Abstract
Purpose of Review
This review article aims to provide an overview of testicular multiparametric ultrasound features in emergency setting, to bring the reader up to date with new technologies and to provide insights into the ultrasound diagnosis of scrotal pathologies.
Recent Findings
Multiparametric ultrasound is increasingly recognized as a fundamental problem-solving technique in testicular diseases. Contrast-enhanced ultrasonography and elastography represent recent technologies that may be advantageous over conventional ultrasonography especially when diagnosis is controversial.
Summary
A wide range of acute diseases, including torsion, infection, ischaemia, and trauma, may affect the testis. These conditions represent frequently surgical emergencies, thus understanding their imaging appearance is imperative to help guide adequate management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiparametric ultrasonography (US) includes in this definition conventional B-Mode US, Colour Doppler ultrasonography (CDUS), contrast-enhanced ultrasonography (CEUS), and elastography. It represents the imaging modality of choice to evaluate testicular diseases, thanks to its highly diagnostic accuracy, cost-effectiveness, large availability, safety, and lack of ionizing radiation [1,2,3]. A wide range of disorders, including infection, ischaemia, and trauma, may affect the testis. These conditions represent frequently surgical emergencies, thus timely recognition on imaging is crucial for a prompt treatment, maintaining fertility, hormonal activity, and erectile function. In this review, we focus on the imaging appearance of testicular emergencies with special emphasis on the role of multiparametric US, starting from the scrotal anatomy and scanning techniques.
Anatomy
The testes are paired oval-shaped organs with smooth surface and homogeneous texture that measure 35–50 mm in length, 25–35 mm in width, and 15–25 mm in height [4]. Each testicle is located within a hemiscrotum and is enveloped by a fibrous connective tissue called tunica albuginea. It gives rise to septa that extend deep into the testicle and divide it into lobules. The tunica albuginea as well is surrounded by the cavity of the tunica vaginalis, a two-layered membrane consisting of an inner visceral layer and an outer parietal layer, which contains a small amount of serous fluid. The epididymis is situated on the upper and posterior–lateral aspect of the testis and is divided into three segments: the head, body, and tail. The epididymal head is a round homogeneous structure (thickened 5–12 mm), the body extends down the posterior aspect of the testicle (thickness 2–4 mm), and tail curve at the inferior pole, becoming ductus deferens (2–5 mm) [5]. Four testicular appendages, remnants of embryonic ducts, are noted: the appendix testis, the appendix epididymis, the vas aberrans, and the paradidymis. The appendix testis and the appendix epididymis can be visualized on ultrasound, the first is attached to the upper pole of the testis in the groove between the testicle and the epididymis, whilst the second is connected to the head of the epididymis. The testicular arteries are branches of the abdominal aorta, which provide the primary vascular supply to the testes. They penetrate the tunica albuginea towards its inferior pole and give rise to capsular artery. Their branches pass through the septations of the testicular parenchyma and then flow centripetally into the testicular mediastinum. Arteries that emerge from the mediastinum are called recurrent or centrifugal arteries. The testes and epididymis are supplied by a network of veins known as the pampiniform plexus, which come together in the inguinal canal to form the testicular veins. The right testicular vein empties into the inferior vena cava and the left testicular vein drains into the left renal vein.
Techniques
B-Mode US and CDUS
Scrotal US requires the patient to be in the supine position and the 7.5–12-MHz linear high-frequency transducer to be used to examine the testes. Both transverse and sagittal planes should be examined, with images of both testes for size comparison and observation of echogenicity. Examination should begin on the non-symptomatic side to optimize parameters for grey-scale and colour Doppler imaging. The testicular parenchyma has normally a homogeneous medium echotexture [6] (Fig. 1). The tunica albuginea, which appears as a thin hyperechoic line around the testes, can be distinguished on ultrasound images. There is usually a small amount of fluid between the two membranes of the tunica vaginalis which appears as a thin anechoic area. To assess the epididymis, it is preferable to perform a longitudinal scan. In general, the head of the epididymis is comparable in echogenicity to the testicle, whereas the body and tail are generally hypoechoic compared to the testicular parenchyma [7] (Fig. 2). Hydrocele is the main cause in which the appendages of the testis and epididymis can be observed. These structures usually have an oval shape and are isoechoic with the testis; in some cases, they may have cystic characteristics.
