Abstract
Introduction
This was a prospective study to investigate the antimicrobial efficacy of a novel ophthalmic solution comprising ozonated sunflower oil in liposomes plus hypromellose in conjunction with liposomal foam (BlefOX), in patients undergoing intravitreal injection, in comparison to povidone iodine 5%.
Methods
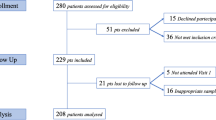
The study employed a paired-eye design with n = 195 patients and a total of n = 390 eyes divided into two groups. Conjunctival swabs were collected from both eyes of each patient at baseline (T0—3 days before the injection). The study group underwent home therapy, which included instilling two drops of an isotonic ophthalmic solution containing 0.5% ozonated sunflower oil in liposomes plus hypromellose (Ozodrop) four times daily and applying liposomal foam twice daily to the eye undergoing intravitreal injections. In contrast, the control group (contralateral eyes) received treatment with povidone iodine 5%. This treatment regimen was maintained for 3 days. At T1 (10 min before injection), all patients instilled one drop of a topical solution of povidone iodine 5% into the conjunctival sac of both eyes. After 30 seconds had elapsed, a conjunctival swab was obtained for each eye in both study groups.
Results
The results, derived from conjunctival swabs, exhibited a significant reduction in the microbial load of the study group on both chocolate agar and blood agar (p ≤ 0.007). The study demonstrated that the combination of povidone iodine 5% + Ozodrop + BlefOX provides a greater reduction in microbial load than povidone iodine 5% alone on both chocolate agar (141 [72.31%] vs. 98 [50.26%], p < 0.0001) and blood agar (130 [66.67%] vs. 97 [49.74%], p = 0.0007). The combination of povidone iodine 5% + Ozodrop + BlefOX resulted in the killing of approximately 41% to 49% of bacteria compared to povidone iodine 5% alone on the chocolate agar and blood agar, respectively.
Conclusions
Liposomal ozonated oil treatment, coupled with liposomal foam, in patients undergoing intravitreal injection led to a substantial reduction in conjunctival microbial load compared to eyes treated solely with povidone iodine 5%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Ozone is recognized as the most potent natural oxidizing agent, possessing extensively documented antiseptic and anti-inflammatory properties. A specific formulation designed for ophthalmic use has been recently developed, comprising 0.5% ozonated oil in liposomes along with hypromellose (Ozodrop, FB VISION S.p.A.). |
The objective of this study was to assess the antimicrobial efficacy of a novel ophthalmic solution comprising ozonated sunflower oil in liposomes plus hypromellose coupled with liposomal foam (BlefOX, FB VISION S.p.A.) in patients undergoing intravitreal injection in comparison to a control group. |
What was learned from the study? |
Our findings revealed that the topical application of liposomal ozonated oil treatment coupled with liposomal foam in patients undergoing intravitreal injection led to a good reduction in conjunctival microbial load compared to eyes treated solely with povidone iodine (PI) 5%. This reduction in the presence of pathogens holds the potential to minimize the risk of endophthalmitis. The antiseptic properties, combined with the absence of toxicity or allergenic effects, indicate that liposomal ozonated oil with liposomal foam may be regarded as a safe and effective adjunct in-home prophylaxis. |
In the present study, the administration of liposomal ozonated sunflower oil and hypromellose, accompanied by liposomal foam for a duration of 3 days preceding the anti-vascular endothelial growth factor (anti-VEGF) injection, led to a substantial reduction in the proportion of potentially pathogenic bacteria. This reduction was found to be significant when compared to the control group, which underwent treatment with a single drop of a topical solution containing PI at a concentration of 5%, administered into the conjunctival sac 10 minutes before the injection. |
Introduction
The significant rise in antimicrobial resistance among common bacterial pathogens has evolved into a critical clinical emergency. The World Health Organization has unequivocally identified antibiotic resistance as one of the top three public health threats in the twenty-first century [1]. Under physiological conditions, the ocular surface of the eye harbors a substantial microbial load known as the “ocular microbiota” [2, 3]. This microbiota consists of Gram-positive resident microorganisms such as staphylococci, streptococci, corynebacteria, and propionibacteria, along with Gram-negative bacteria. [4]
As a result, it is imperative to prioritize the use of presurgical prophylaxis to minimize the bacterial load on periocular skin and ocular conjunctiva before surgical procedures [5]. Postoperative endophthalmitis is commonly attributed to the patient's conjunctival bacterial flora [6]. Despite the implementation of surgical site disinfection and antisepsis of the eyelids and conjunctiva before surgery, there has been a notable increase in the incidence of endophthalmitis in recent decades [7,8,9].
