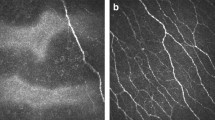
Abstract
Purpose of Review
Confocal microscopy and aethesiometry have allowed clinicians to assess the structural and functional integrity of corneal nerves in health and disease. This review summarizes literature on nerves in dry eye disease (DED) and discusses how this data can be applied to DED diagnosis and treatment.
Recent Findings
Subjects with DED have a heterogeneous symptom and sign profile along with variability in nerve structure and function. Most studies have reported lower nerve density and sensitivity in aqueous tear deficiency, while findings are more inconsistent for other DED subtypes. Examining nerve status, along with profiling symptoms and signs of disease, can help categorize subjects into disease phenotypes (structural and functional patterns) that exist under the umbrella of DED. This, in turn, can guide therapeutic decision-making.
Summary
Due to the heterogeneity in symptoms and signs of DED, corneal nerve evaluations can be valuable for categorizing individuals into disease sub-types and for guiding clinical decision-making.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Erie JC, McLaren JW, Patel SV. Confocal microscopy in ophthalmology. Am J Ophthalmol. 2009;148(5):639–46.
Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–42.
Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37(4):476–88.
Liu YC, Lin MT, Mehta JS. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen Res. 2021;16(4):690–1.
Simsek C, Karalezli A, Dogru M, Kojima T. In vivo confocal microscopy evaluation in dry eye and related diseases. Current Ophthalmology Reports. 2019;7(3):187–95.
Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87(2):225–36.
Petroll WM, Robertson DM. In vivo confocal microscopy of the cornea: new developments in image acquisition, reconstruction, and analysis using the HRT-Rostock Corneal Module. The ocular surface. 2015;13(3):187–203.
Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–85.
Patel DV, McGhee CNJ. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93(7):853–60.
Mantopoulos D, Cruzat A, Hamrah P. In vivo imaging of corneal inflammation: new tools for clinical practice and research. Semin Ophthalmol. 2010;25(5-6):178–85.
Jiao H, Naranjo Golborne C, Dando SJ, McMenamin PG, Downie LE, Chinnery HR. Topographical and morphological differences of corneal dendritic cells during steady state and inflammation. Ocul Immunol Inflamm. 2020;28(6):898–907.
Maruoka S, Inaba M, Ogata N. Activation of dendritic cells in dry eye mouse model. Invest Ophthalmol Vis Sci. 2018;59(8):3269–77.
Schaumburg CS, Siemasko KF, de Paiva CS, Wheeler LA, Niederkorn JY, Pflugfelder SC, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187(7):3653–62.
Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5-6):138–48.
Al-Aqaba MA, et al. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762.
Yang AY, Chow J, Liu J. Corneal innervation and sensation: the eye and beyond. The Yale journal of biology and medicine. 2018;91(1):13–21.
Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32(6):1025–43.
Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet–Bonnet aesthesiometer. Exp Eye Res. 2011;92(5):408–13.
Lum E, Murphy PJ. Effects of ambient humidity on the Cochet–Bonnet aesthesiometer. Eye. 2018;32(10):1644–51.
Chao C, et al., Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optometry and Vision Science, 2015. 92(2).
Stapleton F, Tan ME, Papas EB, Ehrmann K, Golebiowski B, Vega J, et al. Corneal and conjunctival sensitivity to air stimuli. Br J Ophthalmol. 2004;88(12):1547–51.
Erie, JC, et al., The effect of age on the corneal subbasal nerve plexus. Cornea, 2005. 24(6).
Patel DV, et al., Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea, 2009. 28(7).
Niederer RL, Perumal D, Sherwin T, McGhee CNJ. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91(9):1165–9.
Parissi M, Karanis G, Randjelovic S, Germundsson J, Poletti E, Ruggeri A, et al. Standardized baseline human corneal subbasal nerve density for clinical investigations with laser-scanning in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2013;54(10):7091–102.
Murphy PJ, Lawrenson JG, Patel S, Marshall J. Reliability of the non-contact corneal aesthesiometer and its comparison with the Cochet–Bonnet aesthesiometer. Ophthalmic Physiol Opt. 1998;18(6):532–9.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
Ganesalingam K, Ismail S, Sherwin T, Craig JP. Molecular evidence for the role of inflammation in dry eye disease. Clin Exp Optom. 2019;102(5):446–54.
Patel S, et al. Corneal nerve abnormalities in ocular and systemic diseases. Exp Eye Res. 2020:108284.
Kloosterboer A, Dermer HI, Galor A. Diagnostic tests in dry eye. Expert Review of Ophthalmology. 2019;14(4-5):237–46.
