Abstract
Purpose of Review
We briefly review post-intensive care syndrome (PICS) and the morbidities associated with critical illness that led to the intensive care unit (ICU) liberation movement. We review each element of the ICU liberation bundle, including pediatric support data, as well as tips and strategies for implementation in a pediatric ICU (PICU) setting.
Recent Findings
Numerous studies have found children have cognitive, physical, and psychiatric deficits after a PICU stay. The effects of the full ICU liberation bundle in children have not been published, but in adults, bundle implementation (even partial) resulted in significant improvement in survival, mechanical ventilation use, coma, delirium, restraint-free care, ICU readmissions, and post-ICU discharge disposition.
Summary
Although initially described in adults, children also suffer from PICS. The ICU liberation bundle is feasible in children and may ameliorate the effects of a PICU stay. Further studies are needed to characterize the benefits of the ICU liberation bundle in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The landscape of pediatric intensive care has changed immensely since the advent of the first pediatric intensive care units (PICUs) in the 1950s and 1960s [1]. Through advances in mechanical support, medications, and procedures, mortality has been greatly reduced [2], but many pediatric survivors of critical illnesses will experience long-term disabilities, higher readmission rates, and overall poorer health status [3,4,5,6,7,8]. Such knowledge of the harm of an ICU stay, initially described in the adult ICU population, led the movement to reevaluate ICU clinical practice patterns and culture. With increasing awareness of the long-term detriments of an ICU stay, the term post-intensive care syndrome (PICS) was coined to describe the combination of negative cognitive, psychological, and physical effects after critical illness [9, 10•].
PICS has been described in children as well, although the true incidence is difficult to determine [11, 12]. Studies have shown up to 25% of children may display negative psychological and behavioral outcomes in the first year following PICU discharge including ongoing fears, changes in memory, attention span, cognitive functioning, self-esteem, or self-confidence, and a large proportion of PICU survivors may suffer from post-traumatic stress symptoms [13,14,15]. Several studies have described significant motor deficits and exacerbation of baseline physical disabilities in children post-ICU stay [4, 6, 16]. A systematic review published in 2017 found 19 studies documenting deficits in all three PICS domains in PICU survivors [12]. Although these pediatric cohort studies have found significant morbidities, it is difficult to compare data and estimate a true incidence due to varied outcomes scales and measures [12]. In addition to patient deficits, parents or family members of critically ill children can experience depression or post-traumatic stress disorder (PTSD) symptoms as well [13, 17].
Given these findings, recent focus has shifted from solely improving mortality to better understanding and preventing the long-term psychologic, social, and physical impairment experienced by critically ill patients and their families. A compelling body of literature, mostly adult, surfaced to support several changes in clinical care to ameliorate PICS and the effects of an ICU stay. The ICU Liberation Collaborative was a quality improvement initiative hosted by the Society of Critical Care Medicine among 76 hospitals (67 adult and 9 pediatric) formed to implement and assess changes in clinical practice aimed at improving patient outcomes. The collaborative worked to integrate the ICU liberation bundle, also known as the ABCDEF bundle, in the care of their patients to mitigate the effects of an ICU stay [10•]. Bundle implementation resulted in substantial improvements among adult ICU patients [18••, 19]. In two large multicenter studies at varied types of ICUs [18••, 19], even partial bundle implementation resulted in improvement in survival, mechanical ventilation use, coma, delirium, restraint-free care, ICU readmissions, and post-ICU discharge disposition. Furthermore, the data supported a dose-response relationship, in which a higher proportion of bundle compliance correlated with improved clinical outcomes. Although pediatric data is limited, these results have further supported the use of the ABCDEF bundle in all ICU patients, including PICU patients.
Systematic Approach to Liberation
The ICU liberation bundle, also known as the ABCDEF bundle (Table 1), is an evidence-based guideline to liberate patients from the harmful effects of an ICU stay. This large-scale quality improvement strategy offers guidance for the daily care of critically ill patients that can reduce pain, agitation, and delirium, in an effort to prevent physical, psychological, and cognitive morbidities that limit or prolong recovery. The components of the bundle include assessment, prevention, and management of pain; both spontaneous awakening and breathing trials; choice of sedation and analgesia; delirium assessment, prevention and management; early mobility and exercise; and family engagement and empowerment.
The ICU Liberation Bundle
Assess, Prevent, and Manage Pain
Pain and agitation are prevalent issues for pediatric patients during critical illness, and the overall goal of pain management within the PICU should be to maintain children in a calm, comfortable state that minimizes pain, but in which the patient is also able to remain alert and lucid during recovery. A key first step in managing pain is to correctly assess a patient’s pain level. Self-report is a reliable indicator of pain; however, it has been shown that a large proportion of pediatric patients in the PICU are unable to self-report their pain [20]. The heterogenous ages and developmental levels of the patients, in addition to the use of invasive support, can make adequate pain assessment challenging. To ameliorate this, a reliable and valid pain scale, appropriate for different ages, should be used for assessing pain and titrating medications when self-reporting is not possible.