A povidone iodine 5% (PI 5%) solution is commonly employed perioperatively to achieve antisepsis in the periocular and ocular regions [6, 10]. The topical application of eye drops containing 0.6% PI significantly reduces the bacterial load on the conjunctiva and enhances the disinfectant activity of PI 5% [11]. Nevertheless, PI antisepsis does not entirely eliminate the risk of endophthalmitis following intravitreal therapy. The incidence after PI application varies from 0.02% to 0.3% [12], and a cumulative rate throughout the treatment series has been reported in up to 1% of patients [13]. Studies on conjunctival swabs after PI antisepsis revealed a notable decrease in bacterial load on the ocular surface. It is crucial to note that complete eradication was not attained, with the lowest rate of culture-positive swabs being 3% [5, 14].
Therefore, incorporating an additional prophylactic treatment administered in the days preceding the injection could offer further assistance in controlling the microbial load on the conjunctiva, potentially resulting in a further reduction of the risk of endophthalmitis [5]. The identification of novel molecules that are well tolerated by biological tissues and exhibit effective antibacterial properties without contributing to antibiotic resistance may present a promising strategy for ophthalmologists.
Ozone is recognized as the most potent natural oxidizing agent, possessing extensively documented antiseptic and anti-inflammatory properties [15]. However, ozonated oils can be highly irritating to corneal tissue. Therefore, a specific formulation designed for ophthalmic use was recently developed, comprising 0.5% ozonated oil in liposomes along with hypromellose (Ozodrop®, FB VISION S.p.A.) [16,17,18,19]. Importantly, ozone triggers the synthesis of hydrogen peroxide and lipoperoxide, resulting in the liberation of free oxygen radicals that play a role in bacterial lysis and death. Furthermore, ozone has a negative regulatory effect on mitochondrial activity in bacteria and interferes with viral lytic enzymes [20]. Initial findings have indicated that ozone proves to be a safe and effective antiseptic agent in both in vitro and in vivo studies involving both animals and humans [21, 22].
The objective of this study was to assess the antimicrobial efficacy of a novel ophthalmic solution comprising ozonated sunflower oil in liposomes plus hypromellose in combination with liposomal foam (BlefOX®, FB VISION S.p.A.) in patients undergoing intravitreal injection in comparison to a control group.
Methods
Study Design
This was a prospective, interventional, monocentric, non-randomized, paired-eye-designed phase 4 clinical study with the title “Liposomal ozonated oil associated with PI 5% ensures a further reduction in the microbial load compared to PI 5% alone before IVT: the ‘OPERA’ study.”
Study Participants and Antiseptic Protocol
This prospective cohort study was carried out in the Department of Translational Biomedicine Neuroscience at the University of Bari Aldo Moro from September 2022 to September 2023. The research adhered to the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Ethical approval was granted in September 2022 by the Scientific Technical Committee of the Department of Translational Biomedicine Neuroscience at the University of Bari Aldo Moro, Bari, Italy (Protocol number: 94513). Prior to any study-related procedures, informed consent was obtained from all participants, and a comprehensive explanation of the nature and purpose of the investigation was provided.
The study population comprised individuals aged 18 years and older who had undergone intravitreal injections. Exclusion criteria were as follows: the use of topical or systemic antibiotics, topical antiseptics, ongoing treatment with topical therapies that could not be halted during the study, ocular or systemic inflammatory conditions, infectious processes, or hypersensitivity to the components of the study product.
