: dark-colored urine, light-colored stools, profound weakness, weight loss, nausea, vomiting, fever, or night sweats. Dark-colored urine was the most commonly reported symptom. Yellow skin, scleral icterus, and pruritus were also reported. Most patients were hemodynamically stable on presentation; one case showed mildly elevated blood pressure at > 140/80 mmHg in a patient with chronic hypertension [64]. The diagnosis of KIH was made on the basis of exclusion of all other causes of hepatotoxicity. An extensive workup for acute viral hepatitis A, B, and C and assays of hepatotoxic substances such as salicylates, acetaminophen, and CNS medications were used to determine if the exhibited hepatotoxic effects were correlated. Four case reports studied the likelihood of KIH using association scales in which all found a ‘probable’ association [62,63,64,65]. Scales used included the Roussel Uclaf Causality Assessment Method, World Health Organization Uppsala Monitoring Centre and the International Organizations of Medical Sciences Scale. Jaundice was reported in four cases where the total bilirubin was the highest of all KIH cases, ranging from 6.5 mg/dL to 33.7 mg/dL (reference < 1 mg/dL) [58, 59, 63, 64]. After kratom cessation, total bilirubin decreased within 2 days to 7 days [58,59,60,61,62,63,64]. The highest alkaline phosphatase recorded was 790 units/L (reference 35–129 units/L). Complete normalization of total bilirubin and alkaline phosphatase occurred approximately 2 months after cessation [61, 65]. Early reports of KIH confirmed intrahepatic cholestasis based on hepatic function tests, abdominal and hepatic imaging, and histological findings. More recently, two case reports highlighted the possibility of a hepatocellular injury [62, 65]. Both of these two cases had confounding causes for hepatocellular injury, including positive IgM for cytomegalovirus [65] and positive urine cannabinoid [62].
In two case reports, N-acetylcysteine (NAC) was offered as hepatoprotective agent. Mousa and colleagues case reported using intermittent bolus infusion regimen (140 mg/kg followed by 70 mg/kg for 18 doses) in which the patient received the full 18 doses over a 4-day course and authors reported resolution of symptoms and down-trending hepatic enzymes [61]. Tayabali and colleagues started NAC with a loading dose of 150 mg/kg but the patient developed an anaphylactoid reaction soon after the loading dose was administered resulting in a termination of infusion within an hour after initiation [64]. Although signs and symptoms were reported to be improved in 2 days, the role of NAC in KIH is not fully understood or established.
Central Nervous System Effects: Posterior Reversible Encephalopathy Syndrome and Seizure [51, 66, 67]
Posterior reversible encephalopathy syndrome and seizure (PRES) has been reported in patients that combined kratom use with marijuana, fluoxetine, and quetiapine [66]. In this patient, supportive care and lowering blood pressure with nicardipine infusion helped to improve the patient’s condition. Two cases reported of seizure activity within 30 min of kratom ingestion in addition to animal studies previously mentioned [25], indicate that kratom may be a pro-convulsant [41, 67]. These cases also detected levels of other substances including modafinil and antidepressants which are known to lower seizure threshold. The dose and duration of kratom use in these cases was not reported. Standard medications for seizure control such as lorazepam and phenytoin were used successfully in reversing the onset of the seizure. In one case, the urine concentration of MG was measured at 0.167 mg/L. Interestingly, this level is half the MG urine concentration in reported fatalities (range from 0.37 to 3.47 mg/L) [72, 74, 76, 77].
Acute Respiratory Distress Syndrome [68, 69]
Two patient cases have reported respiratory compromise leading to acute respiratory distress syndrome (ARDS) and subsequent need for mechanical ventilation. In both cases, all other causes of respiratory failure from infectious or hemorrhagic causes were ruled out. In the case reported by Pathak and colleagues, the patient was witnessed to have ingested both kratom and alcohol [68]. The duration for mechanical ventilation was for 2 weeks in this patient. In the case reported by Jaliawala and colleagues, mechanical ventilation was needed for 3 days [69]. Both cases reported no attempt to reverse kratom with naloxone.