The choice of pain scale used will depend on patient age and the verbal and cognitive capacity of the patient. In pediatrics, validated pain scales include the Face, Legs, Activity, Crying, Consolability (FLACC) Scale for nonverbal children 0 to 6 years of age [21], the Individualized Numeric Rating Scale (NRS) for nonverbal cognitively impaired children aged 6 years and older [22], and the Wong-Baker Faces Pain Scale (FACES) for verbal children 3 years or older [23]. In each of these scales, a score of 0 to 10 can be assigned, with higher scores indicating more pain. In addition to the use of medications to treat acute pain, nonpharmacologic interventions should be considered as adjuncts. Examples include repositioning, distraction, increasing caregiver presence, heat/cold compresses, or the use of massage therapy, music therapy, and child life therapy [24,25,26].
Tips for Implementation
Start by choosing a validated assessment tool to systematically evaluate levels of pain (in pediatrics, this includes the NRS, FACES, and FLACC Scales) and incorporate this into daily nursing assessments. Discuss among key stakeholders how higher pain scores should be addressed with medications and nonpharmacologic interventions and be sure to include reassessment of pain scores after intervention.
Both Spontaneous Awakening Trials and Spontaneous Breathing Trials
Prolonged mechanical ventilation is associated with increased patient morbidity and mortality and there is evidence that reducing the duration of mechanical ventilation through ventilator weaning protocols can improve clinical outcomes [27, 28•]. Therefore, it is imperative to recognize early those patients who are ready for discontinuation of mechanical respiratory support. A spontaneous breathing trial (SBT) is a systematic clinical assessment of the respiratory pattern, adequacy of gas exchange, hemodynamic stability, and subjective patient comfort that can be used to prompt consideration for ventilator discontinuation [29, 30]. In practice, systematic usage of SBT leads to earlier discontinuation of mechanical ventilation and it has been shown that up to 77% of critically ill adult patients who tolerated SBT were able to be successfully extubated [30]. While similar outcome data for SBT does not yet exist in pediatrics, and there remains controversy over both the optimal technique to perform an SBT and the criteria defining a successful SBT, investigation is currently underway in pediatrics.
Patients requiring mechanical ventilation are often maintained on continuous sedative infusions; thus, it is important to pay attention to the effects of sedation on respiratory drive and how the level of sedation may affect a patient’s success of liberation from mechanical ventilation. Deep sedation has been associated with longer duration of mechanical ventilation and reduced 6-month survival [31]. One such option to systematically assess a patient’s sedation requirements and ability to be more awake while mechanically ventilated is through a trial of daily sedation interruption, or a “sedation holiday.” This daily sedation interruption is often referred to as a spontaneous awakening trial (SAT). A SAT used alone or paired together with SBT has been shown to lead to earlier discontinuation of mechanical ventilation, decreased ICU length of stay, and improved 1-year survival in critically ill adult patients [28•, 32].
Tips for Implementation
Start by forming a multidisciplinary team of physicians, respiratory therapists (RT), and registered nurses (RN) to agree on selection criteria that would allow patients to begin SBT trials, set the criteria that determine trial failure, and determine what next steps will be for patients who have passed the SBT. Once agreed upon criteria are established, trial a huddle each morning to discuss which of the current patients in the PICU meet criteria. Be sure to coordinate the SAT/SBT trial with RN/RT availability. Over time, consider tracking the percentage of patients that qualify for SAT/SBT who pass and are able to be successfully extubated.
Choice of Sedation
The use of deep sedation has been shown to be associated with worse short-term and long-term outcomes [31, 33]. Whenever feasible, the goal of sedation should be to have our patients be as close to alert and calm as safely possible. Numerous adult studies have demonstrated significant benefit in optimizing pain treatment in critically ill patients versus only providing sedatives. This practice, termed “analgosedation,” has been shown to decrease duration of mechanical ventilation and shorten ICU length of stay in adults [34]. As new drugs emerge and we continue to learn about the detrimental effects of long-term sedation and neuromuscular blockade, sedation and analgesia for our patients become an increasingly important and complex choice.
The first choice when determining a sedative and analgesic regimen for a critically ill patient is to choose the degree/depth of sedation targeted. The Richmond Agitation Sedation Scale (RASS), the State Behavioral Scale (SBS), and the COMFORT behavioral scale (COMFORT-B) are validated sedation scales for use in pediatrics, with the RASS and the COMFORT-B having the advantage of having been validated in both intubated and non-intubated patients [35,36,37].
Once the depth of sedation is chosen, it is important to focus on the selection of specific sedative and analgesic medications. In the last decade, an overwhelming amount of data has shown that benzodiazepines are independently associated with the incidence of delirium [38•, 39]. In light of this evidence, benzodiazepines should not be used as a first-line sedative in critically ill children. The use of dexmedetomidine has increased in the PICU over the last decade and may shorten length of mechanical ventilation as well as lower opioid requirements and incidence of delirium [40, 41].
