Abstract
This commentary article delves into the transformative role of optical coherence tomography angiography (OCTA) in diagnosing and managing a wide array of eye conditions, including diabetic retinopathy, age-related macular degeneration, retinal vein occlusions, and white dot syndromes. Developed in 2005, OCTA has emerged as a non-invasive, high-resolution imaging technique that offers advantages over traditional fluorescein angiography (FA), providing quicker and safer monitoring of ocular conditions with similar diagnostic accuracy. In diabetic retinopathy, OCTA has been instrumental in early identification of retinal changes, offering quantifiable metrics including perfused capillary density (PCD) for assessing vascular alterations. For age-related macular degeneration (AMD), OCTA has deepened our understanding of non-exudative neovascular AMD, allowing for more effective monitoring and potential earlier initiation of treatment. In cases of retinal vein occlusions, OCTA can reveal specific microvascular features and allow for depth-resolved measurements of the foveal avascular zone, providing significant prognostic implications. OCTA has also been invaluable in studying rare white dot syndromes, enabling nuanced differentiation between conditions that often present similarly. Emerging research also suggests that OCTA can have potential utility in neurodegenerative diseases like Alzheimer’s, where retinal vascular patterns could offer diagnostic insights. While OCTA is revolutionizing ophthalmic care, further clinical trials and standardization are needed for its broader adoption into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Optical coherence tomography angiography (OCTA) is a non-invasive imaging technique developed in 2005 that provides high-resolution images of vascular layers in the retina and choroid. |
OCTA provides a new level of insight into a variety of ophthalmic conditions including diabetic retinopathy, age-related macular degeneration, retinal vein occlusions and white dot syndromes. |
OCTA aids in early identification of retinal changes, providing metrics such as perfused capillary density (PCD) for assessing vascular changes and offering advantages over traditional fluorescein angiography (FA) including non-invasive and faster imaging. |
While OCTA offers high-resolution, non-invasive imaging capabilities that streamline clinical workflows, it is constrained by its static imaging limitations and inability to showcase vascular dynamics in real time. |
Introduction
Optical coherence tomography angiography (OCTA) is an innovative imaging technique that can quickly and non-invasively capture high-resolution images of all the vascular layers of the retina and choroid. The technology was developed in 2005, and utilizes properties of the Doppler signal to visualize blood flow using swept-source OCT (SS-OCT) [1]. Over the years, this technology has evolved and is now widely accessible to healthcare professionals, offering valuable insights for patient diagnosis and ongoing care. This commentary will focus on the critical role OCTA plays in managing various eye conditions, including diabetic retinopathy, age-related macular degeneration, retinal vein occlusions, white dot syndromes, and emerging research in neurodegenerative diseases. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Strengths of OCTA
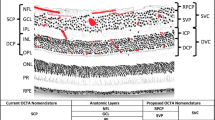
OCTA provides in vivo visualization of retinal blood vessels in a depth-resolved fashion, closely resembling the detail seen in histological studies [2]. By segmenting the volumetric data, it allows for identification of individual retinal capillary plexuses. Compared to fluorescein angiography (FA) and indocyanine green angiography (ICGA), OCTA gives a clearer view of the deep capillary plexus and choroid [3]. One of the major advantages of OCTA is the lack of dye required for vessel visualization. This means images have higher contrast without being clouded by dye leakage, resulting in sharper retinal vasculature depictions. Additionally, without the need for contrast dye, patients avoid potential risks ranging from mild allergies to severe anaphylactic reactions [4]. This makes OCTA especially suitable for patients who might react adversely to dye imaging, such as those with significant kidney pathology or challenging intravenous access. The fast imaging process is beneficial for patients needing regular follow-up, such as those receiving anti-vascular endothelial growth factor (anti-VEGF) treatments for conditions like wet age-related macular degeneration (wet AMD) or diabetic macular edema (DME).
Limitations of OCTA
While OCTA does have numerous advantages, it does also have certain limitations. One key challenge is that OCTA requires multiple scans at a single location for flow detection, which can increase scan duration in larger scan areas. OCTA imaging can be affected by artifacts stemming from the processes of image capture and processing. Additionally, there can be significant variation in both hardware and software across different OCTA manufacturers, which can influence imaging outcomes. Another factor to consider is image size, which can influence the clarity of vascular details captured [5]. Furthermore, the while dye-free nature of OCTA is generally safer, it comes with its own set of drawbacks. OCTA imaging is vulnerable to artifacts that arise from image acquisition and processing. It is also a “static” form of imaging that does not provide dynamic information like in FA. For example, OCTA cannot detect dye leakage, or offer insights into transit time or how the vessels are filling in real time [6].