Labbé A, Alalwani H, van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53(8):4926–31.
Choi EY, et al., Corneal microstructural changes in non-Sjˆgren dry eye using confocal microscopy: clinical correlation. 2015. 56: 680-686.
Labbé A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, et al. Corneal nerve structure and function in patients with non-Sjögren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54(8):5144–50.
Villani E, et al., In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci, 2013. 90(6).
Zhang M, et al., Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea, 2005. 24(7).
Shetty R, et al. Corneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in evaporative dry eye disease. Biomed Res Int. 2016;2016:4369750.
Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60(7):2532–42.
Parra A, et al. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. PAIN®. 2014;155(8):1481–91.
Rahman EZ, et al. Corneal sensitivity in tear dysfunction and its correlation with clinical parameters and blink rate. Am J Ophthalmol. 2015;160(5):858–866.e5.
Benítez-del-Castillo JM, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173–81.
Bourcier T, Acosta MC, Borderie V, Borra´s F, Gallar J, Bury T, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341–5.
Sade De Paiva C, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137(1):109–15.
Simsek C, Kojima T, Dogru M, Tsubota K. Alterations of murine subbasal corneal nerves after environmental dry eye stress. Invest Ophthalmol Vis Sci. 2018;59(5):1986–95.
Simsek C, Kojima T, Nagata T, Dogru M, Tsubota K. Changes in murine subbasal corneal nerves after scopolamine-induced dry eye stress exposure. Invest Ophthalmol Vis Sci. 2019;60(2):615–23.
Leonard BC, Stewart KA, Shaw GC, Hoehn AL, Stanley AA, Murphy CJ, et al. Comprehensive clinical, diagnostic, and advanced imaging characterization of the ocular surface in spontaneous aqueous deficient dry eye disease in dogs. Cornea. 2019;38(12):1568–75.
Schiffman RM, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21.
Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60.
•• Farhangi M, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain. 2019;160(7):1541–50 This study is very important because it validated a neuropathic pain questionnaire that can be used in individuals with ocular pain, the NPSI-Eye.
•• Qazi, Y., et al., Validity and reliability of a Novel Ocular Pain Assessment Survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology, 2016. 123(7): p. 1458-1468. This study is very important because it validated a pain questionnaire that can be used in individuals with ocular pain
Tepelus TC, Chiu GB, Huang J, Huang P, Sadda SVR, Irvine J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1771–8.
Denoyer A, Landman E, Trinh L, Faure JF, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122(4):669–76.
Spierer O, Felix ER, McClellan AL, Parel JM, Gonzalez A, Feuer WJ, et al. Corneal mechanical thresholds negatively associate with dry eye and ocular pain symptoms. Invest Ophthalmol Vis Sci. 2016;57(2):617–25.
Liang Q, et al. Ocular surface epithelial thickness evaluation in dry eye patients: clinical correlations. J Ophthalmol. 2016;2016:1628469.
Galor A, et al., Corneal nerve pathway function in individuals with dry eye symptoms. Ophthalmology, 2020.
Tanaka A, et al. In vivo confocal microscopy in patients with dry eye disease demonstrates decreased peripheral corneal nerve density and correlation to clinical signs. Invest Ophthalmol Vis Sci. 2017;58(8):3753.
Bron AJ, Yokoi N, Gaffney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. The Ocular Surface. 2009;7(2):78–92.
Villani E, Bonsignore F, Cantalamessa E, Serafino M, Nucci P. Imaging biomarkers for dry eye disease. Eye Contact Lens. 2020;46(Suppl 2):S141–s145.
IASP Terminology. 2020; Available from: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698.
Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle∗. The Ocular Surface. 2012;10(1):2–14.
Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, et al. Neuropathic pain and dry eye. The ocular surface. 2018;16(1):31–44.
Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clinical ophthalmology (Auckland, NZ). 2014;8:571–9.
Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye. 2006;20(7):837–9.
Kalangara JP, Galor A, Levitt RC, Covington DB, McManus KT, Sarantopoulos CD, et al. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye Contact Lens. 2017;43(3):192–8.
Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31–44.
• Galor, A., et al., Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain, 2016. 17(3): p. 310-318. This study is important because it evaluated dry eye symptoms and signs in individuals chronic pain
• Farhangi M, et al. Individuals with migraine have a different dry eye symptom profile than individuals without migraine. Br J Ophthalmol. 2020;104(2):260–4 This study is important because it evaluated dry eye symptoms and signs in individuals with and without migraine.