One emerging area of interest in the last decade has been the use of sedation protocols. The Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) trial showed that protocolized sedation was feasible and led to fewer days of opioid administration and exposure to fewer sedative classes and that patients were more often awake and calm. However, protocolized sedation was not found to reduce the duration of mechanical ventilation and did increase days with any report of pain and agitation [42]. Subsequent studies of nurse-driven sedation protocols have continued to show its safety and efficacy, as well as its ability to reduce benzodiazepine administration, shorten duration of mechanical ventilation, and decrease the occurrence of withdrawal symptoms [43, 44].
Tips for Implementation
Start by incorporating a validated tool for sedation in the nursing assessment and educate key stakeholders (nursing, residents/fellows) on the benefit of analgosedation. Incorporate and discuss sedation targets/goals at least daily on rounds. Form a multidisciplinary team to develop and implement a sedation protocol; addressing analgesia first and avoiding benzodiazepines are the first-line choice of sedative.
Delirium: Assess, Prevent, and Manage
Delirium is a prevalent and serious complication of critical illness. This complication affects 25–47% of critically ill children in the PICU [45•, 46, 47], with an even higher prevalence in children following cardiac surgery and cardiopulmonary bypass [48, 49] and in children requiring extracorporeal membrane oxygenation support [50]. The development of delirium in critically ill children has been shown to be associated with increased morbidity and mortality, longer duration of mechanical ventilation, increased length of stay, as well as higher resource utilization and medical cost [51,52,53].
There are three motoric subtypes of delirium: hyperactive, hypoactive, and mixed-type delirium. In critically ill children, the hypoactive subtype is by far the most common. Hypoactive delirium is characterized by inattention, decreased responsiveness, and lethargy, and without standardized use of validated pediatric delirium screening tools is the most likely to be missed or misdiagnosed as oversedation. There are three validated screening tools for use in critically ill children: the Pediatric Confusion Assessment Method for the Intensive Care Unit (pCAM-ICU) and the Preschool Confusion Assessment Method for the ICU (psCAM-ICU), the Cornell Assessment of Pediatric Delirium (CAPD), and the Sophia Observation Withdrawal Symptoms-Pediatric Delirium (SOS-PD) scale [46, 47, 54, 55]. The use of these screening tools is paramount to the assessment and diagnosis of delirium in critically ill children.
In the last decade, modifiable and nonmodifiable risk factors for the development of pediatric delirium have been identified. Younger children, especially under the age of 2, are at higher risk of delirium, as are children with underlying developmental delay, preexisting conditions, and higher severity of illness at PICU admission [45•, 46, 47]. The use of benzodiazepine has been shown to be an independent risk factor for the development of delirium, with a dose-response effect [38, 53, 56]. A recent study found that children receiving benzodiazepines had over three times the likelihood of becoming delirious, after controlling for cognitive status, mechanical ventilation, and opiate use [39]. Other potentially modifiable risk factors include the use of restraints [45•], anticholinergic medications [57, 58], and red blood cell transfusions [59].
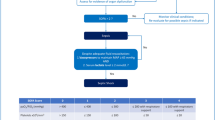
Delirium has multifactorial etiologies and triggers; it can develop as a complication of the underlying illness and organ dysfunction, and be precipitated by sedation, uncontrolled pain, withdrawal, sleep disruption, and the abnormal ICU environment and immobility. The clinical symptomatology of delirium can be difficult to distinguish from pain and iatrogenic withdrawal syndrome, which makes the use of a validated screening tool paramount in its diagnosis (Fig. 1). Preventing and managing delirium therefore starts with a careful identification of triggers and the underlying etiology. There is a paucity of evidence to truly guide the prevention and management of pediatric delirium. Studies in adult ICU patients have shown that implementation and adherence to the ABCDEF liberation bundle had a significantly lower risk of delirium [18••]. Recent studies in adults have shown that antipsychotics are not effective in significantly decreasing the duration of delirium [60]. Antipsychotics can be helpful for symptomatic management when a child continues to experience delirium despite the optimal management of the underlying illness and minimizing iatrogenic triggers. Although this represents off-label use, quetiapine was shown to be safe and effective in a randomized controlled trial [61], and case studies suggest the same for risperidone and olanzapine [62,63,64].
Tips for Implementation
Start by choosing a validated screening tool for delirium and integrating it into the nursing assessment. Avoid unnecessary sedation and avoid the use of benzodiazepines whenever possible. Once delirium is diagnosed, a careful evaluation of inciting events and etiology is warranted. The off-label use of atypical antipsychotics can be helpful in symptomatic management if delirium persists.
Early Mobility and Exercise
ICU-acquired weakness is a well-described phenomenon in adults [65,66,67] and children [68,69,70,71]. Early mobility (EM) [72], the practice of physical and occupational therapy early during critical illness, is used to prevent and treat ICU-acquired weakness. Biopsies in adults with septic shock suggest early mobility may maintain muscle fibers and lessen the muscle atrophy associated with critical illness [73]. Adult studies have shown several other benefits of EM, including decreased delirium incidence, decreased ICU length of stay, decreased hospital length of stay, decreased ventilator days, and earlier attainment of activities of daily living [74,75,76,77].