Toxidromes and Fatalities [70,71,72,73,74,75,76,77,78,79,80]
Kratom toxicity is presumed to resemble an opioid toxidrome: manifested by mydriasis, depressed respiratory function, altered mental status, hypotension, and hypothermia. Two recent case reports showed the role of naloxone reversal on the kratom toxidrome, although it was administered due to suspected co-intoxication with an opioid [70, 71]. In the case of Overbeek and colleagues, the patient received two prehospital doses of naloxone 0.4 mg which led to improvement in respiratory rate and mental status [70]. With supportive care and intravenous fluids, the patient continued to improve over a of 24-h period and ultimately survived. Shekar and colleagues reported a case where additional measures were needed following the naloxone reversal dose [71]. The patient was discharged to a rehabilitation institute 2 weeks after they were admitted to the inpatient facility. The patient’s urine 7-HMG was greater than 500 mcg/L.
In the fatal case reported by Aggarwal and colleagues, the patient presented to the hospital with cardiac arrest [78]. Initially, return of spontaneous circulation (ROSC) was achieved within 1 h of hospital arrival with advanced cardiac life support (ACLS) including vasopressors, inotropes, and sodium bicarbonate. Naloxone was given as an opioid antagonist, but its effect was non-discernible. A standard lipid emulsion dose (1.5 mL/kg intravenous bolus) was also administered with an observed response for approximately 1 h, which resulted in a 16% improvement in alveolar-arterial oxygenation lasting for only a few minutes followed by a 30% reduction in epinephrine requirement lasting approximately 1 h. However, the patient’s cardiorespiratory function subsequently deteriorated leading to death 12 h after ROSC. The author reported modest cardioprotective properties of intralipid on assumption of cardiotoxicity from kratom components. Neither qualitative nor quantitative serum assays for kratom were investigated, and the amount of the reported ingestion was unknown. The urine was negative for MG but positive for codeine, which the patient allegedly took only one standard dose.
Most fatalities were young males with a history of substance abuse or psychiatric disorder. All fatalities were pronounced dead at the scene, except two cases in which the patients were brought to an emergency department. Attempts with ACLS were unsuccessful [73, 78]. In both cases that presented to the emergency department, kratom was suspected to be ingested within 24 h prior to presentation [73, 78]. In the majority of fatal cases, the cause of death was attributed to pulmonary congestion and/or edema. Other causes of death were liver steatosis, brain edema, seizure, hyperthermia, and mechanical asphyxia. All autopsy blood samples confirmed the presence of at least one other CNS depressant [78]. There is speculation that these fatalities may be caused by mixed drug ingestions leading to synergistic or additive effects, yet fatalities have also occurred from kratom alone. Significant variability in extraction methods and assays, as well as the timing of autopsies post mortem, makes it difficult to conclude a lethal blood concentration of MG. Blood concentrations in fatal cases have ranged from 10 to 4800 mcg/L. Of note, the report by Karinen and colleagues is the only case that tested for and reported positive blood concentrations of the other potent component in kratom, 7-HMG at 2200 mcg/L [76].
Assessing Kratom in Active Users and Managing Associated Complications
Case reports and series are reported voluntarily; therefore, there is inconsistency among reports for assessment and management. Based on available evidence from these reports, we propose a stepwise approach to assess kratom safety and efficacy in active users (Fig. 2) and a summary of kratom-associated adverse events (Table 2).
Conclusion
Kratom has been used both medicinally and recreationally and its use has continued to increase. Case reports and case series of kratom-associated adverse events, toxicities, and fatalities are alarming. Healthcare professionals’ awareness of trends in use as well as associated risks is necessary to discuss risks and benefits of use with patients and provide prompt management of adverse events. Controlled studies are needed in the future to examine the impacts of kratom use and provide insight into optimal management and regulation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
United States. Drugs of abuse: a DEA resource guide. Washington, D.C.: U.S. Dept. of Justice, Drug Enforcement Administration. Available Online at; 2017. https://www.hsdl.org/?view&did=806030 (Accessed 3/25/19)
Gong F, Gu HP, Xu QT, Kang WY. Genus Mitragyna: ethnomedicinal uses and pharmacological studies. Phytopharmacol. 2012;3(2):263–72.