The patients were stratified into two groups, and the study adopted a paired-eye design. In each patient, one eye underwent treatment with ozonated oil in liposomes and hypromellose, while the contralateral eye was treated with PI 5% and considered as control. At baseline (T0—3 days before the injection day), conjunctival swabs (eSwab, Copan Diagnostics, Murrieta, CA, USA) were collected from both eyes of each patient. Subsequently, the study group, comprising patients undergoing intravitreal injections, underwent home therapy that involved instilling two drops of an isotonic ophthalmic solution containing 0.5% ozonated sunflower oil in liposomes plus hypromellose (Ozodrop) four times daily (at 8:00, 12:00, 16:00, and 20:00) and applying liposomal foam (BlefOX) twice daily (at 8:00 and 20:00) to the eye undergoing intravitreal surgery. The treatment lasted for 3 days. On the day of the injection, the patients completed a therapy adherence questionnaire to assess their compliance to the treatment. At T1 (10 min before injection), all patients instilled one drop of a topical solution of PI 5% into the conjunctival sac of both eyes. After 30 seconds had elapsed, a conjunctival swab was acquired for each eye in both study groups. Swabs collected at T0 were sent to the laboratory within 24 hours of collection, and swabs collected at T1 were similarly sent to the laboratory within 24 hours of collection. Adverse events were documented throughout the study period. It is noteworthy that ozonated oil in liposomes plus hypromellose is classified as a sterile class 2b medical drug and bears a CE mark issued by Eurofins (CE 0477). This product has been available in the market since September 2017.
Bacterial Cultures
Between September 2022 and September 2023, a total of 390 eSwabs were admitted to the C.S. Microbiology and Virology Unit at the University Hospital Città della Salute e della Scienza di Torino in Turin, Italy. An eSwab consists of a sterile package with two components: a pre-labeled polypropylene screw-cap tube featuring a conical or round bottom, filled with 1 mL of liquid transport medium, and a specimen collection swab with a tip flocked with soft nylon fiber. The transport medium serves as a maintenance medium and is composed of inorganic phosphate buffer, calcium and magnesium salts, and sodium chloride in a reduced environment due to the presence of sodium thioglycolate. This medium is designed to sustain the viability of a variety of organisms.
Upon arrival at the laboratory through refrigerated transport, the samples were promptly placed on a stirrer to homogenize the transport medium. Subsequently, 300 mL of the homogenized medium was plated on chocolate agar, and another 300 mL was plated on BD BBL CDC [Centers for Disease Control] anaerobe agar supplemented with 5% sheep blood (SB) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Chocolate agar serves as a nonselective medium designed for isolating fastidious organisms from clinical specimens. It consists of proteose peptone, digested liver, and yeast extract as sources of nitrogen and vitamins. Osmotic balance is maintained through the inclusion of sodium chloride. Heat-denatured horse blood is incorporated to provide various nutrients, along with factor X (heme) and factor V (nicotinamide adenine dinucleotide, NAD). BD CDC anaerobe agar supplemented with 5% SB functions as a nonselective medium for the isolation and cultivation of fastidious obligate anaerobes from clinical specimens. This enriched and nonselective medium is designed for the isolation and culture of a diverse array of obligate anaerobic bacteria. It comprises trypticase soy agar enriched with agar to serve as a nutritive base. Osmotic balance was maintained using sodium chloride. Essential growth factors including sheep blood, hemin, cystine, and vitamin K1 were provided to support the growth of obligate anaerobes. A carefully measured volume of 300 mL was used to ensure that the operator could obtain an accurate count of bacterial colonies. Values either less than or greater than 300 mL would not permit a correct estimation of the bacterial load present in the conjunctiva. The plates were then incubated for 48 hours, with the CDC anaerobe + 5% SB plates in anaerobic conditions and the chocolate agar plates at 37 °C with 5% CO2. Macroscopic colony counting was conducted, followed by the identification of colony-forming microorganisms using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. MALDI-TOF is an important analytical mass spectrometry technique that measures the mass of molecules in a sample embedded in a matrix. This is achieved by using a laser to ablate and desorb molecules with minimal fragmentation. To conduct the MALDI-TOF analysis, a colony was selected from a culture plate and transferred to a spot on a MALDI-TOF target plate. Multiple strains could be tested simultaneously on the same slide. Following this, 1 mL of matrix was added. The matrix, rich in protons to facilitate sample ionization and highly absorbent to efficiently absorb laser radiation, was then applied to the plate. Subsequently, the plate was positioned in the ionization chamber of the mass spectrometer. In the ensuing hot plume of ablated gases, the sample molecules were ionized and directed into a time-of-flight (TOF) mass spectrometer, which recorded the ion mass-to-charge (m/z) ratio. This measurement was obtained by determining the time it took for the ions to traverse a known length under the influence of an electric field with a known strength. The resulting mass spectrum was generated based on the pattern (i.e., position and relative intensity) of the detected m/z peaks, creating a unique profile for a specific sample. The distinctiveness of mass spectra could be utilized for identification purposes, particularly when a comparison reference spectrum was available. All obtained results were compared with a database of mass spectra using software, leading to the identification of the organism.
