Abstract
Purpose of Review
To review the MRI appearance of physiologic lactational changes, common benign pathologies, and malignancies in the lactating breast.
Recent Findings
The prevalence of pregnancy-associated breast cancer has increased as more women delay childbirth and lactation. There is a transient increase in breast cancer risk after delivery when women may be lactating. MRI is more sensitive than mammography and ultrasound for the evaluation of the extent of disease in lactating women.
Summary
Understanding the key MRI findings of benign and malignant pathologies in the lactating breast is critical for accurate diagnosis and prompt evaluation of pregnancy-associated breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hormonal influences during lactation lead to physiologic breast changes and benign pathologic lesions [1•]. These changes can pose potential diagnostic challenges in the lactating breast. As a result, pregnancy-associated breast cancer (PABC) may be under-diagnosed in this population. Accurate diagnosis and evaluation of PABC are critical as it is often associated with an aggressive biologic profile and poor prognosis [2•, 3•]. While gadolinium is avoided during pregnancy, contrast-enhanced MRI can be safely performed during lactation. MRI remains highly sensitive for the diagnosis and locoregional staging of breast cancer in the lactating breast. Familiarity with the characteristic MRI features of benign and malignant pathologies in the lactating breast can assist breast imagers in the diagnosis and evaluation of PABC.
Physiological Lactational Changes
Lactogenesis describes the transition of the breast from a proliferative state to a secretory state in preparation for milk production. Breast architecture and composition undergo significant changes under the influence of varying hormones. Starting in the first trimester of pregnancy, increased circulating serum estrogen and progesterone induce new ductal formation. Estrogen promotes the involution of stromal adipose tissue and increased glandular vascularity.
Progesterone also induces lobular hyperplasia and provides secretory capability to alveolar cells for milk synthesis. By the second and third trimesters, increased level of prolactin results in synthesis of colostrum and milk in the alveolar cells, but milk release continues to be inhibited by the high levels of estrogen and progesterone. After delivery, there is a precipitous drop in estrogen and progesterone. The loss of inhibitory effect on prolactin allows for copious milk production and release. Physical stimulation during breastfeeding results in continued release of oxytocin and prolactin, which promote maintenance of milk production. These hormonal changes are accompanied by increased breast size, firmness, and nodularity in the lactating breast, and will persist until approximately three months after cessation of breastfeeding.
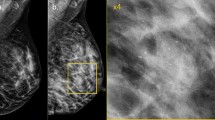
Breast imagers should be familiar with the MRI appearance of benign lactational changes to avoid misinterpretation as suspicious findings in the lactating breast. Increased fibroglandular tissue induced by hormonal changes present as increased breast density on MRI. Background parenchymal enhancement will typically be diffusely increased with fast initial phase kinetic curve because of increased vascularity and hormonal effects on the breast tissue (Fig. 1) [14•]. Milk production leads to diffusely increased T2 signal in the breasts and dilated ducts. On T1-weighted sequences, breast tissue may appear more hypointense due to increased fluid content with hyperintense signal within ducts corresponding to fatty and proteinaceous contents in breast milk. On post-contrast images, benign lactational changes may demonstrate fast initial and plateau delayed enhancement kinetics compared to the slow and persistent enhancement curve typically seen in normal breast tissue [15]. While benign lactational changes are usually diffuse and bilateral, occasionally these findings are asymmetric based on lactation pattern and may be potentially misleading (Fig. 2).
34-year-old woman with BRCA 1 mutation with screening MR performed at baseline and during lactation. STIR (Short Tau Inversion Recovery) and T1 post-contrast subtraction images performed during lactation demonstrate increased background parenchymal enhancement, breast density, and parenchymal T2 signal from benign lactation changes
MR Evaluation
Hormonal fluctuations throughout the menstrual cycle are linked with breast parenchymal and perfusion changes [4, 5]. In lactating women who are menstruating, performing breast MRI during the follicular phase of the menstrual cycle (days 3–14) has been previously suggested to help reduce false positive findings related to hormonal fluctuations [6]. However, the recommendation for performing breast MRI during the follicular phase is gradually evolving to any time during the menstrual cycle. Recent studies have found that timing breast MRI did not reliably decrease background parenchymal enhancement or improve diagnostic performance [7, 8∙].