Diabetic Retinopathy
In the context of diabetic retinopathy (DR), OCTA serves as a useful tool in the diagnosis, ongoing monitoring, and potential early identification of retinal changes in patients with diabetes [7,8,9]. Both qualitative and quantitative vascular changes are markers that correlate with the clinical features of DR, including diabetic macular ischemia. In both non-proliferative DR (NPDR) and proliferative DR (PDR), many vascular abnormalities can be visualized on OCTA, including choriocapillaris flow impairment, clustered capillaries, dilated capillary segments, tortuous capillaries, capillary dropout, reduced capillary density, abnormal capillary loops, and enlargement of the foveal avascular zone (FAZ). Furthermore, in PDR, neovascularization above the inner limiting membrane may be seen [10]. Quantitative OCTA parameters can play a key role in assessing prognosis for patients with DR. With regards to DR, foveal shape is specifically affected as a result of parafoveal vessel dropout and foveal ischemia that worsens with increasing severity of the condition. The FAZ has been demonstrated to be a sensitive marker to differentiate between high and low severities of NPDR [11]. The parafoveal vessel density of the deep capillary plexus has also been identified as a significant predictor of the severity of DR progression [12]. The vessel density of the deep perifoveal area has also been shown to decrease with increased severity of DR [13].
A 2019 study employed OCTA to contrast the perfused capillary density (PCD) between individuals with diabetes and healthy controls. Within the diabetic cohort, patients were further classified into three categories, specifically NPDR, PDR, or no DR. The study found that patients with diabetes reporting no clinical DR symptoms exhibited a notably higher PCD compared to those in the control group, offering a quantifiable metric for early vascular changes in the retina. Researchers speculated that this PCD increase might be due to enhanced capillary recruitment and dilation [7]. The study also found that PCD progressively declined in the NPDR and PDR groups.
Specific to DR, OCTA offers distinct advantages over the existing standard for vascular imaging which is FA. Specifically, PDR is marked by retinal ischemia and the emergence of new blood vessels (neovascularization or NV) at the vitreoretinal interface. OCTA can detect these new vessels by observing flow signals above the internal limiting membrane (ILM) or through ILM outpouching. A study conducted in 2020 compared the use of widefield OCTA to ultra-widefield FA (UWF-FA) and ultra-widefield color fundus photography (UWF-CF) for detecting retinal NV in eyes affected by PDR. The results showed that widefield OCTA could identify NVs that were not yet visible on UWF-CF, offering a quicker and safer monitoring alternative for patients to UWF-FA with similar diagnostic accuracy [8].
OCTA also enables the distinction of subtle forms of NV and microaneurysms, which can appear similar on FA imaging. Another study highlighted the value of widefield OCTA by incorporating flow overlays on cross-sectional B-scans for both staging and predicting the course of DR [14]. Intraretinal microvascular abnormalities (IRMAs) are visible on OCTA as collateral vessels within the retinal layers. Since the appearance of IRMAs marks the shift to severe non-proliferative DR (NPDR), widefield OCTA can be instrumental in identifying eyes at high risk for advanced DR.
However, there are certain limitations when it comes to OCTA and DR as well. As OCTA is based on motion detection, it requires patient fixation, which can be difficult in patients with diabetes that have macular involvement [15]. However, eye tracking and motion correction techniques may help manage these issues. OCTA images are also susceptible to artifacts such as vignetting and segmentation errors, particularly in patients with media opacities or diabetic macular edema, respectively [15]. These artifacts can complicate the differentiation between pathological capillary dropout and low-intensity areas. Finally, segmentation errors, which tend to increase with DR severity, can affect the accuracy of retinal layer identification and vascular density measurements. While it may be possible to manually correct these with built-in software, doing so for large numbers of images would be tedious and inefficient.