Colour Doppler ultrasound is an essential tool for visualizing and assessing intratesticular blood flow, especially in cases of possible vascular emergencies [8]. A transverse colour Doppler scan of both testicles is essential to determine the presence and uniformity of intratesticular flow. It is important to use appropriate colour Doppler settings to accurately depict slow flow [9]. High-frequency transducers, low-pulse repetition frequency, and high gain with minimal filter are some of the recommended settings [10]. In adults, both centripetal and centrifugal arteries appear as short vessels or coloured dots (Fig. 3). It is more difficult to visualize flow in paediatric patients due to the physiological hypovascularization of their testes. Power Doppler imaging is particularly advantageous when used in paediatric patients as it is more sensitive to low flow conditions and does not require any adjustments due to the angle of the probe [11].
CEUS
Contrast-enhanced ultrasound is executed with a linear array transducer and a low mechanical index (MI between 0.05 and 0.08) [12]. The US contrast agent consists of very small (less than 10 microns in diameter) microbubbles with gas and organic shells, such as sulphur hexafluoride, encapsulated in phospholipid shells. This solution is injected intravenously in a dose range of 2.4 to 4.8 mL. Following the injection, static and multi-frame cine clips are acquired until the microbubbles dissipate. The testis and epididymis can be seen to enhance rapidly on imaging, with the arteries enhancing first, followed by a thorough and progressive filling of testicular parenchyma within a few seconds [13•]. This enhancement process typically subsides within two to three minutes. CEUS has been shown to be useful in the diagnosis of testicular disease. Microbubbles follow the parenchymal microcirculation and are able to detect intraparenchymal abnormalities within the testis, mainly in the diagnosis of testicular lesions [14, 15] and acute scrotum [16].
Elastography
Elastography is an advanced type of ultrasound technology used to measure tissue stiffness. It can be divided into two branches: strain and shear wave elastography. Strain elastography (SE) is used to measure the degree of deformation in a specific target tissue relative to the surrounding tissue by repeated manual compression of the transducer. This is represented by a colour pattern [17] (Fig. 4). Shear wave elastography (SWE) uses an acoustic radiation force pulse to create a longitudinal strain and the speed at which the shear waves propagate is calculated to accurately quantify tissue stiffness [18••]. Elastography is increasingly being used to diagnose testicular disease as it has the potential to provide solutions in situations where conventional ultrasound provides inconclusive results [19]. The visual elasticity score (VES), a scoring system adapted from breast elastography, is used to measure the stiffness of a testicular abnormality. This system scores elasticity on either a 5-point or a 6-point scale. In order to differentiate testicular lesions according to their stiffness, a cut-off point of 3 VES can be set. Anything above three is considered ‘stiff’, whereas anything at or below this threshold would be termed ‘soft’ [20].
Testicular Torsion
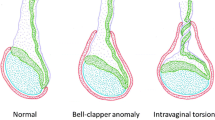
Testicular torsion is a twisting of the spermatic cord and its contents leading to impairment of testicular blood flow. It occurs annually in one per 4,000 males younger than 25 years [21]. There is an age distribution of this condition: neonates and adolescents going through puberty are the group more affected [22]. It represents the most frequent cause of acute scrotum (86%) in adolescents [23]. Extravaginal torsion predominates in neonates, whereas in older children and adults, testicular torsion is usually found within the tunica vaginalis [24]. The so-called ‘bell clapper deformity’ is the most common cause of testicular torsion [25]. In this condition, the tunica vaginalis covers also the spermatic cord allowing the testis to rotate freely within the tunica vaginalis. There are several other reasons that can lead to a higher risk of testicular torsion, such as undescended testicles, which occur when a testicle is not in its normal position, an increase in testicular size during puberty or previous trauma or twisting of the spermatic cord [26]. Testicular torsion can take many forms, from partial to complete. The process starts with an increase in venous pressure, which is then balanced by an equalization of arterial pressure. Unfortunately, this compromises arterial flow and leads to testicular ischaemia. Clinically testicular torsion occurs with a severe unilateral testicular pain associated with nausea and vomiting [27, 28]. The scrotal sac containing the affected testis may appear higher due to twisting of the spermatic cord and larger due to venous congestion. However, the clearest sign of this condition is the absence of the cremasteric reflex [29, 30]. In cases of suspected torsion, surgical exploration is recommended as the best course of action. Ultrasound should be used if a definitive diagnosis cannot be made [31]. Ultrasound is an effective diagnostic tool for testicular torsion, with a sensitivity of 84–99% and a specificity of 93–99% [32]. In addition, the ‘whirlpool or pseudomass sign’, which presents as an enlarged twisted spermatic cord, can be seen on scans (Fig. 5a) [33], making it an easily identifiable indicator. It may extend down to epididymis which itself can also become swollen and difficult to differentiate from the adjacent cord [34, 35]. Therefore, sonographic evaluation of the spermatic cord, including transverse and longitudinal images of cord twisting, is essential. Grey-scale ultrasound findings in testicular torsion are variable. Initially, testicular torsion may have a regular grey-scale appearance. However, over time the testicle may enlarge and show hypoechogenicity due to oedema and swelling. If left untreated, a twisted testis can become more complicated after 24 h. The affected area may appear with increased echogenicity, usually due to congestion, lack of oxygen, and bleeding. Doppler US can be used to measure