Lactating women should nurse or pump immediately before imaging to reduce potentially confounding background T2 signal [9]. The American College of Radiology (ACR) and the American College of Obstetrics and Gynecology (ACOG) recommend lactating women to continue breastfeeding after receiving intravenous gadolinium-based contrast agents [10, 11]. Women are not required to discontinue breastfeeding as only 0.04% of the maternal gadolinium dose is excreted into breast milk [12]. The amount absorbed by the infant would be less than 1% of the permitted dose for neonates [12]. If lactating women remain concerned about the potential effects of gadolinium exposure to the infant, they can consider expressing and discarding breast milk for 24 h after gadolinium administration then resuming lactation [13].
Galactocele
Galactoceles are the most common benign lesion in lactating or recently lactating women [16]. They are milk-filled retention cysts that usually form from lactiferous duct occlusion leading to milk retention, ductal dilation, and cyst formation. Galactoceles often present as painless, palpable masses occurring over weeks to months in women with recent abrupt cessation of breastfeeding or decreased frequency of nursing [17]. They have a predilection for occurring in the sub-areolar region and can be multiple and bilateral [18]. The majority of galactoceles will spontaneously resolve. Superinfection is relatively common due to the rich nutrient content of stagnant breast milk contained within galactoceles. If galactoceles are symptomatic or superinfected, aspiration can be both diagnostic and therapeutic [19].
The MRI appearance of galactoceles are round or oval circumscribed, cystic masses with pathognomonic fat-fluid levels (Fig. 3). The fat-fluid level represents the separation of fat and water content in breast milk. The less dense fatty content rises and appears closer to the chest wall in prone-positioned patients. Rim-enhancement may be seen with secondary infection of galactoceles with abscess formation or inflammatory reaction due to cyst rupture in the appropriate clinical setting. However, the presence of enhancement should raise suspicion for possible malignancy as necrotic masses can mimic galactoceles in lactating women (Fig. 4). A persistent or enlarging mass with enhancement and lack of the classic fat-fluid level is worrisome and warrants further evaluation. Galactoceles with predominantly fatty contents will have T1 hyperintense appearance with or without a fat-fluid level and corresponding appearance of a pseudolipoma on mammogram. In cases where the high viscosity of old milk does not allow the physical separation of fat and fluid, the characteristic fat-fluid level may not be appreciated, and the lesion may have the appearance of a pseudohamartoma on mammogram [16].
40-year-old breastfeeding woman presenting with a palpable lump in the right breast. MRI demonstrates an oval, circumscribed mass (arrow) with fat-fluid level in the right breast consistent with galactocele. On T1 non-fat-saturated and T2 fat-saturated images, the fatty content (arrowhead) within the galactocele rises toward the chest wall in the prone patient. Post-contrast images demonstrate no associated enhancement (arrow)
33-year-old breastfeeding woman with palpable abnormality in the right breast. A Targeted ultrasound demonstrated a round, well-circumscribed, avascular mixed cystic and solid mass. Findings were initially presumed to represent a galactocele; however, persistence and growth of mass prompted biopsy which demonstrated triple-negative invasive ductal carcinoma. B MRI demonstrates a round, well-circumscribed cystic and solid mass without fat-fluid level. On T1-weighted images, there is no intrinsically T1 hyperintense material within the cystic component (arrow). On T2-weighted images, the lesion demonstrates a large cystic component that is hyperintense (arrow). On T1 post-contrast subtraction images, the solid and cystic mass demonstrates rim enhancement. Necrotic mass can mimic a galactocele in a lactating patient, however enhancement and absence of classic fluid-fluid level is suspicious and warrants further evaluation
Fibroadenoma
Fibroadenomas are a common benign breast lesion that arises from the epithelium and stroma of the terminal duct lobular unit [20•]. They typically present as palpable, mobile, and painless masses. Fibroadenomas are hormone-sensitive and may demonstrate secretory hyperplasia or lactational changes during pregnancy and lactation [21]. As a result, they can demonstrate rapid growth and present as new or enlarging masses in the peripartum or postpartum period. In some cases, rapid growth of fibroadenomas can lead to infarction due to outpacing of the vascular supply. Unlike typical fibroadenomas, infarcted fibroadenomas can present as tender, nonmobile masses [22]. When evaluating an enlarging fibroadenoma, the growth rate may be helpful in identifying when additional tissue sampling is necessary. In nonpregnant women, fibroadenomas diagnosed by fine-needle aspiration and demonstrating growth of less than 20% in all three dimensions within a 6-month period can be continued to be followed on imaging and may not warrant excisional biopsy [23]. This criterion could also be applied to pre-existing fibroadenomas that demonstrate growth during pregnancy or lactation in the absence of suspicious features.