Jansen KL, Prast CJ. Ethnopharmacology of kratom and the Mitragyna alkaloids. J Ethnopharmacol. 1988;23(1):15–119.
Shellard EJ. Ethnopharmacology of kratom and the Mitragyna alkaloids. J Ethnopharmacol. 1989;25:123–4. https://doi.org/10.1016/0378-8741(89)90053-6.
Singh D, Narayanan S, Vicknasingam B. Traditional and non-traditional uses of Mitragynine (kratom): a survey of the literature. Brain Res Bull. 2016;126(1):41–6. https://doi.org/10.1016/j.brainresbull.2016.05.004.
Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792–9.
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, et al. From kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37(2):138–51. https://doi.org/10.1016/j.neubiorev.2012.11.012.
Tanguay P. Kratom in Thailand: decriminalisation and community control? Series on Legislative Reform of Drug Policies No. 13, Transnational Institute. International Drug Policy Consortium (IPDC). 2011. Available Online at: https://www.tni.org/files/download/kratom-briefing-dlr13.pdf (Accessed 12/8/2018).
Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, et al. Following “the roots” of kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. Biomed Res Int. 2015;2015:1–11. https://doi.org/10.1155/2015/968786.
Kratom fact sheet, from American Kratom Association website available at: https://www.americankratom.org/images/file/Myths_Facts-on-Kratom-Legislative-Day-Handout-Final-2.pdf. (Accessed 12.15.2018).
•• Ulbricht C, Costa D, Dao J, Isaac R, LeBlanc YC, Rhoades J, et al. An evidence-based systematic review of kratom (Mitragyna speciosa) by the natural standard research collaboration. J Diet Suppl. 2013;10(2):152–70. https://doi.org/10.3109/19390211.2013.793541This is a systematic review of randomized controlled trials regarding Kratom.
•• Grundmann O. Patterns of kratom use and health impact in the US—results from an online survey. Drug Alcohol Depend. 2017;176:63–70. https://doi.org/10.1016/j.drugalcdep.2017.03.007This is a survey of active Kratom users in the US.
Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340–8. https://doi.org/10.1016/j.drugalcdep.2017.08.034.
Fox J, Smith A, Yale A, Chow C, Alaswad E, Cushing T, et al. Drugs of abuse and novel psychoactive substances at outdoor music festivals in Colorado. Subst Use Misuse. 2018;53(7):1203–11. https://doi.org/10.1080/10826084.2017.1400067.
Swogger MT, Hart E, Erowid F, Erowid E, Trabold N, Yee K, et al. Experiences of kratom users: a qualitative analysis. J Psychoactive Drugs. 2015;47(5):360–7. https://doi.org/10.1080/02791072.2015.1096434.
Kruegel AC, Grundmann O. The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacol. 2018;134:108–20. https://doi.org/10.1016/j.neuropharm.2017.08.026.
•• Chin KY, Mark-Lee WF. A review on the Antinociceptive effects of Mitragyna speciosa and its derivatives on animal model. Curr Drug Targets. 2018;19(12):1359–65. https://doi.org/10.2174/1389450118666170925154025This is a review of from in-vitro and in-vivo studies regarding kratom safety and effects.
Swogger MT, Walsh Z. Kratom use and mental health: a systematic review. Drug Alcohol Depend. 2018;183:134–40. https://doi.org/10.1016/j.drugalcdep.2017.10.012.
Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130(1):127–38. https://doi.org/10.1007/s00414-015-1279-y.
Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M. Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacol. 2015;232(13):2227–38. https://doi.org/10.1007/s00213-015-3866-5.
Chittrakarn S, Keawpradub N, Sawangjaroen K, Kansenalak S, Janchawee B. The neuromuscular blockade produced by pure alkaloid, mitragynine and methanol extract of kratom leaves (Mitragyna speciosa Korth.). J Ethnopharmacol. 2010;192(3):344–9. https://doi.org/10.1016/j.jep.2010.03.035.