the intrascrotal blood flow. One of the telltale signs of testicular torsion on ultrasound is a decrease or complete absence of blood flow compared to an uninjured testis [31, 36]. If blood flow is absent on Doppler US, immediate surgical exploration is indicated [22]. However, false-negative results may be seen due to intermittent torsion or early torsion where only venous outflow is obstructed [25]. CEUS has become a useful tool in the diagnosis of testicular torsion. It provides a definitive answer, confirming the lack of perfusion, in scenarios where conventional ultrasound and CDUS are inconclusive (Fig. 5b). It is extremely useful in detecting vascularity, especially in paediatric testes, which are often small and have low flow [37]. In addition, real-time strain elastography can be used in conjunction with CDUS to examine testicular tissue in torsion setting [38, 39]. Clinical and animal studies have shown a significant increase in SWE values within the testicular parenchyma during testicular torsion [40, 41]. When ultrasound results are equivocal as in the case of partial testicular torsion, elastography images and strain ratios could be useful in determining testicular viability and help surgeons make more informed decisions regarding the appropriate surgical technique [38].
20-year-old patient with high-degree right testicular torsion investigated with multiparametric US after the onset of acute scrotal pain. B-MODE ultrasound image (a) shows the pseudomass (arrowed) above the epididymis. The right testicle has normal echogenicity and echotexture. CEUS image (b) confirms the complete lack of vascularity of the testis
Scrotal Trauma
Scrotal trauma accounts for less than 1% of all trauma-related injuries, peaking in the 10–30-year age group [42]. Blunt trauma is the most common type of scrotal injury and typically affects only one side of the scrotum [43]. It represents a common occurrence in male athletes and sports activities are the most common cause, accounting for over 50% of all cases. Motor vehicle collisions are also common causes of scrotal injury [44, 45]. The right testicle is more likely to be injured in this way because it is anatomically slightly higher than the left in most men [46]. Physical examination can usually detect scrotal swelling and pain, but imaging studies are essential for appropriate clinical management that may save the affected testicle. Grey-scale and CDUS are considered the most important techniques in the diagnosis of testicular trauma. CEUS and elastography represent the advanced techniques in situations where the results of the initial evaluation are inconclusive [47••]. Trauma-induced post-traumatic hydrocele, haematocele, intra- and extra-testicular haematomas, testicular fractures, and ruptures are all possible outcomes. Most people with scrotal injury develop a hydrocele—a collection of anechoic fluid between the two layers of the tunica vaginalis. This is usually small and goes away on its own. A haematocele, on the other hand, is caused by a build-up of blood inside the tunica vaginalis. Ultrasound shows a complex heterogeneous fluid collection with low echogenicity, fluid levels, and septations (Fig. 6). A distinctive feature of a haematocele is the presence of internal echoes—this can be seen on ultrasound and can help to distinguish it from a simple hydrocele. It is known to be associated with echogenic changes in the testicular parenchyma and may indicate testicular contusion. Scrotal trauma can frequently cause haematomas in the testis; these are seen either alone or in association with other scrotal injuries. The appearance of haematomas in the US can vary depending on when they are assessed after the injury. Initially, they may have a similar appearance to the testis and be difficult to identify [36], so a repeat US may help to identify it and rule out further complications. Chronic haematomas appear more hypoechoic and tend to shrink as they heal. Ultrasound imaging is an invaluable tool in distinguishing between haematomas and tumours, as they differ in their vascularity. Acute and chronic haematomas do not show internal colour flow on CDUS, whereas tumours do. A testicular fracture appears as a hypoechoic line cutting through the testicular tissue and represents a break in the normal testicular architecture. This abnormality is usually treated conservatively if regular flow can be seen on CDUS. Thus, the main objective of the US examination is to evaluate vascular perfusion and testicular integrity, distinguishing testicular rupture from other lesions to determine whether a patient requires surgical intervention [47••]. Testicular rupture is usually identified by an irregular testicular morphology and discontinuity of the echogenic line of the tunica albuginea, often protruding due to extrusion of the testicular parenchyma (Fig. 7a) [48, 49]. Additional US findings include heterogeneous echotexture which may indicate intratesticular haematoma, fracture lines, absence or reduction of blood flow on CDUS, scrotal thickening, and haematocele formation. CDUS is essential in the assessment of post-traumatic scrotums because there is almost always a rupture of the tunica albuginea along with the capsular arteries. This results in a lack of vascularity in the underlying parenchyma (Fig. 7b). However, CDUS may be less effective in detecting acute conditions as it may not be able to detect low flow, especially in paediatric patients [50]. CEUS represents a reliable technique for difficult-to-diagnose cases where conventional US is inconclusive. It effectively highlights the difference between vital and non-vital tissues, fracture lines, and intra- and extra-testicular haematoma [50,51,52] (Fig. 7c). In addition, CEUS has a significant advantage over other imaging modalities in determining which lesions are avascular versus poorly vascularised. This allows clinicians to differentiate between testicular haematoma and tumours, which can sometimes be identified at the time of trauma [47••]. Finally, SE can help ensure accuracy in diagnostic tasks by providing an easier way to detect and differentiate tumours (‘hard’ lesions) from haematomas which present with lower strain ratio values [53].