On MRI, fibroadenomas typically appear as oval masses with circumscribed margins. On T1-weighted imaging, fibroadenomas are isointense to the surrounding breast parenchyma. On T2-weighted imaging, fibroadenomas are usually hyperintense, however the signal can vary with the lesion content [24]. Non-enhancing hypointense internal septa are seen in more than 95% of fibroadenomas, however the absence of internal septa does not exclude the diagnosis of a fibroadenoma [22]. They can demonstrate secretory hyperplasia and lactational changes with internal accumulation of milk, mimicking galactoceles [25]. On dynamic post-contrast imaging, progressive enhancement is often seen. In cases of hemorrhagic infarct, fibroadenomas may appear intrinsically T1 hyperintense [26]. Central necrosis is the most common pattern with heterogenous T2 hyperintensity and irregular margins [21]. On post-contrast images, infarcted fibroadenomas can demonstrate rim enhancement in areas of viable tissue.
Lactating Adenoma
Lactating adenomas are benign adenomatous breast lesions associated with hormonal surges [16]. They occur most commonly in the third trimester or postpartum during lactation but have also been reported in the first and second trimesters [28]. There are several theories regarding the histogenesis of lactating adenomas. Some propose they develop from pre-existing fibroadenoma, tubular adenoma, or lobular hyperplasia under the influence of physiologic hormonal changes [29]. Others suggest that lactating adenomas arise de novo due to increased estrogen stimulation during pregnancy and lactation [14•, 30]. Histologically, lactating adenomas differ from fibroadenomas as they consist predominantly of epithelial elements with very minimal stromal component and lack the marked myoepithelial proliferation seen in fibroadenomas [31]. However, pathologic differentiation between lactating adenoma and fibroadenoma with lactational changes can be difficult [16]. Both can demonstrate foci of infarcts, especially in the setting of rapid growth.
Lactating adenomas have similar presentation as fibroadenomas, often presenting as painless, palpable, and mobile lesions [16, 22]. Those demonstrating rapid growth and pain may be associated with infarction. Lactating adenomas usually spontaneously regress after cessation of breastfeeding, and may recur in subsequent pregnancies. Bromocriptine can help decrease the size of the lesion by decreasing lactational changes. If lactating adenomas persist and cause discomfort, surgical excision may be considered after lactational changes have abated. While lactating adenomas are generally considered to be benign, there are rare cases reporting the coexistence of lactating adenomas and invasive carcinomas [29, 32,33,34]. Therefore, close followup to confirm complete resolution to exclude co-existent malignancy is suggested, even though the chance is remote.
When lactating adenoma is suspected based on presentation and clinical history, but sonographic and histological evaluation are inconclusive, MRI may be helpful in further characterization. Lactating adenomas often present as oval, circumscribed mass with intermediate T2 hyperintensity (Fig. 5). On T1-weighted imaging, they may demonstrate intermediate or hyperintense signal due to presence of milk fat or proteinaceous content. On post-contrast imaging, heterogenous internal enhancement may be present [35]. In larger lesions, internal nonenhancing, low signal bands may be visualized that correspond to fibrous septa [36, 37].
36-year-old breastfeeding woman with history of biopsy-proven lactating adenoma in the left breast and presenting for screening MR. MR demonstrates an oval, circumscribed mass in the left breast. The mass demonstrates intermediate hyperintensity on T2-weighted imaging (arrow) and intrinsic hyperintensity on T1-weighted imaging (arrow). No associated enhancement on T1 post-contrast subtraction images (arrow)
Hamartoma
Hamartomas are benign lesions that consist of proliferation of fibrous, glandular, and fatty tissue surrounded by collagen pseudoencapsulation [31]. They can be readily diagnosed on mammogram by their characteristic “breast within a breast” appearance, a circumscribed mass of mixed fatty and soft tissue elements and thin pseudocapsule [38]. Diagnosis may be more challenging when there are atypical features associated with infarction [39]. Hamartomas are frequently asymptomatic, but can present as palpable masses. They are generally slow-growing but are hormonally sensitive with the potential to rapidly increase in size during pregnancy and lactation.