Ocular Adverse Events
As previously reported by Spadea et al. [19], conjunctival hyperemia was graded into five levels: 0, normal conjunctival vessels without engorgement (none); 1, trace flush, reddish pink, minimally dilated blood vessels, and normal underlying sclera easily visible (trace); 2, mild flush, reddish pink, mildly increased density of dilated deep blood vessels, and pink appearance of the sclera (mild); 3, bright red color, significantly tortuous and engorged deep blood vessels, and minimal white scleral tissue visible (moderate); 4, deep bright diffuse redness, a dense network of engorged vessels, and no normal white scleral tissue visible (severe).
Patient satisfaction was evaluated using a 10-point visual analog scale (0 = very comfortable, 1–3 = mild discomfort, 4–6 = moderate discomfort, and 7–10 = severe discomfort) at post-baseline visit.
Statistical Analysis
The study was configured as a paired-eye design. Since the SDdiff (standard deviation of the difference between the preoperative and postoperative differences) was not known, a pilot study was conducted to obtain these unknown data. Applying the proc mixed method, we obtained the within-subject standard deviation which, divided by the square root of 2, gives us the standard deviation [23] necessary to calculate the sample size and is equal to ± 6.53. Considering a mean difference d = 4 (clinically reliable) between the combination of PI 5% + Ozodrop + BlefOX vs. PI 5% before and after the surgery we obtain, applying the proc power twosamplemeans test = diff, with α = 0.01 and power of 90% a sample size n = 162.
With a dropout of 20%, the number of patients to be enrolled becomes n = 195.
The comparisons between the two therapies (PI 5% vs. PI 5% + Ozodrop + BlefOX) with respect to chocolate agar count (baseline and 3 days after treatment) and blood agar count (baseline and 3 days after treatment) were evaluated using the Brunner-Munzel test. Data are expressed as mean ± standard deviation (SD) or standard error (SE) and 95% confidence interval (95% CI), and categorical variables are presented as absolute frequencies and percentages.
In the presence of high positive skewness, a low percentage of high values and large proportion of zeros justify the application the zero-inflated negative binomial (ZINB) regression with pre/post–treatment data. ZINB regression is a mixture of two distributions: (1) binary distribution with zero excesses, and (2) a counting distribution as the negative binomial. ZINB regression produces two sets of coefficients: the first predicting the frequency of event occurrence (count data: negative binomial), and the second predicting whether the event occurred (excess zeros: logistic part).
Comparisons between the values of hyperemia and VAS were evaluated by the chi-squared test.
Statistical analysis and sample size calculation were performed using SAS version 9.4 TS Level 1 M8 and JMP PRO version 17 (SAS Institute Inc., Cary, NC, USA).
A p value < 0.05 was considered statistically detectable.
Results
Characteristics of Patients Included in the Analysis
The analysis included a total of n = 390 eyes from n = 195 participants in the study. Of these, 94 (48.21%) were female and 101 (51.79%) were male. The mean age was 69.57 ± 10.41 years. The predominant diagnosis was age-related macular degeneration (AMD), observed in 47% of cases, while diabetic macular edema was present in 27% of patients [23]. Macular edema attributed to retinal vein occlusion (RVO) and myopic choroidal neovascularization (CNV) accounted for 13% and 5% of cases, respectively. The remaining participants received injections for conditions such as posterior uveitis, Irvine–Gass syndrome, and pachychoroid neovascularization.
Figure 1A–D shows the percentage distribution of bacterial counts in chocolate and blood agar at baseline and after 3 days for the PI 5% group. Figure 2E–H shows the percentage distribution of bacterial counts in chocolate and blood agar at baseline and after 3 days for the PI 5% + Ozodrop + BlefOX group.
In both figures, high positive asymmetry is observed, but in general, in Fig. 1 the four panels show a fairly similar trend when using PI 5%, while Fig. 2 highlights a reduction in bacterial count between baseline and after 3 days of treatment with PI 5% + Ozodrop + BlefOX.