36-year-old man after a blunt trauma. B-Mode US image shows the presence of a heterogeneous fluid collection with low-level echogenicity and septations consistent for a haematocele of moderate size. A heterogeneous appearance of testicular parenchyma attributable to an intratesticular contusion is associated
17-year-old patient with testicular rupture after a blunt trauma to the groin. B-Mode US image (a) shows the interruption of the tunica albuginea with protrusion of echogenic material (arrowed). CDUS image (b) shows that the testicle has lost its oval morphology with an avascular area at the breaking point. CEUS image (c) conclusively delineates the fracture line and confirms the lack of enhancement in the traumatized, non-vascularized section of testicular parenchyma
Inflammatory Diseases
Acute scrotal pain is mainly caused by epididymitis and epididymo-orchitis in adolescent and adult males. Retrograde bacterial infections can cause epididymo-orchitis, particularly in adolescents and young men, with Chlamydia trachomatis and Neisseria gonorrhoeae responsible for the majority of cases secondary to sexual transmission. In older people, Escherichia coli and Proteus mirabilis are the most common causes [54]. Rare causes include sarcoidosis, brucellosis, and tuberculosis [55, 56]. Considering the retrograde spread, the epididymal tail usually is the first site of infection and then it progresses to the body and head. Approximately, 20–40% of cases also develop orchitis due to direct spread. Clinical diagnosis can be challenging because epididymo-orchitis arises with non-specific findings, including scrotal swelling, redness, and pain, may not be clear-cut signs. B-mode imaging typically shows a larger volume of either the epididymis alone or the epididymis combined with the testis. The structure may be hypoechoic due to oedema or hyperechoic, possibly due to haemorrhage. In addition to direct signs of inflammation, the presence of a reactive hydrocele associated with scrotal wall thickening has been noted [7] (Fig. 8). The acutely inflamed regions are characteristically hypervascular, thus in CDUS studies, an increased blood flow to the epididymis and testis is considered a key indicator of epididymo-orchitis. This criterion has a high sensitivity of almost 100% [57] (Fig. 9). Inflammation can lead to various complications, such as venous compression, hypovascularity, or parenchymal ischaemia, increasing the risk of abscess formation. When post-inflammatory ischaemia develops, the testicle becomes hypovascular, despite clinical signs of inflammation (Fig. 10a). The use of CEUS in this clinical setting can facilitate the accurate diagnosis of abscesses and venous infarction [58, 59] (Fig. 10b). An abscess is usually surrounded by a rim of increased enhancement with a lack of internal brightness. In some cases, intracavitary gas bubbles may be seen. Epididymo-orchitis appears firm on elastography, whereas abscesses tend to be softer (Fig. 10c). In addition, a developing abscess may be stiffer on the outside with inhomogeneous firmness in the core, representing small areas of liquefaction [61].