Lu J, Wei H, Wu J, Jamil MF, Tan ML, Adenan MI, et al. Evaluation of the cardiotoxicity of mitragynine and its analogues using human induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9(12):e115648. https://doi.org/10.1371/journal.pone.0115648.
Saidin NA, Randall T, Takayama H, Holmes E, Gooderham NJ. Malaysian kratom, a phyto-pharmaceutical of abuse: studies on the mechanism of its cytotoxicity. Toxicol. 2008;1(253):19–20. https://doi.org/10.1016/j.tox.2008.07.024.
Pantano F, Tittarelli R, Mannocchi G, Zaami S, Ricci S, Giorgetti R, et al. Hepatotoxicity induced by “the 3Ks”: kava, kratom and khat. Int J Mol Sci. 2016;17(4):580. https://doi.org/10.3390/ijms17040580.
Kong WM, Chik Z, Ramachandra M, Subramaniam U, Aziddin RE, Mohamed Z. Evaluation of the effects of Mitragyna speciosa alkaloid extract on cytochrome P450 enzymes using a high throughput assay. Molecules. 2011;16(9):7344–56. https://doi.org/10.3390/molecules16097344.
Avery BA, Boddu SP, Sharma A, Furr EB, Leon F, Cutler SJ, et al. Comparative pharmacokinetics of mitragynine after oral administration of Mitragyna speciosa (kratom) leaf extracts in rats. Planta Med. 2019;85(04):340–6.
Sabetghadam A, Navaratnam V, Mansor SM. Dose–response relationship, acute toxicity, and therapeutic index between the alkaloid extract of mitragyna speciosa and its main active compound mitragynine in mice. Drug Dev Res. 2013;74(1):23–30. https://doi.org/10.1002/ddr.21052.
Harizal SN, Mansor SM, Hasnan J, Tharakan JK, Abdullah J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. J Ethnopharmacol. 2010;131(2):404–9. https://doi.org/10.1016/j.jep.2010.07.013.
Suhaimi FW, Yusoff NH, Hassan R, Mansor SM, Navaratnam V, Muller CP, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126:29–40. https://doi.org/10.1016/j.brainresbull.2016.03.015.
Daud MS, Mossadeq MS. Effect of short-term ingestion of the methanolic extract of mitragyna speciosa on sperm quality in mice.
•• Post S, Spiller HA, Chounthirath T, Smith GA. Kratom exposures reported to United States poison control centers: 2011–2017. Clin Toxicol. 2019;21:1–8. https://doi.org/10.1080/15563650.2019.1569236This is a report from the US poison control center (PCC).
Gottlieb S Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom, underscoring its potential for abuse: additional adverse events associated with kratom use identified. Silver Spring, MD: Food and Drug Administration, 2018.
Singh D, Muller CP, Vicknasingam BK. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132–7. https://doi.org/10.1016/j.drugalcdep.2014.03.017.
Singh D, Narayanan S, Vicknasingam BK, Prozialeck WC, Ramanathan S, Zainal H, et al. Severity of pain and sleep problems during kratom (Mitragyna speciosa Korth.) cessation among regular kratom users. J Psychoactive Drugs. 2018;50:1–9. https://doi.org/10.1080/02791072.2018.1443234.
Saingam D, Assanangkornchai S, Geater AF, Lerkiatbundit S. Validation of Krathom (Mitragyna speciosa Korth.) dependence scale (KDS): a dependence screen for internationally emerging psychoactive substance. Subst Abus. 2014;35(3):276–83. https://doi.org/10.1080/08897077.2014.924464.
Abdullah MF, Singh D, Kasinather BV, Azman N. Validation of the malay version of the kratom dependence scale (KDS) among malaysian kratom (Mitragyna Speciosa korth) users. Asian J Psychiatr. 2018;19(1). DOI: https://doi.org/10.1080/08897077.2014.924464.