Distribution of percentages of bacterial counts in chocolate and blood agar. E and F Blood agar at baseline and after 3 days, respectively, using Povidone Iodine 5% + Ozodrop + Blefox group. (G and H) Chocolate agar at baseline and after 3 days, respectively, using Povidone Iodine 5% + Ozodrop + Blefox group
In Figs. 3 and 4, at baseline the two groups (PI 5% vs. PI 5% + Ozodrop + BlefOX) show a similar trend and variability on both chocolate (20.63 ± 29.14, 95% CI [16.51–24.75] vs. 20.91 ± 26.90, 95% CI [17.11–24.71]; p = 0.97) and blood (25.91 ± 31.37, 95% CI [21.47–30.34] vs. 25.79 ± 33.60, 95% CI [21.04–30.54]; p = 0.62) agar count. After 3 days of treatment there was a detectable difference between the two groups for both chocolate (17.60 ± 25.80, 95% CI [13.96–21.24] vs. 7.14 ± 15.98, 95% CI [4.89–9.40]; p < 0.0001) and blood (21.28 ± 31.69, 95% CI [16.81–25.76] vs. 9.49 ± 19.76, 95% CI [6.70–12.28]; p < 0.0001) agar count. In fact, the half-violin plot shows a much more marked peak for the group using PI 5% + Ozodrop + BlefOX than the group using PI 5% alone, and this reduction is evident above all by observing the box plots.
Figure 3 shows the change form baseline for chocolate and blood agar counts. In both panels it is clear that the PI 5% + Ozodrop + BlefOX treatment achieved a higher percentage of reduction than the PI 5% alone: chocolate agar count (PI 5% + Ozodrop + BlefOX: 141 [72.31%] vs. PI 5%: 98 [50.26%], p < 0.0001) and blood agar (PI 5% + Ozodrop + BlefOX: 130 [66.67%] vs. PI 5%: 97 [49.74%], p = 0.0007).
In the presence of excess zeros, a zero-inflated model is suitable for analyzing the data to avoid model misspecification, which may result in biased or inconsistent estimators. In fact, as shown in Figs. 1, 2, 3, and 4, data show high positive skewness and a large proportion of zeros. The presence of excess zeros indicates a high probability of overdispersion. Analysis of the data showed that the variance was higher with respect to mean (chocolate agar at 3 days after treatment: PI 5% + Ozodrop + BlefOX group, mean = 7.14 and variance = 255.33, PI 5% group, mean = 17.60 and variance = 665.54; blood agar at 3 days after treatment: PI 5% + Ozodrop + BlefOX group, mean = 9.49 and variance = 390.58, PI 5% group, mean = 21.28 and variance = 1004.28). This scenario shows overdispersion using zero-inflated Poisson (ZIP) regression; therefore, this model was not adopted. In order to avoid biased estimates, ZINB regression was used with pre/post data.
Tables 1 and 2 report the parameters (β and γ) of ZINB regression for both chocolate and blood agar count. In both tables we corrected for the baseline data, and both models show a positive association (p < 0.0001 and p = 0.004, respectively) with the end-of-study data. The β1 values are very low and the relative percentage is very low (0.7% and 0.6%, respectively), confirming that no differences between patient groups are present at baseline. In both ZINB regressions, in the negative binomial part there is a detectable association with the therapy (PI 5% + Ozodrop + BlefOX vs. PI 5%) (β2: −0.52 ± 0.19, p = 0.007 for chocolate agar count and −0.67 ± 0.20, p < 0.0001 for blood agar count). The β1,2 coefficients of the count part of the ZINB are interpreted by exponentiating them so the values obtained are translated into the original scale. The sign of these coefficients (β2) indicates a good reduction in both chocolate and blood agar bacterial counts: approximately 41% in chocolate agar count and approximately 49% for blood agar count when using PI 5% + Ozodrop + BlefOX vs. PI 5%.
The logistic part in Tables 1 and 2 for both chocolate and blood agar count predicted the nonoccurrence of the outcome. Both tables include logit coefficients (γ1,2) for predicting excess zero-species. The coefficients for the logistic regression were on the logit scale, thus exponentiating the transformed values to odds ratio. The positive sign in γ2 indicates that log odds of being an excessive zero-species would increase by 17.76 (p < 0.0001) in chocolate agar count and 2.96 (p = 0.07) in blood agar count in the group using PI 5% + Ozodrop + BlefOX vs. PI 5%.