Age-Related Macular Degeneration
In recent times, the extensive clinical application of OCTA has deepened our understanding of age-related macular degeneration (AMD) that features macular neovascularization (MNV) without fluid or leakage as seen on FA [16, 17]. This specific condition, first identified in the 1970s, is known as non-exudative neovascular AMD [16, 17]. MNV can be effectively identified and tracked by using en face OCTA in conjunction with B-scan flow overlays [16]. Eyes with this condition are at a heightened risk for developing exudation compared to those with non-neovascular AMD [16, 18,19,20]. Studies have estimated that the incidence of new exudation in eyes where the other eye already had exudative AMD was around 25% over various follow-up periods ranging from 6 to 20 months [17]. The growth of MNV is suggested as a potential biomarker for predicting the transition to exudative AMD, although more long-term research is needed to confirm this, as findings have been inconsistent across studies [17]. In addition to the areas of choroidal neovascularization (CNV), the diameter, branching pattern, and morphology of neovessels can be well assessed with OCTA imaging. OCTA can showcase the size, structure, configuration, and location of neovessels clearly. Thicker diameter has been shown to be associated with untreated AMD [21]. Other features associated with CNV include poorly circumscribed vessels, loop-like or tree-like membranes, areas of hyperreflective microvascular tufts at the outer capillary level, and denser vascular networks on the en face image [22].
While no randomized clinical trials have been conducted specifically on the management of non-exudative neovascular AMD, the consensus is that it should be monitored rather than treated [16, 17]. Some research even suggests that this type of neovascularization may offer a protective effect against geographic atrophy (GA). Some studies have shown a slower rate of GA lesion growth and better survival of retinal pigment epithelium over areas of MNV with adjacent atrophy [16, 17, 19]. OCT and OCTA technologies enable ophthalmologists to monitor patients with this condition closely, allowing for timely initiation of treatment if exudation occurs.
Yet, there exist certain limitations that must be addressed in the usage of OCTA for AMD. Like with DR, background noise from media opacities and artifacts from patient movement can interfere with signal detection [23]. Segmentation errors and projection artifacts also negatively impact OCTA accuracy. While techniques may exist to help, like slab subtraction and projection-resolved OCTA, they are not perfect. The narrow field of view of OCTA results in a limited ability to detect changes in the retinal periphery. Finally, OCTA does not provide functional information, such as fluorescein leakage, when compared to traditional angiographic examination methods; this highlights the need for continuous improvement in OCTA technology for AMD evaluation.
Retinal Vein Occlusions
In cases of retinal vein occlusions (RVO), OCTA has proven to be especially useful for its capacity to reveal specific microvascular features in both the superficial and deep retinal plexuses. It also allows for depth-resolved measurements of the FAZ. Unlike the traditional clinical standard of FA, OCTA enables the measurement of the FAZ size in both the superficial and deep vascular plexuses separately [24]. Studies have found that the size of the FAZ is notably larger in eyes affected by RVO compared to their unaffected counterparts. Furthermore, an enlarged FAZ in the superficial vascular plexus (SVP) has been linked to worse visual outcomes in RVO cases [24]. While both OCTA and FA can assess regions of central and peripheral non-perfusion, OCTA offers enhanced precision in determining the scope and severity of non-perfusion, which carries significant prognostic implications. While macular edema is frequently the culprit behind vision loss in RVO cases, sustained poor vision can also result from photoreceptor damage due to non-perfusion [25]. Specifically, poor visual outcomes are associated with grade 4 macular ischemia as per the Bradley classification, whereas grades 2 and 3 do not show a significant correlation with poor vision [25]. OCTA further enables the observation of subtle microvascular alterations, such as vascular tortuosity, telangiectasia, and the formation of collateral vessels [26].
While OCTA provides a variety of benefits for RVO, it is similarly plagued by some of the key issues faced with OCTA in DR and AMD, like segmentation errors, artifacts, limited field of view, and the inability to detect leakage among others [26]. Additionally, microaneurysms imaged with FA may not be apparent on OCTA if they have flow rates below the detection threshold.
White Dot Syndromes
The application of OCTA in the study of white dot syndromes has shed new light on the underlying mechanisms, diagnosis, and treatment of these conditions [27,28,29,30]. Although these syndromes are relatively rare and often present with similar clinical features, OCTA has enabled more nuanced differentiation between them. For instance, multiple evanescent white dot syndrome (MEWDS) and acute posterior multifocal placoid pigment epitheliopathy (APMPPE) appear clinically similar, but OCT and OCTA can be utilized to reveal distinct underlying processes [27,28,29,30,31]. In MEWDS, normal OCTA findings in the choriocapillaris suggest that the condition is primarily an inflammatory process primarily affecting the photoreceptors, rather than a pathology of the choriocapillaris [28]. On the other hand, OCTA in APMPPE shows patchy flow deficits in the choriocapillaris, co-located with inflammatory lesions, pointing to primary ischemia in the choriocapillaris with secondary effects on the outer retinal layers and retinal pigment epithelium [29]. Distinguishing between MEWDS and APMPPE is crucial, as MEWDS can mimic other conditions like multifocal choroiditis, syphilis, and vitreoretinal lymphoma [27]. APMPPE, although generally self-limiting, can occasionally lead to complications like central nervous system vasculitis [27]. The utility of OCTA in detecting choroidal neovascularization is well established and proves useful in white dot syndromes like birdshot chorioretinopathy and idiopathic multifocal choroiditis (iMFC) with panuveitis, which can develop neovascularization due to ischemia and may require treatment [27, 29]. Other white dot syndromes, such as acute idiopathic maculopathy and serpiginous choroiditis, show choriocapillaris flow deficits or reduced flow on OCTA [27, 29]. In contrast, acute macular neuroretinopathy is linked to ischemia in the deep capillary plexus [27].