Assanangkornchai S, Muekthong A, Sam-Angsri N, Pattanasattayawong U. The use of Mitragynine speciosa (“Krathom”), an addictive plant, in Thailand. Subst Use Misuse. 2007;42(14):2145–57. https://doi.org/10.1080/10826080701205869.
Singh D, Muller CP, Vicknasingam BK, Mansor SM. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125–31. https://doi.org/10.1080/02791072.2015.1012610.
Laboratory analysis of kratom products for heavy metal, from FDA website, available at: https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm635097.htm (accessed 4/12/19).
Company announcement: Sunstone Organics issues voluntary nationwide recall of select kratom products due to potential contamination by Salmonella, from FDA website, available at https://www.fda.gov/Safety/Recalls/ucm632554.htm (accessed on 4/22/2019).
Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction. 2008;103(6):1048–50. https://doi.org/10.1111/j.1360-0443.2008.02209.x.
McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229–31. https://doi.org/10.1159/000320288.
Sheleg SV, Collins GB. A coincidence of addiction to “kratom” and severe primary hypothyroidism. J Addict Med. 2011;5(4):300–1. https://doi.org/10.1097/ADM.0b013e318221fbfa.
Galbis-Reig D. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49–52.
Tavakoli HR, Buchholz AC, Kabir IK, Deb A, Gayk JN. Kratom: a new product in an expanding substance abuse market. Fed Pract. 2016;33(11):32–6.
Jayadeva V, Bunnag A, Meyen R, Fernando I. Kratom (Mitragyna speciosa) use in a veteran with chronic pain. Am J Psychiatry. 2017;12(3):13–5. https://doi.org/10.1176/appi.ajp-rj.2017.120305.
Lydecker A, Zuckerman M, Hack J, Becker B, Cherkes J, Boyer E. Intravenous kratom use in a patient with opioid dependence. J Toxic Pharm. 2017;1:003.
Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121–2.
Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481–3. https://doi.org/10.1097/ADM.0000000000000428.
Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493–5. https://doi.org/10.1097/ADM.0000000000000435.
Stanciu CN, Gnanasegaram SA, Ahmed S, Penders T. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12–8. https://doi.org/10.1080/02791072.2018.1562133.
Smid MC, Charles JE, Gordon AJ, Wright TE. Use of kratom, an opioid-like traditional herb, in pregnancy. Obstet Gynecol. 2018;132(4):926–8. https://doi.org/10.1097/AOG.0000000000002871.
Pizarro-Osilla C. Introducing… kratom. J Emerg Nurs. 2017;43(4):373–4. https://doi.org/10.1016/j.jen.2017.03.016.
Eldridge WB, Foster C, Wyble L. Neonatal abstinence syndrome due to maternal kratom use. Pediatrics. 2018;142(6):e20181839. https://doi.org/10.1542/peds.2018-1839.
Davidson L, Rawat M, Stojanovski S, Chandrasekharan P. Natural drugs, not so natural effects: Neonatal abstinence syndrome secondary to ‘kratom’. J Neonatal-Perinatal Med. 2018;(preprint). https://doi.org/10.3233/NPM-1863.
Murthy P, Clark D. An unusual cause for neonatal abstinence syndrome. Paediatr Child Health. 2019;24(1):12–4. https://doi.org/10.1093/pch/pxy084.
LaBryer L, Sharma R, Chaudhari KS, Talsania M, Scofield RH. Kratom, an emerging drug of abuse, raises prolactin and causes secondary hypogonadism: case report. J Investig Med High Impact Case Rep. 2018;6:1–3. https://doi.org/10.1177/2324709618765022.
Kapp FG, Maurer HH, Auwarter V, Winkelmann M, Hermanns-Clausen M. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227–31. https://doi.org/10.1007/s13181-011-0155-5.
Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086–7. https://doi.org/10.1002/hep.27612.
Riverso M, Chang M, Soldevila-Pico C, Lai J, Liu X. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79. https://doi.org/10.14740/gr990e.