On MRI, hamartomas typically appear as oval or round masses with circumscribed margins and a hypointense rim on T1 and T2-weighted sequences that correspond to the pseudocapsule (Fig. 6). They demonstrate heterogenous signal intensities on T1- and T2-weighted sequences corresponding to varying proportions of fatty components interspersed with nodular fibroglandular elements [40,41,42,43]. The fatty elements appear hyperintense on T1 with loss of signal on fat-saturated sequences. The fibroglandular elements can undergo lactational changes demonstrating increased T2 hyperintensity and enhancement. On dynamic contrast-enhanced images, hamartomas demonstrate slow progressive enhancement [35,36,37,38]. On DWI, hamartomas have ADC values similar to that of normal breast tissue [44]. On MR spectroscopy, no significance difference has been reported between hamartomas and normal breast tissue [38].
40-year-old breastfeeding woman presenting with an enlarging palpable lump in the right breast and MR findings compatible with a hamartoma. MR demonstrates an oval, circumscribed mass with central T1 hyperintensity (arrow) that suppresses on T2 fat-saturated images (arrow). The mass demonstrates a T1 and T2 hypointense rim (arrowhead). Areas within the mass demonstrate hyperintensity on T2-weighted imaging with corresponding enhancement (arrow) on T1 post-contrast subtraction images consistent with internal lactation changes
Pregnancy-Associated Breast Cancer
Pregnancy-associated breast cancer (PABC) is defined as breast malignancy occurring during pregnancy, within 12 months postpartum, or during lactation [39]. Incidence of PABC is increasing as more women are delaying childbearing to their forties [45, 46•, 47, 48]. While pregnancy lowers the lifetime risk of breast cancer, there is a transient increase in breast cancer risk after delivery, when women may be lactating. The transient increase peaks at 6 years and persists until approximately 10 years post-partum [49]. PABC is associated with worse prognosis than breast cancer in women who are not pregnant or lactating [3, 50•, 51∙]. There are several factors which may contribute to the poor outcome associated with PABC. Breast engorgement and increase breast tissue density can limit clinical and imaging evaluation of breast cancer and result in delayed diagnosis and treatment [16]. Studies have also suggested that PABC has more aggressive tumor biology as it is associated with younger age at diagnosis, BRCA positivity, higher pathologic stage, higher histologic grade, and triple-negative receptor status [2, 3]. Pregnant or lactating women with breast cancer are more likely to present with advanced disease and axillary nodal metastasis compared to age-adjusted nonpregnant women [52, 53]. Even after adjusting for age at diagnosis, extent of disease, and diagnostic periods, women with breast cancers diagnosed during lactation have an increased risk of cause-specific death [54].
Given the rising incidence and aggressive nature of PABC, accurate diagnosis and evaluation of the extent of disease is of growing importance. The MR appearance of PABC is similar to that of breast cancer in nonpregnant women. PABC typically demonstrate hypointensity on T2-weighted imaging and fast initial contrast enhancement with washout on dynamic post-contrast imaging (Figs. 7 and 8) [27, 55•]. Given the overrepresentation of triple-negative breast cancers, PABC may be more likely to demonstrate areas of necrosis [15]. There is concern that marked background parenchymal enhancement of lactating tissue may decrease lesion conspicuity and limit the sensitivity of MRI [56•]. However, several studies on dynamic contrast-enhanced MRI have found that background parenchymal enhancement of lactating tissue resulted in minimal to no decrease in lesion conspicuity as malignant lesions and the background parenchyma can be differentiated by enhancement kinetics and morphology [14•, 27, 57, 58•, 59]. Supplemental subtraction images that subtract the latest post-contrast dynamic series from an early post-contrast dynamic series have been reported to assist in differentiating tumor enhancement from glandular parenchymal enhancement particularly in women with marked background parenchymal enhancement. These supplemental subtraction images improve visualization of areas of wash-out for detection of malignant lesions and reduce plateau enhancement typical of lactational changes [60•]. Diffusion-weighted imaging has also been reported to have additive value in the detection of breast cancer in lactating patients [56, 61•]. In particular, diffusion tensor imaging demonstrated 138% increased tumor conspicuity compared to conventional dynamic contrast-enhanced MRI and may be a potential adjunctive modality [56•]. If needed, weaning may help decrease the background enhancement in glandular parenchyma to aid in visualization of small enhancing masses and non-mass enhancement [58•].