The ZINB regressions appropriately fitted the data because the dispersions were approximately 1, the variances (s2u and s2v) were low, and all gradients were lower than 10–3.
Tables 3 and 4 show the distribution of the different bacterial species subdivided by time and therapy.
Finally, in Table 5, only six (3.19%) patients reported ocular adverse events in the treated eye; more specifically, five (2.66%) patients reported traces, while mild discomfort was reported in only one (0.53%) patient regarding conjunctival hyperemia. No other signs were reported. In VAS analysis, 159 (84.57%) patients did not experience any ocular discomfort. Mild discomfort occurred in 28 (14.89%) patients.
Discussion
The periocular zone and conjunctiva harbor substantial microbial loads, collectively forming the ocular microbiota. This microbiota plays a crucial role in preserving the equilibrium of the ocular surface [24]. The ocular flora is widely recognized as the primary origin of microbes responsible for a majority of intraocular infections [6]. Consequently, implementing strategies to reduce microbial presence on the ocular surface and eliminate potential pathogenic flora during the preoperative phase proves beneficial in lowering the risk of endophthalmitis [24]. However, various studies have examined the antibiotic resistance pattern of conjunctival bacterial flora in patients undergoing cataract surgery, and have found an increase in bacterial resistance, attributed to the inappropriate and widespread use of antibiotics. Consequently, these studies advocate for the exploration of alternative prophylactic methods [19, 22, 25].
In recent years, there has been a significant shift toward utilizing various disinfectants to broadly reduce the microbial load on the conjunctiva. Among these, PI has become a leading antiseptic and disinfectant, especially prevalent in preoperative eye surgery preparations, most commonly used at a 5% concentration [26]. Likewise, ozone, in both gaseous and aqueous phases, has been proven to be a potent and dependable antimicrobial agent effective against a broad spectrum of bacteria. It demonstrates the ability to eradicate various types of bacteria, encompassing both Gram-positive and Gram-negative strains. This includes notoriously resistant bacteria such as Escherichia coli and multidrug-resistant Staphylococcus aureus [27, 28].
In this study, administration of liposomal ozonated sunflower oil and hypromellose along with liposomal foam for 3 days before the anti-vascular endothelial growth factor (anti-VEGF) injection resulted in a significant decrease in the proportion of potentially pathogenic bacteria. This reduction was found to be significant when compared to the control group, which underwent treatment with a single drop of a topical solution containing PI at a concentration of 5%, administered into the conjunctival sac 10 minutes before the injection. Specifically, our findings indicated that the pre-injection treatment for 3 days with Ozodrop® in conjunction with BlefOX® resulted in a detectable reduction (Δ T1–T0) = 72.31% in the number of swab cultures showing positive bacterial growth on chocolate agar. This decrease was notably greater than the PI 5% group, which exhibited a reduction of 50.26% (p = < 0.0001). Likewise, on the blood agar, the study group exhibited a noteworthy reduction of (Δ T1–T0) = 66.67% in the microbial load compared to eyes treated solely with PI 5%: 49.74% (p = 0.0007).
The noted protective effect of liposomal ozonated sunflower oil can be elucidated by the unique activity of this molecule in averting inflammation and combating microbial infections. Its antimicrobial efficacy is linked to ozonolysis, involving the breakdown of the bacterial cell envelope by oxidizing phospholipids and lipoproteins, along with impairment of the bacterial cytoplasmic membrane [28]. This action is nonspecific and selectively targets microbial cells, potentially offering a significant benefit in instances involving resistant bacteria [27].
The antiseptic effectiveness of liposomal ozonated oil has been established in the treatment of various ocular infections. Celenza et al. [29] conducted a study assessing the antifungal efficacy of 0.5% ozonated oil in liposomes plus hypromellose against four clinical Candida species: Candida albicans, Candida glabrata, Candida krusei, and Candida orthopsilosis. The results indicated that all Candida isolates were susceptible to 0.5% ozonated oil in liposomes plus hypromellose. Following a 1-hour exposure at the minimum inhibitory concentration, approximately 30% of cells were killed, and this percentage increased to approximately 70% at the highest concentration of ozonated oil (0.5%) in liposomes plus hypromellose. Furthermore, the use of liposomal ozonated oil has also been shown to facilitate wound healing and effectively treat certain inflammatory and infectious pathologies in the anterior segment, in both humans and animals. This includes conditions such as vernal conjunctivitis, granulomatous conjunctivitis, and persistent dystrophic corneal ulcers [30, 31]. The action of ozonated oil is linked, in part, to its antimicrobial effects, as well as its capacity to stimulate certain growth factors, activate local antioxidant mechanisms, and facilitate tissue repair. This multifaceted action could offer significant advantages compared to PI and chlorhexidine.