41-year-old patient with acute epididymo-orchitis presenting with right testicular pain and swelling. CDUS image (a) shows no flow in the context of ischaemic lesion with perilesional hypervascularization. CEUS image (b) confirms the lack of vascularization of ischaemic lesion with a hypervascularization of the testicle. At strain elastography (c), the texture of the lesion is inhomogeneous mainly soft due to necrosis haemorrhage
Testicular Infarction
Testicular infarction is a rare condition that causes acute scrotal pain [62]. It is usually caused by several conditions, including trauma, acute epididymo-orchitis, haematological conditions, and sometimes idiopathic/iatrogenic factors. Segmental infarction may not be readily apparent on ultrasound in the early stages, but later tends to appear as a wedge-shaped area of reduced echogenicity in the testicular parenchyma [63, 64]. Higher echogenic spot consistent with haemorrhage may be seen inside the affected area. In addition, at CDUS segmental testicular infarction is hypovascular or avascular [64, 65]. Conversely the adjacent undamaged testicular parenchyma shows normal blood flow. The absence of Doppler signal in the infarct zone allows differentiation from tumours [66]. The differential diagnosis between a testicular infarction with a hypovascular tumour may be challenging in small lesions and when blood flow is not completely absent. The use of CEUS can make it easier to diagnose segmental testicular infarction and confirm the absence of flow from the lesion [58,59,60,61,62,63,64,65,66,67]. In addition, CEUS has the ability to detect a characteristic perilesional rim enhancement, which is an indicator of segmental testicular infarction [68]. On elastography, segmental testicular infarction is usually seen as firm in the outer regions and soft in the centre, due to the presence of oedema and bleeding [69].
Conclusion
Ultrasonography is well established as the mainstay imaging technique for scrotal acute pathology. It is essential for accurate diagnosis and for guiding appropriate management. CEUS is gaining increasing attention as a helpful problem-solving tool when the US diagnosis is unclear and remains inconclusive. Elastography is becoming a key component of multiparametric ultrasound examinations, providing a more accurate way of assessing tissue properties, thereby increasing the operator’s confidence in the diagnosis.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Avery LL, Scheinfeld MH. Imaging of penile and scrotal emergencies. Radiographics. 2013;33(3):721–40. https://doi.org/10.1148/rg.333125158.
Kühn AL, Scortegagna E, Nowitzki KM, Kim YH. Ultrasonography of the scrotum in adults. Ultrasonography. 2016;35(3):180–97. https://doi.org/10.14366/usg.15075.
Crawford P, Crop JA. Evaluation of scrotal masses. Am Fam Physician. 2014;89(9):723–7.
Ragheb D, Higgins JL Jr. Ultrasonography of the scrotum: technique, anatomy, and pathologic entities. J Ultrasound Med. 2002;21(2):171–85.
Gorman B. The scrotum. In: Rumack CM, editor. Diagnostic ultrasound. 4th ed. Philadelphia: Elsevier; 2011. p. 840–77.
Langer JE. Ultrasound of the scrotum. Semin Roentgenol. 1993;28(1):5–18. https://doi.org/10.1016/s0037-198x(05)80109-9.
Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227(1):18–36. https://doi.org/10.1148/radiol.2271001744.
Siegel MJ. The acute scrotum. Radiol Clin North Am. 1997;35(4):959–76.
Dudea SM, Ciurea A, Chiorean A, Botar-Jid C. Doppler applications in testicular and scrotal disease. Med Ultrason. 2010;12(1):43–51.
Alkhori NA, Barth RA. Pediatric scrotal ultrasound: review and update. PediatrRadiol. 2017;47(9):1125–33. https://doi.org/10.1007/s00247-017-3923-9.
Hamper UM, DeJong MR, Caskey CI, Sheth S. Power doppler imaging: clinical experience and correlation with color Doppler US and other imaging modalities. Radiographics. 1997;17(2):499–513. https://doi.org/10.1148/radiographics.17.2.9084086.
Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). UltraschallMed. 2018;39(2):e2–44.
•Tenuta M, Sesti F, Bonaventura I, Mazzotta P, Pofi R, Gianfrilli D, Pozza C. Use of contrast enhanced ultrasound in testicular diseases: a comprehensive review. Andrology. 2021;9(5):1369–1382. doi: https://doi.org/10.1111/andr.13057. Important reference which allows to understand the role of contrast enhanced ultrasound in scrotal diseases, providing a more accurate diagnosis in testicular lesions and acute scrotum
Isidori AM, Pozza C, Gianfrilli D, et al. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology. 2014;273(2):606–18. https://doi.org/10.1148/radiol.14132718.
Luzurier A, Maxwell F, Correas JM, et al. Qualitative and quantitative contrast-enhanced ultrasonography for the characterisation of non-palpable testicular tumours. Clin Radiol. 2018;73(3):322.e1-322.e9. https://doi.org/10.1016/j.crad.2017.10.007.