While OCTA has multiple uses in white dot syndromes, it also has limitations in evaluating these conditions. For example, OCTA distinguishes between MEWDS and APMPPE by revealing normal choriocapillaris findings in MEWDS and patchy flow deficits in APMPPE [32]. However, like with other use cases, OCTA’s ability to detect subtle flow changes may be compromised by artifacts from patient movement, segmentation errors, difficulty detecting flow in the slow-flow system of the choriocapillaris, and the limited field of view, which can result in missing peripheral lesions. Additionally, while OCTA is useful in detecting choroidal neovascularization in syndromes like birdshot chorioretinopathy and iMFC, its inability to quantify blood flow and potential for shadowing artifacts from opacities can hinder accurate assessment as well.
Neurodegenerative Diseases
The application of OCTA in studying neurodegenerative diseases like Alzheimer’s dementia (AD) is a burgeoning field of research [33,34,35,36,37]. Given the similarities in microvasculature between the retina and the brain, retinal vascular patterns observed through OCTA could offer diagnostic or predictive insights into neurodegenerative conditions [33]. In the case of AD, some studies have shown significant differences in OCTA metrics when compared to control groups, and others have identified correlations between OCTA measurements and cognitive scores, such as those from the Montreal Cognitive Assessment [34, 36, 38, 39]. Specifically, reduced vessel density in the superficial vascular plexus (SVP) has been reported in the eyes of patients with AD [34]. While some research has also reported a decrease in the density of the deep vascular plexus (DVP) and radial peripapillary capillary levels, these findings have not been consistently replicated [34,35,36]. A machine learning model that utilized multimodal retinal imaging and patient data was able to detect AD with a reasonable degree of accuracy, focusing on factors like the size of the FAZ and SVP density for differentiation [39]. In individuals without a cognitive impairment or dementia diagnosis, SVP density was found to correlate with cognitive test scores, suggesting a potential role for OCTA in early detection of cognitive changes [33]. To fully realize OCTA’s potential in the realm of neurocognitive disorders, further studies employing standardized OCTA techniques and longitudinal research approaches are needed [34, 36]. For the future, one should also consider how OCTA results may be confounded by artifacts or poor-quality scans, which may be due to poor subject compliance and deficits with fixation—something that may be more prevalent in those with certain neurodegenerative conditions [40]. Therefore, there also exists a continued need for research and technological advancements to fully solidify OCTA’s potential role in diagnosing and monitoring neurodegenerative conditions.
Practical Considerations of OCTA
From a practical standpoint, the use of OCTA has certain advantages within an ophthalmology clinic. The non-invasive and comparatively short timeframe required to acquire OCTA imaging compared with FA saves a lot of time and can make clinic workflow very efficient. The level of detail and the image resolution in OCTA is high, does not require focusing like traditional angiography, and does not get obscured through leakage from damaged vessels. However, there are also several barriers to consider with regards to OCTA use in the clinic. The primary barrier is the cost and the reimbursement implications of using a new imaging technology, which can vary according to the financial policies of certain sites and locations. Secondly, training technicians to use, understand, and interpret this new technology requires investment and specific training resources. This can be a limiting factor that can delay uptake of the technology into clinic workflow.