Varied developmental stages and a broad spectrum of ages can make EM more challenging in pediatrics [78]. In addition to protocolization and equipment barriers, many studies have found staff perceptions to be a significant barrier [78, 79]. However, a systematic review of 11 pediatric EM studies and over 1100 patients found only 1% of patients had any type of adverse event related to EM, suggesting EM is also safe in the PICU population [80]. Staff perceptions may be amenable to change with education and development of a multidisciplinary protocol [79]. EM outcomes data in pediatrics is sparse, but several studies have demonstrated feasibility [78, 81,82,83,84, 85•]. Two pediatric studies have found significant clinical benefits related to EM. In a single-center pre-post cohort of pediatric liver transplant patients, implementation of an EM program resulted in faster ambulation and shorter hospital length of stay [86•]. Simone et al. instituted delirium screening, protocolized sedation, and an EM protocol in a staged approach in single-center PICU and noted decreased incidence of delirium after implementation of EM [85•]. Further studies are needed to elucidate the clinical benefits of EM in the PICU population.
Tips for Implementation
Engage key stakeholders early and build a multidisciplinary committee (i.e., physical therapy, occupational therapy, nursing, respiratory therapy, administration, physicians and nurse practitioners or physician assistants, child life specialists, speech therapists) to create, champion, and implement a unit-wide protocol. Protocols should take into consideration unit- and hospital-specific needs and resources, and always make patient safety a priority. Engage bedside staff with education, hands-on experience, and success stories to encourage protocol adherence.
Family Engagement and Empowerment
Patient- and family-centered care (PFCC) is not a novel concept to pediatricians, as it is a core value in the field of pediatrics given the importance of family engagement to the successful care of children. PFCC is rooted in the understanding that involving patients and families in their own care or their loved one’s care is a mutually beneficial experience that will result in improved patient satisfaction, decreased patient anxiety, confusion, and agitation, and potentially higher quality care and safer care [87,88,89]. The core PFCC values in the ICU liberation bundle are keeping patients and families informed, actively involving patients and families in decision-making, actively involving patients and families in self-management, providing both physical comfort and emotional support to patient and families, and maintaining a clear understanding of patients’ concepts of illness and cultural beliefs [90].
Tips for Implementation
Form a patient and family counsel to help identify areas for improvement and guide change. Consider a family survey to understand baseline family engagement in your PICU. Common ways to institute PFCC include open visitation policies, family-centered rounds, allowing family presence during codes, providing education to families on how they can participate in care, encouraging families to be part of your unit’s safety culture, providing ICU diaries, and practicing shared decision-making to create a partnership between the ICU team and families.
Challenges to Bundle Implementation
Implementation of the ABCDEF bundle can be daunting since it requires a multidisciplinary collaborative approach and a real culture change. A recent review on the existing barriers to ABCDE bundle implementation in adult ICU identified four distinct domains: (1) patient-related, (2) clinician-related, (3) protocol-related, and (4) ICU contextual barriers [91]. These domains are consistent with domains of the Consolidated Framework for Implementation Research (CFIR), a widely-used framework in implementation science [92]. Each PICU will likely face different barriers within each domain, and the first step to implementation should be to identify our own unit’s barriers.
One of the most common hurdles to protocol implementation is provider buy-in. Bedside providers may have concerns about patient safety (especially for early mobility and the use of an analgosedation model) and may think that the risk it poses outweighs potential benefits. With effective education on the safety and feasibility of bundle implementation [79, 81] and ongoing feedback to providers with up to date data on how usage of the bundle is helping their patients, providers can feel empowered and motivated to lead bundle reliability performance. Involving multidisciplinary bedside providers and other key stakeholders early on during protocol design is also essential to ensure protocol feasibility and will help buy-in.
Another frequently encountered barrier to protocol implementation is resource limitations, especially the availability of personnel such as physical and respiratory therapists, and equipment. Support and engagement of senior health care executive can help secure and allocate the appropriate resources. The support of these groups is vital to the success of ABCDEF bundle implementation and function, as they can play an important role in motivating teams, helping to solve complex problems involving culture change within the hospital and ICU environment [93]. A summary of these and other commonly encountered barriers can be found in Table 2.
Future Directions
While the improvements in clinical outcomes using the ICU liberation bundle in the critically ill adult population is promising, similar outcome data for bundle implementation in pediatrics is lacking. Further emphasis should be placed on investigating the effect the ICU liberation bundle, as well as each individual bundle element, has on survival and morbidity related to critical illness in pediatrics. Similarly, PICS may be a significant issue for survivors of pediatric illness and their families, but no unified scales exist in the literature to be able to truly quantify the incidence of this problem. Unified outcomes scales are needed in order to better understand both the factors that predispose patients to developing PICS as well as improve long-term outcomes from those affected by PICS after hospital discharge.