Lobianco R, Regine R, De Siero M, Catalano O, Caiazzo C, Ragozzino A. Contrast-enhanced sonography in blunt scrotal trauma(). J Ultrasound. 2011;14(4):188–95. https://doi.org/10.1016/j.jus.2011.09.003.
Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303–29. https://doi.org/10.7150/thno.
••Huang DY, Pesapane F, Rafailidis V, Deganello A, Sellars ME, Sidhu PS. The role of multiparametric ultrasound in the diagnosis of paediatric scrotal pathology. Br J Radiol. 2020;93(1110):20200063. https://doi.org/10.1259/bjr.20200063. Very important reference which explain the role of multiparametric ultrasound in the diagnosis of scrotal pathology, showing how it could increase operator diagnostic confidence with the potential to influence the diagnostic and therapeutic decisions
Bertolotto M, Muça M, Currò F, Bucci S, Rocher L, Cova MA. Multiparametric US for scrotal diseases. Abdom Radiol (NY). 2018;43(4):899–917. https://doi.org/10.1007/s00261-018-1510-7.
Konstantatou E, Fang C, Romanos O, et al. Evaluation of intratesticular lesions with strain elastography using strain ratio and color map visual grading: differentiation of neoplastic and nonneoplastic lesions. J Ultrasound Med. 2019;38(1):223–32. https://doi.org/10.1002/jum.14686.
Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989;142(3):746–8. https://doi.org/10.1016/s0022-5347(17)38875-4.
Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835–40.
Aso C, Enríquez G, Fité M, Torán N, Piró C, Piqueras J, Lucaya J. Gray-scale and color Doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197–214. https://doi.org/10.1148/rg.255045109.
Witherington R, Jarrell TS. Torsion of the spermatic cord in adults. J Urol. 1990;143(1):62–3. https://doi.org/10.1016/s0022-5347(17)39866-x.
Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74(10):1739–43.
Palmer LS, Palmer JS. 2016 Management of abnormalities of the external genitalia in boys. Campbell-Walsh urology. 11th ed.Philadelphia: Elsevier. Amsterdam. p. 3384–97
Davis JE, Silverman M. Scrotal emergencies. Emerg Med Clin North Am. 2011;29(3):469–84. https://doi.org/10.1016/j.emc.2011.04.011.
Boettcher M, Bergholz R, Krebs TF, Wenke K, Aronson DC. Clinical predictors of testicular torsion in children. Urology. 2012;79(3):670–4. https://doi.org/10.1016/j.urology.2011.10.041.
Srinivasan A, Cinman N, Feber KM, Gitlin J, Palmer LS. History and physical examination findings predictive of testicular torsion: an attempt to promote clinical diagnosis by house staff. J Pediatr Urol. 2011;7(4):470–4. https://doi.org/10.1016/j.jpurol.2010.12.010.
Beni-Israel T, Goldman M, Bar Chaim S, Kozer E. Clinical predictors for testicular torsion as seen in the pediatric ED. Am J Emerg Med. 2010;28(7):786–9. https://doi.org/10.1016/j.ajem.2009.03.025.
Yagil Y, Naroditsky I, Milhem J, Leiba R, Leiderman M, Badaan S, Gaitini D. Role of doppler ultrasonography in the triage of acute scrotum in the emergency department. J Ultrasound Med. 2010;29(1):11–21. https://doi.org/10.7863/jum.2010.29.1.11.
Ota K, Fukui K, Oba K, Shimoda A, Oka M, Ota K, Sakaue M, Takasu A. The role of ultrasound imaging in adult patients with testicular torsion: a systematic review and meta-analysis. J Med Ultrason. 2019;46(3):325–34. https://doi.org/10.1007/s10396-019-00937-3.
Vijayaraghavan SB. Sonographic differential diagnosis of acute scrotum: real-time whirlpool sign, a key sign of torsion. J Ultrasound Med. 2006;25(5):563–74. https://doi.org/10.7863/jum.2006.25.5.563.
Munden MM, Williams JL, Zhang W, Crowe JE, Munden RF, Cisek LJ. Intermittent testicular torsion in the pediatric patient: sonographic indicators of a difficult diagnosis. AJR Am J Roentgenol. 2013;201(4):912–8. https://doi.org/10.2214/AJR.12.9448.
Rebik K, Wagner JM, Middleton W. Scrotal ultrasound. Radiol Clin North Am. 2019;57(3):635–48. https://doi.org/10.1016/j.rcl.2019.01.007.
Bhatt S, Dogra VS. Role of US in testicular and scrotal trauma. Radiographics. 2008;28(6):1617–29. https://doi.org/10.1148/rg.286085507.