40-year-old breastfeeding woman with biopsy-proven adenocarcinoma in the left breast. MR demonstrates a large irregular mass with spiculated margins. On T2-weighted imaging, the mass appears hypointense (arrow) relative to hyperintense background of lactational changes. Post-contrast T1 subtracted images demonstrate fast, early enhancement with washout kinetics (arrow)
42-year-old breastfeeding woman with biopsy-proven invasive lobular carcinoma in the left breast. MR demonstrates an irregular mass with spiculated margins. On T2-weighted imaging, the mass appears hypointense (arrow) relative to hyperintense background of lactational changes. Post-contrast T1 subtracted images demonstrate fast, early enhancement with washout kinetics (arrow)
For pregnant and lactating women with newly diagnosed breast cancer, post-partum contrast-enhanced MRI is recommended for locoregional staging. In a small series of women with PABC, 45% of whom were lactating, MRI identified greater extent of disease than identified on mammography and ultrasound in 23% of women [57]. In addition, MRI changed surgical management in 28% of post-partum patients with PABC. Similarly in another small series of nine lactating women with breast cancer, MRI identified additional lesions in three patients and defined tumor extent more accurately than mammography or ultrasound [62•]. This study also reported limited evidence that MRI may also be helpful in evaluation of response after neoadjuvant chemotherapy [62•].
There are scarce data on MRI screening in high-risk women who are lactating. In a series of seven lactating women including five with invasive carcinomas, all cancers were visible on MRI despite the presence of physiologic lactational changes [27]. Given the limited amount of supporting evidence, screening MRI may be considered by lactating women and referring clinicians. It may be reasonable to perform screening breast MRI in high-risk, lactating women who plan to breastfeed for more than six months. For those who plan to breastfeed for less than 6 months, it may be reasonable to wait until 6–8 weeks after cessation of breastfeeding before resuming MRI screening [63].
Procedural Considerations
Suspicious breast masses can be safely sampled via percutaneous biopsy in lactating women, however breast imagers should inform patients of certain procedural risks. Increased vascularity in the lactating breast increases the risk of biopsy-associated bleeding and infection [16]. Breast imagers can mitigate these risks by paying close attention to post-procedural hemostasis and aseptic technique. Core needle biopsy in the third trimester can rarely cause milk fistula between the dilated high-pressure duct and the biopsy tract, resulting in chronic milk drainage [64]. This risk can be lowered using higher-gauge core biopsy needles or, alternatively, fine-needle aspiration for initial sampling.
When performing fine-needle aspiration in pregnancy or lactating women, breast imagers should be cognizant of the higher incidence of false positive and false negative diagnoses in this patient population [65,66,67,68]. Differentiating lactational changes and malignancy can be a diagnostic challenge. The hormonal milieu of pregnancy and lactation result in epithelial hyperplastic changes and atypical nuclear findings including nuclear enlargement, hyperchromia, and increased mitotic figures that can be misinterpreted as malignant (Fig. 9) [69]. Conversely, breast malignancies with round nuclei and central prominent nucleoli may be misinterpreted as benign lactational changes, resulting in false negative diagnoses. Given the potential overlapping features of benign and malignant pathologies, it is critical that breast imagers provide clinical history of pregnancy or lactation for accurate cytologic interpretation. In addition, maintaining a high index of suspicion for malignancy and awareness of the potential pitfalls of cytologic evaluation in lactating women are key to attaining the correct diagnosis. When atypical cellular findings are identified, core biopsy and possibly MRI may be helpful next steps for evaluation.
Comparison of histologic appearance of lactational change and invasive ductal carcinoma on Papanicolaou (Pap) stain and May Grunwald-Giemsa (MGG) stain. A Lactational change viewed with Pap stain at ×60. Ductal epithelium shows homogeneously enlarged nuclei with prominent nucleoli and intermixed myoepithelial cells (red arrow). Foamy macrophage in the background (brown arrow). B Invasive ductal carcinoma viewed with Pap stain at ×60. Loosely cohesive invasive ductal carcinoma cells with enlarged pleomorphic nuclei, markedly irregular nuclear contours, inconspicuous nucleoli, and reduced cytoplasm (green arrow). Strands of mucin in the background (orange arrow). C Lactational change viewed with MGG stain at ×60. Ductal epithelium with intracytoplasmic granules consistent with mitochondria (blue arrow) and bubbly background of proteinaceous fluid from milk (black arrow). D Invasive ductal carcinoma viewed with MGG stain at ×60. Enlarged, pleomorphic nuclei (teal arrow) in a bloody background (magenta arrow)
Conclusion
Physiologic lactational changes have a distinct appearance on MRI. Hormonal influences during lactation can also lead to new or enlarging benign pathologic lesions including galactoceles, lactating adenomas, hamartomas, and fibroadenomas. It is important to differentiate these benign entities from pregnancy-associated breast cancer to avoid necessary tissue sampling as well as potential delays in diagnosis. Complementary to mammography and ultrasound, MRI has high sensitivity for the detection of breast cancer in lactating women. MRI is recommended for the evaluation of the extent of disease in lactating women with breast cancer and may be considered for supplemental screening in lactating women with high breast cancer risk.