As the survivorship of patients in the PICU increases, more focus will need to be placed on improving post-ICU functional status with intentional after-ICU support and interventions. One novel and developing approach to improving the lives of survivors of critical illness is the development of ICU follow-up clinics. This outpatient support has been used as part of post-ICU recovery models for some adult units after ICU discharge, though its impact on recovery remains largely unknown. This intervention has not been adopted widely, nor has it been studied extensively in the pediatric population. With advances in technology and the availability of telemedicine services, future research should explore whether a model for ICU follow-up can further improve the after-ICU recovery for critically ill pediatric patients and their families.
Conclusions
Medical advancements have led to a steady increase in survival among pediatric patients suffering from critical illness, though morbidity related to long-term physical, cognitive, and psychological effects persists for many pediatric patients and their families after hospital discharge. Use of systematic care bundles such as the ICU liberation bundle may further improve survival in pediatric patients as well as decrease the incidence of PICS. Widespread ICU liberation bundle implementation in pediatrics with systematic outcome monitoring and analysis will be essential for further advances in outcomes for pediatric survivors of critical illness.
Change history
23 June 2020
Table 2 of the original version of this article unfortunately was not formatted well, making it unclear for readers.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Epstein D, Brill JE. A history of pediatric critical care medicine. Pediatr Res. 2005. https://doi.org/10.1203/01.PDR.0000182822.16263.3D.
Namachivayam P, Shann F, Shekerdemian L, Taylor A, Van Sloten I, Delzoppo C, et al. Three decades of pediatric intensive care: who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010. https://doi.org/10.1097/PCC.0b013e3181ce7427.
Ong C, Lee JH, Leow MKS, Puthucheary ZA. Functional outcomes and physical impairments in pediatric critical care survivors: a scoping review. Pediatr Crit Care Med. 2016. https://doi.org/10.1097/PCC.0000000000000706.
Jones S, Rantell K, Stevens K, Colwell B, Ratcliffe JR, Holland P, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United Kingdom. Pediatrics. 2006.
Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-term function after pediatric critical illness: results from the survivor outcomes study∗. Pediatr Crit Care Med. 2017. https://doi.org/10.1097/PCC.0000000000001070.
Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014. https://doi.org/10.1097/PCC.0000000000000250.
Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study.
Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU. Pediatr Crit Care Med. 2014. https://doi.org/10.1097/PCC.0000000000000199.
Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. 2017. p. 225–43. Doi:https://doi.org/10.1016/j.ccc.2016.12.005
• Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017. https://doi.org/10.1097/CCM.0000000000002175A review article highlighting the science behind the ICU liberation bundle.
Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in children - the PICS-p framework. Pediatr Crit Care Med. 2018. https://doi.org/10.1097/PCC.0000000000001476.
Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: a systematic review. World J Crit Care Med. 2017. https://doi.org/10.5492/wjccm.v6.i2.124.
Rennick JE, Dougherty G, Chambers C, Stremler R, Childerhose JE, Stack DM, et al. Children’s psychological and behavioral responses following pediatric intensive care unit hospitalization: the caring intensively study. BMC Pediatr. 2014. https://doi.org/10.1186/1471-2431-14-276.
Colville G, Pierce C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med. 2012. https://doi.org/10.1007/s00134-012-2612-2.
Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med. 2012. https://doi.org/10.1097/PCC.0b013e3182196a8f.
Choong K, Fraser D, Al-Harbi S, Borham A, Cameron J, Cameron S, et al. Functional recovery in critically ill children, the “weeCover” multicenter study. Pediatr Crit Care Med. 2018;19:145–54. https://doi.org/10.1097/PCC.0000000000001421.
Morris A, Gabert-Quillen C, Delahanty D. The association between parent PTSD/depression symptoms and child PTSD symptoms: a meta-analysis. J Pediatr Psychol. 2012. https://doi.org/10.1093/jpepsy/jss091.
•• Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle. Crit Care Med [Internet]. 2018;1. https://doi.org/10.1097/CCM.0000000000003482Landmark large multicenter study showing benefits of ICU liberation bundle in adults.
Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at Seven California Community Hospitals: implementing PAD guidelines via the ABCDEF bundle in 6064 patients. Crit Care Med. 2017. https://doi.org/10.1097/CCM.0000000000002149.
Laures E, LaFond C, Hanrahan K, Pierce N, Min H, McCarthy AM. Pain assessment practices in the pediatric intensive care unit. J Pediatr Nurs. 2019. https://doi.org/10.1016/j.pedn.2019.07.005.
Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997.
Solodiuk J, Curley MAQ. Pain assessment in nonverbal children with severe cognitive impairments: the individualized numeric rating scale (INRS). J Pediatr Nurs. 2003.
Wong DL, Baker CM. Pain in children: comparison of assessment scales. Okla Nurse. 1988.
Moadad N, Kozman K, Shahine R, Ohanian S, Badr LK. Distraction using the BUZZY for children during an IV insertion. J Pediatr Nurs. 2016. https://doi.org/10.1016/j.pedn.2015.07.010.
Fein JA, Zempsky WT, Cravero JP, Shaw KN, Ackerman AD, Chun TH, et al. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2012. https://doi.org/10.1542/peds.2012-2536.