Kitami M. Ultrasonography of pediatric urogenital emergencies: review of classic and new techniques. Ultrasonography. 2017;36(3):222–38. https://doi.org/10.14366/usg.17011.
Herek D, Herek O, Akbulut M, Ufuk F. Role of strain elastography in the evaluation of testicular torsion: an experimental study. J UltrasoundMed. 2016;35(10):2149–58. https://doi.org/10.7863/ultra.15.11038.
Laimer G, Müller R, Radmayr C, Lindner AK, Lebovici A, Aigner F. Multiparametric ultrasound in torsion of the testicular appendages: a reliable diagnostic tool? MedUltrason. 2022;24(1):33–7. https://doi.org/10.11152/mu-3206.
Sun Z, Xie M, Xiang F, Song Y, Yu C, Zhang Y, et al. Utility of real-time shear wave elastography in the assessment of testicular torsion. PLoS One. 2015;10(9):e0138523. https://doi.org/10.1371/journal.pone.0138523.
Zhang X, Lv F, Tang J. Shear wave elastography (SWE) is reliable method for testicular spermatogenesis evaluation after torsion. Int J Clin Exp Med. 2015;8(5):7089–97.
Deurdulian C, Mittelstaedt CA, Chong WK. Fielding JR. US of acute scrotal trauma: optimal technique, imaging findings, and management. Radiographics. 2007;27(2):357–69. https://doi.org/10.1148/rg.272065117.
Kitrey ND, Djakovic N, Hallscheidt P et al (2020) EAU guidelines on urological trauma. Edn. Presented at the EAU Annual Congress, Amsterdam, the Netherlands
Haas CA, Brown SL, Spirnak JP. Penile fracture and testicular rupture. World J Urol. 1999;17(2):101–6. https://doi.org/10.1007/s003450050114.
Munter DW, Faleski EJ. Blunt scrotal trauma: emergency department evaluation and management. Am J Emerg Med. 1989;7(2):227–34. https://doi.org/10.1016/0735-6757(89)90143-5.
Mulhall JP, Gabram SG, Jacobs LM. Emergency management of blunt testicular trauma. Acad Emerg Med. 1995;2(7):639–43. https://doi.org/10.1111/j.1553-2712.1995.tb03604.x.
••Ramanathan S, Bertolotto M, Freeman S, et al. Imaging in scrotal trauma: a European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) position statement. EurRadiol. 2021;31(7):4918–28. https://doi.org/10.1007/s00330-020-07631-w. Very important reference which contains the position statements of the Scrotal and Penile Imaging Working Group of the European Society of Urogenital Radiology with the aim of providing guidance for the use of imaging especially multiparametric US in scrotal trauma
Bhatt S, Ghazale H, Dogra VS. Sonographic evaluation of scrotal and penile trauma. Ultrasound Clin. 2007;2:45–56.
Pepe P, Panella P, Pennisi M, Aragona F. Does color Doppler sonography improve the clinical assessment of patients with acute scrotum? Eur J Radiol. 2006;60(1):120–4. https://doi.org/10.1016/j.ejrad.2006.04.016.
Yusuf G, Konstantatou E, Sellars ME, Huang DY, Sidhu PS. Multiparametric sonography of testicular hematomas: features on grayscale, color doppler, and contrast-enhanced sonography and strain elastography. J Ultrasound Med. 2015;34(7):1319–28. https://doi.org/10.7863/ultra.34.7.1319.
Trinci M, Cirimele V, Ferrari R, Ianniello S, Galluzzo M, Miele V. Diagnostic value of contrast-enhanced ultrasound (CEUS) and comparison with color Doppler ultrasound and magnetic resonance in a case of scrotal trauma. J Ultrasound. 2020;23(2):189–94. https://doi.org/10.1007/s40477-019-00389-y.
Hedayati V, Sellars ME, Sharma DM, Sidhu PS. Contrast-enhanced ultrasound in testicular trauma: role in directing exploration, debridement and organ salvage. Br J Radiol. 2012;85(1011):e65–8. https://doi.org/10.1259/bjr/95600238.
Adlan T, Freeman SJ. Can ultrasound help to manage patients with scrotal trauma? Ultrasound. 2014;22(4):205–12. https://doi.org/10.1177/1742271X14545911.
Horstman WG, Middleton WD, Melson GL. Scrotal inflammatory disease: color doppler US findings. Radiology. 1991;179(1):55–9. https://doi.org/10.1148/radiology.179.1.2006304.