References
• Of importance
Pillay J, Davis TJ. Physiology, Lactation. StatPearls [Internet]. StatPearls Publishing; 2021 [cited 2021 Dec 8]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK499981/
• Gooch JC, Chun J, Kaplowitz E, Guth A, Axelrod D, Shapiro R, et al. Pregnancy-associated breast cancer in a contemporary cohort of newly diagnosed women. Breast J. 2020;26:668–71.
• Madaras L, Kovács KA, Szász AM, Kenessey I, Tokés AM, Székely B, et al. Clinicopathological features and prognosis of pregnancy associated breast cancer—a matched case control study. Pathol Oncol Res. 2014;20:581–90.
Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–44.
Müller-Schimpfle M, Ohmenhäuser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145–9.
Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J. 2005;11:236–41.
• Dontchos BN, Rahbar H, Partridge SC, Lehman CD, DeMartini WB. Influence of menstrual cycle timing on screening breast MRI background parenchymal enhancement and diagnostic performance in premenopausal women. J Breast Imaging. 2019;1:205–11.
• Lee CH, Bryce Y, Zheng J, Sung JS, Comstock CE, Moskowitz C, et al. Outcome of screening MRI in premenopausal women as a function of the week of the menstrual cycle. AJR Am J Roentgenol. 2020;214:1175–81.
Kieturakis AJ, Wahab RA, Vijapura C, Mahoney MC. Current recommendations for breast imaging of the pregnant and lactating patient. Am Roentgen Ray Soc. 2020;216:1462–75.
Patient Safety in Obstetrics and Gynecology |ACOG [Internet]. [cited 2021 Nov 30]. Available from: https://www.acog.org/clinical/clinical-guidance/committeeopinion/articles/2009/12/patient-safety-in-obstetrics-and-gynecology
ACR Manual On Contrast Media. 2021; American College of Radiology [Internet], https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf.
Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T, Seifert B, Puchert E, Wittek S, et al. Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology. 2000;216:555–8.
• diFlorio-Alexander RM, Slanetz PJ, Moy L, Baron P, Didwania AD, Heller SL, et al. ACR Appropriateness Criteria® breast imaging of pregnant and lactating women. J Am Coll Radiol. 2018;15:S263–75.
• Talele AC, Slanetz PJ, Edmister WB, Yeh ED, Kopans DB. The lactating breast: MRI findings and literature review. Breast J. 2003;9:237–40.
Joshi S, Dialani V, Marotti J, Mehta TS, Slanetz PJ. Breast disease in the pregnant and lactating patient: radiological-pathological correlation. Insights Imaging. 2013;4:527–38.
Sabate JM, Clotet M, Torrubia S, Gomez A, Guerrero R, de Las HP, et al. Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics. 2007. https://doi.org/10.3390/brainsci8010003.
Stavros AThomas. Breast ultrasound. Rapp CL, Parker SH, editors. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2004.
Teberian I, Bhimani C, Sciotto M, Wilkes A, Germaine P. Breast masses in pregnancy and lactation. J Am Osteopath Coll Radiol. 2019;8(8):5–16.
Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast, 5e. 2014.
• Abramson L, Massaro L, Alberty-Oller JJ, Melsaether A. Breast imaging during pregnancy and lactation. J Breast Imaging. 2019;1:342–51.
Canny PF, Berkowitz GS, Kelsey J, Livolsi VA. Fibroadenoma and the use of exogenous hormones. A case-control study. Am J Epidemiol. 1988;127:454–61.
Vashi R, Hooley R, Butler R, Geisel J, Philpotts L. Breast imaging of the pregnant and lactating patient: physiologic changes and common benign entities. Am Roentgen Ray Soc. 2013;200:329–36.