Friedrichsdorf SJ, Postier A, Eull D, Weidner C, Foster L, Gilbert M, et al. Pain outcomes in a US children’s hospital: a prospective cross-sectional survey. Hosp Pediatr. 2015. https://doi.org/10.1542/hpeds.2014-0084.
Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet. 2003. https://doi.org/10.1016/S0140-6736(03)12329-X.
• Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008. https://doi.org/10.1016/S0140-6736(08)60105-1Key study providing evidence supporting paired SAT/SBT.
Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996. https://doi.org/10.1056/NEJM199612193352502.
MacIntyre NR. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American college of chest physicians; the American association for respiratory care; and the American college of critical medicine. Chest. 2001. https://doi.org/10.1378/chest.120.6_suppl.375 s.
Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012. https://doi.org/10.1164/rccm.201203-0522OC.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000. https://doi.org/10.1056/NEJM200005183422002.
Stephens RJ, Dettmer MR, Roberts BW, Ablordeppey E, Fowler SA, Kollef MH, et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018. https://doi.org/10.1097/CCM.0000000000002885.
Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. Ann Pharmacother. 2012. https://doi.org/10.1345/aph.1q525.
Kerson AG, DeMaria R, Mauer E, Joyce C, Gerber LM, Greenwald BM, et al. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. J Intensive Care. 2016. https://doi.org/10.1186/s40560-016-0189-5.
Ista E, Van Dijk M, Tibboel D, De Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Pediatr Crit Care Med. 2005. https://doi.org/10.1097/01.PCC.0000149318.40279.1A.
Curley MAQ, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006. https://doi.org/10.1097/01.PCC.0000200955.40962.38.
• Smith HAB, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Thompson JL, et al. Delirium and benzodiazepines associated with prolonged ICU stay in critically ill infants and young children. Crit Care Med. 2017;45:1427–35. https://doi.org/10.1097/CCM.0000000000002515Key study describing the association of benzodiazepine use with the development of delirium.
Mody K, Kaur S, Mauer EA, Gerber LM, Greenwald BM, Silver G, et al. Benzodiazepines and development of delirium in critically ill children. Crit Care Med. 2018. https://doi.org/10.1097/CCM.0000000000003194.
Liu Y, Bian W, Liu P, Zang X, Gu X, Chen W. Dexmedetomidine improves the outcomes in paediatric cardiac surgery: a meta-analysis of randomized controlled trials. Interact Cardiovasc Thorac Surg. 2018. https://doi.org/10.1093/icvts/ivy043.
Pan W, Wang Y, Lin L, Zhou G, Hua X, Mo L. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-Analysis. Paediatr Anaesth. 2016. https://doi.org/10.1111/pan.12820.
Watson RS, Asaro LA, Hertzog JH, Sorce LR, Kachmar AG, Dervan LA, et al. Long-term outcomes after protocolized sedation versus usual care in ventilated pediatric patients. Am J Respir Crit Care Med. 2018. https://doi.org/10.1164/rccm.201708-1768OC.
Gaillard-Le Roux B, Liet JM, Bourgoin P, Legrand A, Roze JC, Joram N. Implementation of a nurse-driven sedation protocol in a PICU decreases daily doses of midazolam. Pediatr Crit Care Med. 2017. https://doi.org/10.1097/PCC.0000000000000998.
Neunhoeffer F, Kumpf M, Renk H, Hanelt M, Berneck N, Bosk A, et al. Nurse-driven pediatric analgesia and sedation protocol reduces withdrawal symptoms in critically ill medical pediatric patients. Paediatr Anaesth. 2015. https://doi.org/10.1111/pan.12649.
• Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, et al. Delirium in critically ill children: an international point prevalence study∗. Crit Care Med. 2017;45:584–90. https://doi.org/10.1097/CCM.0000000000002250Landmark multi-center study describing the prevalence and burden of pediatric ICU delirium.
Smith HAB, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med. 2011. https://doi.org/10.1097/CCM.0b013e3181feb489.
Smith HAB, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, et al. The Preschool Confusion Assessment Method for the ICU. Crit Care Med. 2016. https://doi.org/10.1097/ccm.0000000000001428.
Alvarez RV, Palmer C, Czaja AS, Peyton C, Silver G, Traube C, et al. Delirium is a common and early finding in patients in the pediatric cardiac intensive care unit. J Pediatr. 2018.
Patel AK, Biagas KV, Clarke EC, Gerber LM, Mauer E, Silver G, et al. Delirium in children after cardiac bypass surgery. Pediatr Crit Care Med. 2017;18:165–71. https://doi.org/10.1097/PCC.0000000000001032.
Patel AK, Biagas KV, Clark EC, Traube C. Delirium in the pediatric cardiac extracorporeal membrane oxygenation patient population: a case series. Pediatr Crit Care Med. 2017;18:e621–4. https://doi.org/10.1097/PCC.0000000000001364.