Toyoshima M, Chida K, Masuda M, Eguchi T, Imokawa S, Nakamura Y, Suda T, Nakamura H. Testicular sarcoidosis. Nihon Kokyuki Gakkai Zasshi. 2000;38(1):63–6.
Bayram MM, Kervancioğlu R. Scrotal gray-scale and color Doppler sonographic findings in genitourinary brucellosis. J Clin Ultrasound. 1997;25(8):443–7. https://doi.org/10.1002/(sici)1097-0096(199710)25:8%3c443::aid-jcu6%3e3.0.co;2-j.
Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815–21. https://doi.org/10.1148/radiology.175.3.2188301.
Lung PF, Jaffer OS, Sellars ME, Sriprasad S, Kooiman GG, Sidhu PS. Contrast-enhanced ultrasound in the evaluation of focal testicular complications secondary to epididymitis. AJR Am J Roentgenol. 2012;199(3):W345-54. https://doi.org/10.2214/AJR.11.7997.
Bertolotto M, Cantisani V, Valentino M, Pavlica P, Derchi LE. Pitfalls in imaging for acute scrotal pathology. Semin Roentgenol. 2016;51(1):60–9. https://doi.org/10.1053/j.ro.2016.02.012.
Drudi FM, Valentino M, Di Leo N, Malpassini F, Cantisani V, Gnecchi M, Iori F. Color-/power doppler ultrasound imaging and ultrasound contrast media in acute scrotum - 2. Ultraschall Med. 2013;34(1):72–84. https://doi.org/10.1055/s-0032-1325563.
Huang DY, Sidhu PS. Focal testicular lesions: colour doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br J Radiol. 2012;85(1):S41-53. https://doi.org/10.1259/bjr/30029741.
Sriprasad S, Kooiman GG, Muir GH, Sidhu PS. Acute segmental testicular infarction: differentiation from tumour using high frequency colour Doppler ultrasound. Br J Radiol. 2001;74(886):965–7. https://doi.org/10.1259/bjr.74.886.740965.
Doebler RW, Norbut AM. Localized testicular infarction masquerading as a testicular neoplasm. Urology. 1999;54(2):366. https://doi.org/10.1016/s0090-4295(99)00067-9.
Bilagi P, Sriprasad S, Clarke JL, Sellars ME, Muir GH, Sidhu PS. Clinical and ultrasound features of segmental testicular infarction: six-year experience from a single centre. Eur Radiol. 2007;17(11):2810–8. https://doi.org/10.1007/s00330-007-0674-2.
Eisner DJ, Goldman SM, Petronis J, Millmond SH. Bilateral testicular infarction caused by epididymitis. AJR Am J Roentgenol. 1991;157(3):517–9. https://doi.org/10.2214/ajr.157.3.1872237.
Fernández-Pérez GC, Tardáguila FM, Velasco M, Rivas C, Dos Santos J, Cambronero J, Trinidad C, San MP. Radiologic findings of segmental testicular infarction. AJR Am J Roentgenol. 2005;184(5):1587–93. https://doi.org/10.2214/ajr.184.5.01841587.
Lorenz N, Schuster F, Steinbach F, Heubner G. Segmental testicular infarction after methamphetamine abuse in a 16-year-old—diagnosis by using contrast-enhanced ultrasound (CEUS). Ultraschall Med. 2019;40(2):253–4. https://doi.org/10.1055/a-0810-0636.
Bertolotto M, Derchi LE, Sidhu PS, Serafini G, Valentino M, Grenier N, Cova MA. Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. AJR Am J Roentgenol. 2011;196(4):834–41. https://doi.org/10.2214/AJR.10.4821.
Patel KV, Huang DY, Sidhu PS. Metachronous bilateral segmental testicular infarction: multi-parametric ultrasound imaging with grey-scale ultrasound, doppler ultrasound, contrast-enhanced ultrasound (CEUS) and real-time tissue elastography (RTE). J Ultrasound. 2014;17(3):233–8. https://doi.org/10.1007/s40477-014-0098-1.
Acknowledgements
We thank Dr. Mariano Scaglione and Dr. Luca Saba for reviewing the manuscript.
Funding
Open access funding provided by Università di Foggia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article’s conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or competing interests to disclose.
Human or Animal Rights Consent
This article does not contain any studies with human or animal subject performed by any of the authors.
All the figures used are original.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paparella, M.T., Eusebi, L., Pagliara, M.P. et al. Multiparametric Ultrasound in Testicular Emergencies: State-of-the-Art. Curr Radiol Rep 11, 109–119 (2023). https://doi.org/10.1007/s40134-023-00415-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40134-023-00415-2