Gordon PB, Gagnon FA, Lanzkowsky L. Solid breast masses diagnosed as fibroadenoma at fine-needle aspiration biopsy: acceptable rates of growth at long-term follow-up1. Radiol Soc N Am. 2003;229:233–8.
Basara Akin I, Balci P. Fibroadenomas: a multidisciplinary review of the variants. Clin Imaging. 2021;71:83–100.
de Rosas CHS, de Góes ACA, Saltão LM, da Tanaka AMS, Marques EF, Bitencourt AGV. Pregnancy-lactation cycle: how to use imaging methods for breast evaluation. Radiol Bras. 2020;53:405.
Fukui S, Yamaguchi K, Nakazono T, Egashira R, Nakamura J, Masuda M, et al. Breast MRI findings of fibroadenoma with hemorrhagic infarction. 2018 [cited 2021 Dec 6]; Available from: http://orcid.org/0000-0003-0807-4787
• Espinosa LA, Daniel BL, Vidarsson L, Zakhour M, Ikeda DM, Herfkens RJ. The lactating breast: contrast-enhanced MR imaging of normal tissue and cancer. Radiology. 2005;237:429–36.
Magno S, Terribile D, Franceschini G, Fabbri C, Chiesa F, di Leone A, et al. Early onset lactating adenoma and the role of breast MRI: a case report. J Med Case Rep. 2009;3:1–5.
Breast adenomas - Hertel - 1976 - Cancer - Wiley Online Library [Internet]. [cited 2021 Dec 5]. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/https://doi.org/10.1002/10970142(197606)37:6%3C2891::AID-CNCR2820370647%3E3.0.CO;2-P
James K, Bridger J, Anthony PP. Breast tumour of pregnancy (“lactating” adenoma). J Pathol. 1988;156:37–44.
Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
Saglam A, Can B. Coexistence of lactating adenoma and invasive ductal adenocarcinoma of the breast in a pregnant woman. J Clin Pathol. 2005;58:87.
Geschickter CF. Pregnancy and lactation changes in fibro-adenoma of breast. Br Med J. 1938;1:499.
Kumar H, Narasimha A, Bhaskaran, Divyarani (2015) Concurrent lactating adenoma and infiltrating ductal carcinoma: a case report. J Clin Diagn Res; 9:14–15
Hara Y, Yano H, Yamaguchi R, Iwasaki K. Surgical excision of a lactating adenoma with rapid enlargement: a case report. Int J Surg Case Rep. 2021;89:106544.
Parnes AN, Akalin A, Quinlan RM, Vijayaraghavan GR, History M, Adenoma L. BREAST IMAGING AIRP best cases in radiologic-pathologic correlation. Radiographics. 2013;33:455–9.
Chico MJ, Causa Andrieu PI, Wernicke A, Pesce K. Breast lactating adenoma, an example of the utility of the radiological-pathological correlation. Clin Imaging. 2021;71:136–40.
Helvie MA, Adler DD, Rebner M, Oberman HA. Breast hamartomas: variable mammographic appearance. Radiology. 1989;170:417–21.
Langer A, Mohallem M, Berment H, Ferreira F, Gog A, Khalifa D, et al. Breast lumps in pregnant women. Diagn Interv Imaging. 2015;96:1077–87.
Mohamed A. Breast hamartoma: unusual radiological presentation. Radiol Case Rep. 2020;15:2714.
Nam SY, Han B-K, Lee S, Lee K. MR findings of hamartoma of the breast: a report of two cases. J Korean Soc Magn Reson Med. 2012;16:271–5.
Cucci E, Santoro A, di Gesú C, Ciuffreda M, Maselli G, Pierro A, et al. Integrated imaging of breast hamartoma: two case reports. Breast Dis. 2015;35:53–7.
Kievit HCE, Sikkenk AC, Thelissen GRP, Merchants TE. Magnetic Resonance Image Appearance of Hamartoma of the Breast. Magnetic Resonance Imaging; 11(2): 1993;293–8.
Erdem G, Karakaş HM, Işik B, Firat AK. Advanced MRI findings in patients with breast hamartomas. Diagn Interv Radiol. 2011;17:33–7.
Petrek JA. Breast cancer during pregnancy. Cancer. 1994;74:518–27.
• Stensheim H, Møller B, van Dijk T, Fosså SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45–51.
Andersson TML, Johansson ALV, Hsieh CC, Cnattingius S, Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114:568–72.