Mousa MS, Sephien A, Gutierrez J, O'leary C. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):550–1. https://doi.org/10.1097/MJT.0000000000000631.
Griffiths CL, Gandhi N, Olin JL. Possible kratom-induced hepatomegaly: a case report. J Am Pharm Assoc. 2018;58(5):561–3. https://doi.org/10.1016/j.japh.2018.05.006.
Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2018;0:1–2.
Tayabali K, Bolzon C, Foster P, Patel J, Kalim MO. Kratom: a dangerous player in the opioid crisis. J Community Hosp Intern Med Perspect. 2018;8(3):107–10. https://doi.org/10.1080/20009666.2018.1468693.
Osborne CS, Overstreet AN, Rockey DC, Schreiner AD. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. https://doi.org/10.1177/2324709619826167.
Castillo A, Payne JD, Nugent K. Posterior reversible leukoencephalopathy syndrome after kratom ingestion. Proc (Baylor Univ Med Cent). 2017;30(3):355–7.
Nelsen JL, Lapoint J, Hodgman MJ, Aldous KM. Seizure and coma following kratom (Mitragynina speciosa Korth) exposure. J Med Toxicol. 2010;6(4):424–6. https://doi.org/10.1007/s13181-010-0079-5.
Pathak V, Hahn C, Cabellon M, Aris R. Adult respiratory distress syndrome secondary to the use of herbal drug kratom. Am J Respir Crit Care Med. 2014;189:1.
Jaliawala HA, Abdo T, Carlile PV. Kratom; a potential cause of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:6604.
Overbeek DL, Abraham J, Munzer BW. Kratom (Mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24–6. https://doi.org/10.5811/cpcem.2018.11.40588.
Palasamudram Shekar S, Rojas EE, D’Angelo CC, Gillenwater SR, Martinez Galvis NP. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28–30. https://doi.org/10.1080/02791072.2018.1562591.
Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54–9.
Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242–7.
Neerman MF, Frost RE, Deking J. A drug fatality involving kratom. J Forensic Sci. 2013;58:278–9. https://doi.org/10.1111/1556-4029.12009.
McIntyre IM, Trochta A, Stolberg S, Campman SC. Mitragynine ‘kratom’ related fatality: a case report with postmortem concentrations. J Anal Toxicol. 2014;39(2):152–5. https://doi.org/10.1093/jat/bku137.
Karinen R, Fosen JT, Rogde S, Vindenes V. An accidental poisoning with mitragynine. Forensic Sci Int. 2014;245:29–32. https://doi.org/10.1016/j.forsciint.2014.10.025.
Domingo O, Roider G, Stöver A, Graw M, Musshoff F, Sachs H, et al. Mitragynine concentrations in two fatalities. Forensic Sci Int. 2017;271:1–7. https://doi.org/10.1016/j.forsciint.2016.12.020.
Aggarwal G, Robertson E, McKinlay J, Walter E. Death from kratom toxicity and the possible role of intralipid. J Intensive Care Med. 2018;19(1):61–3. https://doi.org/10.1177/1751143717712652.
Hughes RL. Fatal combination of mitragynine and quetiapine–a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019 Mar;15(1):110–3. https://doi.org/10.1007/s12024-018-0049-9.
Gershman K, Timm K, Frank M, Lampi L, Melamed J, Gerona R, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97–8. https://doi.org/10.1056/NEJMc1811055.
Singh D, Murugaiyah V, Hamid SB, Kasinather V, Chan MS, Ho ET, et al. Assessment of gonadotropins and testosterone hormone levels in regular Mitragyna speciosa (Korth.) users. J Ethnopharmacol. 2018;221:30–6. https://doi.org/10.1016/j.jep.2018.04.005.
Acknowledgments
The authors would like to thank Dr. Glee Lenoir for reviewing their manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pharmacology Care
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Alsarraf, E., Myers, J., Culbreth, S. et al. Kratom from Head to Toe—Case Reviews of Adverse Events and Toxicities. Curr Emerg Hosp Med Rep 7, 141–168 (2019). https://doi.org/10.1007/s40138-019-00194-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00194-1