Traube C, Mauer EA, Gerber LM, Kaur S, Joyce C, Kerson A, et al. Cost associated with pediatric delirium in the ICU. Crit Care Med. 2016;44:e1175–9. https://doi.org/10.1097/CCM.0000000000002004.
Meyburg J, Dill M-L, Traube C, Silver G, von Haken R. Patterns of postoperative delirium in children*. Pediatr Crit Care Med. 2017;18:128–33. https://doi.org/10.1097/PCC.0000000000000993.
Traube C, Silver G, Gerber LM, Kaur S, Mauer EA, Kerson A, et al. Delirium and mortality in critically ill children. Crit Care Med [Internet]. 2017;45:891–8. https://doi.org/10.1097/CCM.0000000000002324.
Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell assessment of pediatric delirium. Crit Care Med [Internet]. 2014;42:656–63. https://doi.org/10.1097/CCM.0b013e3182a66b76.
Ista E, Van Dijk M, De Hoog M, Tibboel D, Duivenvoorden HJ. Construction of the Sophia Observation withdrawal Symptoms-scale (SOS) for critically ill children. Intensive Care Med. 2009. https://doi.org/10.1007/s00134-009-1487-3.
Mody K, Kaur S, Mauer EA, Gerber LM, Greenwald BM, Silver G, et al. Benzodiazepines and development of delirium in critically ill children: estimating the causal effect. Crit Care Med. 2018. https://doi.org/10.1097/CCM.0000000000003194.
Winsnes K, Sochacki P, Eriksson C, Shereck E, Recht M, Johnson K, et al. Delirium in the pediatric hematology, oncology, and bone marrow transplant population. Pediatr Blood Cancer. 2019. https://doi.org/10.1002/pbc.27640.
Madden K, Hussain K, Tasker RC. Anticholinergic medication burden in pediatric prolonged critical illness: a potentially modifiable risk factor for delirium. Pediatr Crit Care Med. 2018. https://doi.org/10.1097/PCC.0000000000001658.
Nellis ME, Goel R, Feinstein S, Shahbaz S, Kaur S, Traube C. Association between transfusion of RBCs and subsequent development of delirium in critically ill children. Pediatr Crit Care Med. 2018. https://doi.org/10.1097/PCC.0000000000001675.
Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1808217.
Joyce C, Witcher R, Herrup E, Kaur S, Mendez-Rico E, Silver G, et al. Evaluation of the safety of quetiapine in treating delirium in critically ill children: a retrospective review. J Child Adolesc Psychopharmacol [Internet]. 2015;25:666–70. https://doi.org/10.1089/cap.2015.0093.
Campbell CT, Grey E, Munoz-Pareja J, Manasco KB. An evaluation of risperidone dosing for pediatric delirium in children less than or equal to 2 years of age. Ann Pharmacother. 2019. https://doi.org/10.1177/1060028019891969.
Kishk OA, Simone S, Lardieri AB, Graciano AL, Tumulty J, Edwards S. Antipsychotic treatment of delirium in critically Ill children: a retrospective matched cohort study. J Pediatr Pharmacol Ther. 2019. https://doi.org/10.5863/1551-6776-24.3.204.
Sassano-Higgins S, Freudenberg N, Jacobson J, Turkel S. Olanzapine reduces delirium symptoms in the critically ill pediatric patient. J Pediatr Intensive Care. 2013;2:49–54. https://doi.org/10.3233/PIC-13049.
Kramer CL. Intensive care unit–acquired weakness. Neurol Clin. 2017;35:723–36. https://doi.org/10.1016/j.ncl.2017.06.008.
Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest [Internet]. Elsevier Inc. 2016;150:1129–40. https://doi.org/10.1016/j.chest.2016.03.045.
Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med [Internet]. 2014;370:1626–35. https://doi.org/10.1056/NEJMra1209390.
Kukreti V, Shamim M, Khilnani P. Intensive care unit acquired weakness in children: critical illness polyneuropathy and myopathy. Indian J Crit Care Med. 2014. https://doi.org/10.4103/0972-5229.126079.
Field-Ridley A, Dharmar M, Steinhorn D, McDonald C, Marcin JP. ICU-acquired weakness is associated with differences in clinical outcomes in critically ill children. Pediatr Crit Care Med. 2016. https://doi.org/10.1097/PCC.0000000000000538.
Banwell BL, Mildner RJ, Hassall AC, Becker LE, Vajsar J, Shemie SD. Muscle weakness in critically ill children. Neurology. 2003. https://doi.org/10.1212/01.WNL.0000098886.90030.67.
Valla FV, Young DK, Rabilloud M, Periasami U, John M, Baudin F, et al. Thigh ultrasound monitoring identifies decreases in quadriceps femoris thickness as a frequent observation in critically ill children. Pediatr Crit Care Med. 2017. https://doi.org/10.1097/PCC.0000000000001235.
Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit – from pathophysiology to clinical trials. Crit Care. 2009. https://doi.org/10.1186/cc7885.
Hickmann CE, Castanares-Zapatero D, Deldicque L, Van den Bergh P, Caty G, Robert A, et al. Impact of very early physical therapy during septic shock on skeletal muscle. Crit Care Med [Internet]. 2018;46:1. https://doi.org/10.1097/CCM.0000000000003263.
Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–42. https://doi.org/10.1016/j.apmr.2010.01.002.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet [Internet]. Elsevier Ltd. 2009;373:1874–82. https://doi.org/10.1016/S0140-6736(09)60658-9.
Schaller SJ, Waak K, Edrich T, Walz JM, Blobner M, Eikermann M. Goal directed early mobilization reduces ICU length of stay and improves functional mobility: an international multi center, randomized, controlled trial (Soms Trial). Anesth Analg [Internet]. 2016;122:S418. https://doi.org/10.1213/01.ane.0000499505.96779.a0.
Al-Qadheeb NS, Skrobik Y, Schumaker G, Pacheco MN, Roberts RJ, Ruthazer RR, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med. 2016;44:583–91. https://doi.org/10.1097/CCM.0000000000001411.
Betters KA, Hebbar KB, Farthing D, Griego B, Easley T, Turman H, et al. Development and implementation of an early mobility program for mechanically ventilated pediatric patients. J Crit Care. 2017;41. https://doi.org/10.1016/j.jcrc.2017.08.004.
Hanna ES, Zhao S, Shannon CN, Betters KA. Changes in provider perceptions regarding early mobility in the PICU. Pediatr Crit Care Med. 2020. https://doi.org/10.1097/PCC.0000000000002177.
Cuello-Garcia CA, Mai SHC, Simpson R, Al-Harbi S, Choong K. Early mobilization in critically ill children: a systematic review. J Pediatr [Internet]. Elsevier Inc. 2018. https://doi.org/10.1016/j.jpeds.2018.07.037.
Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU up! Pediatr Crit Care Med [Internet]. 2016;17:e559–66. https://doi.org/10.1097/PCC.0000000000000983.
Choong K, Awladthani S, Khawaji A, Clark H, Borhan A, Cheng J, et al. Early exercise in critically ill youth and children, a preliminary evaluation: the wEECYCLE pilot trial. Pediatr Crit Care Med. 2017;18:e546–54. https://doi.org/10.1097/PCC.0000000000001329.
Fink EL, Beers SR, Houtrow AJ, Richichi R, Burns C, Doughty L, et al. Early protocolized versus usual care rehabilitation for pediatric neurocritical care patients: a randomized controlled trial. 2019:1–11. https://doi.org/10.1097/PCC.0000000000001881.
Colwell BRL, Williams CN, Kelly SP, Ibsen LM. Mobilization therapy in the pediatric intensive care unit: a multidisciplinary quality improvement initiative. Am J Crit Care. 2018. https://doi.org/10.4037/ajcc2018193.
• Simone S, Edwards S, Lardieri A, Walker LK, Graciano AL, Kishk OA, et al. Implementation of an ICU Bundle: an interprofessional quality improvement project to enhance delirium management and monitor delirium prevalence in a single PICU. Pediatr Crit Care Med. 2017;18:531–540. Doi:10.1097/PCC.0000000000001127. Pediatric QI study showing decreased delirium with the implementation of several parts of the liberation bundle.
• Tsuboi N, Hiratsuka M, Kaneko S, Nishimura N, Nakagawa S, Kasahara M, et al. Benefits of early mobilization after pediatric liver transplantation. Pediatr Crit Care Med. 2019;20:e91–7. https://doi.org/10.1097/PCC.0000000000001815Pediatric cohort study showing physical improvement in pediatric liver transplant patients with EM.
Bell L. Family presence: Visitation in the adult ICU. Crit Care Nurse. 2012.
Meert KL, Clark J, Eggly S. Family-centered care in the pediatric intensive care unit. Pediatr Clin N Am. 2013. https://doi.org/10.1016/j.pcl.2013.02.011.
Everhart JL, Haskell H, Khan A. Patient- and family-centered care: leveraging best practices to improve the care of hospitalized children. Pediatr Clin N Am. 2019. https://doi.org/10.1016/j.pcl.2019.03.005.
Society of Critical Care Medicine. Family engagement and empowerment [Internet]. [cited 2020 Mar 4]. Available from: https://www.sccm.org/ICULiberation/ABCDEF-Bundles/Family-Engagement
Costa DK, White MR, Ginier E, Manojlovich M, Govindan S, Iwashyna TJ, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest. 2017. https://doi.org/10.1016/j.chest.2017.03.054.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009. https://doi.org/10.1186/1748-5908-4-50.
Balas MC, Pun BT, Pasero C, Engel HJ, Perme C, Esbrook CL, et al. Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse. 2019. https://doi.org/10.4037/ccn2019927.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Critical Care
The original version of this article was revised: Table 2 layout was modified to make it clear for readers.
Rights and permissions
About this article
Cite this article
Walz, A., Canter, M.O. & Betters, K. The ICU Liberation Bundle and Strategies for Implementation in Pediatrics. Curr Pediatr Rep 8, 69–78 (2020). https://doi.org/10.1007/s40124-020-00216-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-020-00216-7