Cancer Statistics Review, 1975–2016 - SEER Statistics. [cited 2019 Dec 21]. Available from: https://seer.cancer.gov/csr/1975_2016/
Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–75.
• Myers KS, Green LA, Lebron L, Morris EA. Imaging appearance and clinical impact of preoperative breast MRI in pregnancy-associated breast cancer. American Roentgen Ray Society. 2017;209:W177–83.
• Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat. 2016;160:347–60.
Reed W, Hannisdal E, Skovlund E, Thoresen S, Lilleng P, Nesland JM. Pregnancy and breast cancer: a population-based study. Virchows Arch. 2003;443:44–50.
Barnes DM, Newman LA. Pregnancy-associated breast cancer: a literature review. Surg Clin North Am. 2007;87:417–30.
Stensheim H, Møller B, van Dijk T, Fosså SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45–51.
• Arasu VA, Kannan N, Krishnarao PM, Kuehner G, Kuan MC, Kim JC, et al. Imaging the breast in pregnant or lactating women. Curr Radiol Rep. 2018;6:1–10.
• Nissan N, Allweis T, Menes T, Brodsky A, Paluch-Shimon S, Haas I, et al. Breast MRI during lactation: effects on tumor conspicuity using dynamic contrast-enhanced (DCE) in comparison with diffusion tensor imaging (DTI) parametric maps. Eur Radiol. 2020;30:767–77.
Myers KS, Green LA, Lebron L, Morris EA. Imaging appearance and clinical impact of preoperative breast MRI in pregnancy-associated breast cancer. AJR Am J Roentgenol. 2017;209:W177–83.
• Taylor D, Lazberger J, Ives A, Wylie E, Saunders C. Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol. 2011;55:33–42.
Boivin G, de Korvin B, Marion J, Duvauferrier R. Is a breast MRI possible and indicated in case of suspicion of breast cancer during lactation? Diagn Interv Imaging. 2012;93(11):823–7.
• Taron J, Fleischer S, Preibsch H, Nikolaou K, Gruber I, Bahrs S. Background parenchymal enhancement in pregnancy-associated breast cancer: a hindrance to diagnosis? Eur Radiol. 2019;29:1187–93.
• Sah RG, Agarwal K, Sharma U, Parshad R, Seenu V, Jagannathan NR. Characterization of malignant breast tissue of breast cancer patients and the normal breast tissue of healthy lactating women volunteers using diffusion MRI and in vivo 1H MR spectroscopy. J Magn Reson Imaging. 2015;41:169–74.
• Oh SW, Lim HS, Moon SM, Kim JW, Shin SS, Heo SH, Lee JS, Park MH. MR imaging characteristics of breast cancer diagnosed during lactation. Br J Radiol. 2017;90(1078):20170203.
Carmichael H, Matsen C, Freer P, Kohlmann W, Stein M, Buys SS, Colonna S. Breast cancer screening of pregnant and breastfeeding women with BRCA mutations. Breast Cancer Res Treat. 2017;162(2):225–30.
Schackmuth EM, Harlow CL, Norton LW. Milk fistula: a complication after core breast biopsy. American Public Health Association. 2013;161:961–2.
Gupta RK, McHutchison AGR, Dowle CS, Simpson JS. Fine-needle aspiration cytodiagnosis of breast masses in pregnant and lactating women and its impact on management. Diagnostic cytopathology [Internet]. Diagn Cytopathol; 1993;9:156–9
Grenko RT, Lee KP, Lee KR. Fine needle aspiration cytology of lactating adenoma of the breast. A comparative light microscopic and morphometric study. Acta Cytol. 34:21–6.
Novotny DB, Maygarden SJ, Shermer RW, Frable WJ. Fine needle aspiration of benign and malignant breast masses associated with pregnancy. Acta Cytol. 1991;35:676–86.
Mitre BK, Kanbour AI, Mauser N. Fine needle aspiration biopsy of breast carcinoma in pregnancy and lactation. Acta Cytol. 1997;41:1121–30.
Shabb NS, Boulos FI, Abdul-Karim FW. Indeterminate and erroneous fine-needle aspirates of breast with focus on the ‘True Gray Zone’’: a review’. Acta Cytol. 2013;57:316–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Breast Imaging.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, M., Ruiz-Cordero, R., Lee, A.Y. et al. MRI Evaluation of the Lactating Breast. Curr Radiol Rep 10, 57–67 (2022). https://doi.org/10.1007/s40134-022-00395-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40134-022-00395-9