Crane AM, Feuer W, Felix ER, Levitt RC, McClellan AL, Sarantopoulos KD, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017;101(9):1238–43.
Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–34.
• Dermer H, et al., Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. British Journal of Ophthalmology, 2021: p. bjophthalmol-2020-317891. This study is important because it did not find a relationship between a specific anatomic findings (termed microneuroma) and ocular pain.
Rózsa AJ, Guss RB, Beuerman RW. Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci. 1983;24(8):1033–51.
•• Moein H-R, et al. Visualization of microneuromas by using in vivo confocal microscopy: an objective biomarker for the diagnosis of neuropathic corneal pain? The Ocular Surface. 2020;18(4):651–6 This study is very important because it presented an anatomic nerve finding (termed microneuroma) as a potential marker for corneal neuropathic pain.
Chinnery HR, et al., Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: a systematic review. British Journal of Ophthalmology, 2021: p. bjophthalmol-2020-318156.
• Stepp MA, et al., Corneal epithelial “neuromas”: a case of mistaken identity? Cornea, 2020. 39(7). This study is important because it provided an alternative explanation to the anatomic finding termed microneuroma.
Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137–45.
Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9.
Matsumoto Y, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–20.
Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94(5):584–91.
Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106(8):1504–11.
• Bonini S, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–43 This study is important because it evaluated the efficacy of recombinant NGF in NK.
• Pflugfelder SC, et al. Topical recombinant human nerve growth factor (Cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14–26 This study is important because it evaluated the efficacy of recombinant NGF in NK.
Kim JS, Rafailov L, Leyngold IM, Corneal neurotization for postherpetic neurotrophic keratopathy: initial experience and clinical outcomes. Ophthalmic Plastic & Reconstructive Surgery, 9000. Publish Ahead of Print
Terzis JK, Dryer MM, Bodner BI, Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg, 2009. 123(1).
Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. The Ocular Surface. 2015;13(3):250–62.
Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. The ocular surface. 2018;16(1):132–8.
Bates BD, Mitchell K, Keller JM, Chan CC, Swaim WD, Yaskovich R, et al. Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. PAIN. 2010;149(3):522–8.
Small LR, et al., Oral gabapentinoids and nerve blocks for the treatment of chronic ocular pain. Eye & Contact Lens, 2020. 46(3).
Ozmen MC, et al. Nortriptyline is effective in ameliorating symptoms of neuropathic corneal pain. Invest Ophthalmol Vis Sci. 2019;60(9):4732.
Scholz A, Kuboyama N, Hempelmann G, Vogel W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. J Neurophysiol. 1998;79(4):1746–54.
Diel RJ, Kroeger ZA, Levitt RC, Sarantopoulos C, Sered H, Martinez-Barrizonte J, et al. Botulinum toxin A for the treatment of photophobia and dry eye. Ophthalmology. 2018;125(1):139–40.
Johnson M. Transcutaneous electrical nerve stimulation: mechanisms, clinical application and evidence. Reviews in pain. 2007;1(1):7–11.
Diel RJ, Hwang J, Kroeger ZA, Levitt RC, Sarantopoulos CD, Sered H, et al. Photophobia and sensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. Br J Ophthalmol. 2019;103(8):1024–9.
Sivanesan E, Levitt RC, Sarantopoulos CD, Patin D, Galor A. Noninvasive electrical stimulation for the treatment of chronic ocular pain and photophobia. Neuromodulation: Technology at the Neural Interface. 2018;21(8):727–34.
Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. 2016;61(4):466–77.
Wilkins AJ, Patel R, Adjamian P, Evans BJW. Tinted spectacles and visually sensitive migraine. Cephalalgia. 2002;22(9):711–9.
Patel S, et al., Dysfunctional coping mechanisms contribute to dry eye symptoms. J Clin Med, 2019. 8(6).
Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet. 2010;375(9718):916–23.
Otis JD, Sanderson K, Hardway C, Pincus M, Tun C, Soumekh S. A randomized controlled pilot study of a cognitive-behavioral therapy approach for painful diabetic peripheral neuropathy. J Pain. 2013;14(5):475–82.
Mehra D, Cohen NK, Galor A, Ocular surface pain: a narrative review. Ophthalmology and Therapy, 2020.
Acknowledgements
This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cornea
Rights and permissions
About this article
Cite this article
Patel, S., Mehra, D., Cabrera, K. et al. How Should Corneal Nerves Be Incorporated Into the Diagnosis and Management of Dry Eye?. Curr Ophthalmol Rep 9, 65–76 (2021). https://doi.org/10.1007/s40135-021-00268-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-021-00268-y