Additionally, Spadea et al. [19] demonstrated that the topical application of ozonated liposomal oil treatment in a substantial study population led to a noteworthy decrease in the conjunctival microbial load. This reduction in pathogen burden can contribute to enhanced preoperative prophylaxis procedures, particularly beneficial in the context of cataract surgery.
Crucially, the ozonated liposomal oil treatment demonstrated excellent tolerance, with no safety concerns. No serious systemic adverse events were reported in either group, and there were no discernible differences in ocular adverse events between the study group and the control group. Additionally, no cases of endophthalmitis were identified in either of the groups. This indicates the potential utility of liposomal ozone dispersion as a preoperative cleaning agent, given the absence of adverse events and the high level of satisfaction reported.
The present study has certain limitations that should be acknowledged, primarily the non-randomized design, which has the potential to introduce bias in the data analysis. Additionally, the clinical significance of this treatment with regard to an altered ocular microbial profile was not investigated and will be the emphasis of future large-scale studies.
Conclusions
In conclusion, our study demonstrates that the application of liposomal ozonated oil and liposomal foam significantly reduces the conjunctival microbial load. While this reduction is a positive indicator for potentially lowering infection risk, further studies are necessary to directly correlate these findings with a reduced risk of endophthalmitis. The observed antiseptic properties and absence of toxicity or allergenic effects within the study period suggest that liposomal ozonated oil with liposomal foam has potential as an adjunct in-home prophylaxis. The reduction in microbial load observed in our study suggests potential benefits for enhancing preoperative prophylaxis procedures for eyes undergoing intravitreal injection. Nonetheless, further research is needed to confirm its safety and establish definitive clinical guidelines.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectrum 4
Li JJ, Yi S, Wei L (2020) Ocular microbiota and intraocular inflammation. Front Immunol 11
Cavuoto KM, Stradiotto AC, Galor A. Role of the ocular surface microbiome in allergic disease. Curr Opin Allergy Clin Immunol. 2019;19:482–7.
Aragona P, et al. The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Surv Ophthalmol. 2021;66:907–25.
Halachimi-Eyal O, Lang Y, Keness Y, Miron D. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refractive Surg. 2009;35:2109–14.
Shimada H, Nakashizuka H (2021) Cataract surgery by intraoperative surface irrigation with 0.25% Povidone-Iodine. J Clin Med 10
Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30:597–613.
Tranos P, et al. Current perspectives of prophylaxis and management of acute infective endophthalmitis. Adv Ther. 2016;33:727–46.
Vaziri K, Schwartz SG, Kishor K, Flynn HW (2015) Endophthalmitis: state of the art. Clinical ophthalmology (Auckland, N.Z.) 9: 95–108.
Grzybowski A, Nakashizuka H, Shimada H. Prevention and treatment of postoperative endophthalmitis using povidone-iodine. Curr Pharm Des. 2017;23:574–85.
Reibaldi M et al. (2019) The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-VEGF intravitreal injection: a prospective, randomized study. J Clin Med 8.
Fileta JB, Scott IU, Flynn HW. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45:143–9.
Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Carrim ZI, Mackie G, Gallacher G, Wykes WN. The efficacy of 5% povidone-iodine for 3 minutes prior to cataract surgery. Eur J Ophthalmol. 2009;19:560–4.
Travagli V, Zanardi I, Bocci V. Topical applications of ozone and ozonated oils as anti-infective agents: an insight into the patent claims. Recent Pat Anti-Infect Drug Discovery. 2009;4:130–42.
Passidomo F, Pignatelli F, Addabbo G, Costagliola C. Topical liposomal ozonated oil in complicated corneal disease: a report on three clinical cases. Int Med Case Rep J. 2021;14:327–32.
Ugazio E, Tullio V, Binello A, Tagliapietra S, Dosio F (2020) Ozonated oils as antimicrobial systems in topical applications. Their characterization, current applications, and advances in improved delivery techniques. Molecules 25.
Spadea L, Tonti E, Spaterna A, Marchegiani A. Use of ozone-based eye drops: a series of cases in veterinary and human spontaneous ocular pathologies. Case Rep Ophthalmol. 2018;9:287–98.
Spadea L, Zanotto E, Cavallo R, Campagna G, Giannico MI, Costagliola C. Effectiveness of liposomal ozonized oil in reducing ocular microbial flora in patients undergoing cataract surgery. J Cataract Refract Surg. 2021;47:1548–55.
Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153:1096–100.
Pérez-Santonja JJ et al. Liposomal ozonated oil in ocular infections: a review of preclinical and clinical studies, focusing on its antiseptic and regenerative properties. Clin Ophthalmol (Auckland, N.Z.) 2022;16:1953
Kashiwagi K, Saito K, Wang YD, Takahashi H, Ishijima K, Tsukahara S. Safety of ozonated solution as an antiseptic of the ocular surface prior to ophthalmic surgery. Ophthalmologica. 2001;215:351–6.
Mastropasqua R et al. (2021) Serum microRNA levels in diabetes mellitus. Diagnostics 11
Valacchi G, Lim Y, Belmonte G, Miracco C, Zanardi I, Bocci V, et al. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Repair Regen. 2011;19:107–15.
Ansari MR, Madani H, Ghaderi E. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Pakistan J Med Sci. 2008;24(4):581–85.
Olson RJ, Braga-Mele R, Chen SH, et al. Cataract in the Adult Eye Preferred Practice Pattern®. Ophthalmology. 2017;124(2):P1–P119. https://doi.org/10.1016/J.OPHTHA.2016.09.027
Song M, Zeng Q, Xiang Y, et al. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol Med Rep. 2018;17(2):2449. https://doi.org/10.3892/MMR.2017.8148
Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2(1):66–70. https://doi.org/10.4103/0976-9668.82319
Celenza G, Iorio R, Cracchiolo S, et al. Antimycotic Activity of Ozonized Oil in Liposome Eye Drops against Candida spp. Transl Vis Sci Technol. 2020;9(8):1–11. https://doi.org/10.1167/TVST.9.8.4
Valacchi G, Lim Y, Belmonte G, et al. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Repair Regen. 2011;19(1):107–15. https://doi.org/10.1111/J.1524-475X.2010.00649.X
Kashiwagi K, Saito K, Wang YD, Takahashi H, Ishijima K, Tsukahara S. Safety of ozonated solution as an antiseptic of the ocular surface prior to ophthalmic surgery. Ophthalmologica. 2001;215(5):351–6. https://doi.org/10.1159/000050884
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The journal’s Rapid Service fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Maria Oliva Grassi, Giacomo Boscia and Pasquale Viggiano: Conceptualization, Methodology, Software. Marta Zerbinati, Giovanni Petrara, Ermete Giancipoli and Pasquale Puzo: Data curation. Giuseppe Campagna and Pasquale Viggiano: Writing—Original draft preparation. Giovanni Alessio: Visualization, Investigation. Francesco Boscia: Supervision, Software, Validation, Writing—Review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
Maria Oliva Grassi, Giacomo Boscia, Giovanni Alessio, Marta Zerbinati, Giovanni Petrara, Pasquale Puzo, Ermete Giancipoli, Campagna Giuseppe, Francesco Boscia, and Pasquale Viggiano confirm that they have no conflicts of interest to declare.
Ethical Approval
The research adhered to the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Ethical approval was granted in September 2022 by the Scientific Technical Committee of the Department of Translational Biomedicine Neuroscience at the University of Bari “Aldo Moro”, Bari, Italy (Protocol number: 94513). Prior to any study-related procedures, informed consent was obtained from all participants, and a comprehensive explanation of the investigation's nature and purpose was provided.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Grassi, M.O., Boscia, G., Alessio, G. et al. Liposomal Ozonated Oil Ensures a Further Reduction in the Microbial Load Before Intravitreal Injection: the “OPERA” Study. Ophthalmol Ther 13, 2771–2788 (2024). https://doi.org/10.1007/s40123-024-01006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-01006-w