Acquisition Strategies
A variety of algorithms for OCTA have been developed and implemented in both research and clinical settings for the creation and optimization of OCTA images. Consequently, OCTA images from different devices exhibit varying appearances, leading to potential differences in clinical diagnostic interpretations. Each OCTA algorithm has its own set of limitations, influenced by its specific approach [41]. These include issues like diminished light penetration in deeper layers and the projection of artifacts from superficial to deeper layers. Such artifacts may arise during image acquisition, due to eye movement, throughout image processing, or as a result of display methods. There are several acquisition strategies that can be utilized to optimize OCTA imaging. One of the most important techniques is signal averaging. The quality of OCTA images is significantly influenced by the signal-to-noise ratio. Here, signal refers to the valuable data obtained from the tissue being examined, while noise represents irrelevant, undesirable data produced during the process of image capture and processing. When the signal is weak or the noise is excessive, the overall quality of the image deteriorates [42]. Averaging is a technique that can enhance the signal-to-noise ratio by allowing for the averaging of multiple signals. This can be done by quickly merging several OCT frames or even entire volumes taken at the same sample location. A study conducted by Szkulmowski and Wojtkowski in 2013 analyzed the effects of different averaging methods on signals and noise. Their findings revealed that averaging significantly reduced noise across a variety of different OCTA imaging scenarios [43].
Conclusion: Essential or Gimmick?
OCTA has revolutionized the clinical diagnosis and treatment of retinal diseases, especially in patients with early DR, CNV, RVO, and other vascular conditions. The evolution of OCTA has also given rise to WF-OCTA, which offers comprehensive imaging of the posterior pole extending to the mid-to-distant periphery. These advancements have provided clinicians with dependable, non-invasive imaging solutions. Anticipated enhancements, like montaging capabilities, are on the horizon, promising to further amplify the scope and utility of these systems. Current research consistently shows that OCTA can effectively detect significant lesions in conditions like DR, RVO, and other vascular retinal disorders. This efficacy rivals traditional dye-based angiography while offering a more streamlined experience for both medical staff and patients.
Beyond its applications to retinal conditions, OCTA offers insights into other ocular and even systemic diseases. The body of evidence supporting the role of OCTA in clinical practice continues to grow, with new research emerging annually that sheds light on its application to the assessment and management of various diseases. Compared to existing standards for ophthalmic vascular imaging, OCTA offers the benefits of rapid, non-invasive and reproducible imaging. Yet, that is not to say that OCTA is perfect. There are several issues like susceptibility to artifacts, segmentation errors, a limited field of view, an inability to quantify blood flow accurately, and challenges in distinguishing subtle vascular changes that need to be addressed in the future. Furthermore, additional clinical trials are required to fully integrate this imaging technology into established guidelines for treating various ocular diseases. The standardization of imaging protocols and effective artifact management will be key factors in the broader adoption of this promising technology.
References
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1. https://doi.org/10.1016/J.PRETEYERES.2017.11.0032.
Matsunaga D, Yi J, Puliafito CA, Kashani AH. OCT angiography in healthy human subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):510–5.
Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55.
Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography: results of a prospective study. Ophthalmology. 1991;98(7):1139–42.
Rabiolo A, Gelormini F, Marchese A, et al. Macular perfusion parameters in different angiocube sizes: does the size matter in quantitative optical coherence tomography angiography? Investig Ophthalmol Vis Sci. 2018;59(1):231–7.
Greig EC, Duker JS, Waheed NK. A practical guide to optical coherence tomography angiography interpretation. Int J Retina Vitreous. 2020;6(1):1–17.
Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019;203:103–15. https://doi.org/10.1016/J.AJO.2019.01.0123.
Pichi F, Smith SD, Abboud EB, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1901–9. https://doi.org/10.1007/s00417-020-04773-x4.
Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35(11):2371–6. https://doi.org/10.1097/IAE.00000000000007165.
Choi W, Waheed NK, Moult EM, et al. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37(1):11–21.
Alam M, Zhang Y, Lim JI, Chan RVP, Yang M, Yao X. Quantitative OCT angiography features for objective classification and staging of diabetic retinopathy. Retina (Philadelphia, Pa). 2020;40:322–32.
Rodrigues TM, Marques JP, Soares M, et al. Macular OCT-angiography parameters to predict the clinical stage of nonproliferative diabetic retinopathy: an exploratory analysis. Eye. 2019;33(8):1240–7.
Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124(2):235–44.
Arya M, Sorour O, Chaudhri J, et al. Distinguishing intraretinal microvascular abnormalities from retinal neovascularization using optical coherence tomography angiography. Retin J Retina Vitreous Dis. 2019;00(00):1–10. https://doi.org/10.1097/IAE.00000000000026716.
Chua J, et al. Optical coherence tomography angiography in diabetes and diabetic retinopathy. J Clin Med. 2020;9:1723.
De Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255–66. https://doi.org/10.1016/j.ophtha.2017.08.0307.
Laiginhas R, Yang J, Rosenfeld PJ, Falcão M. Non-exudative macular neovascularization—a systematic review of prevalence, natural history, and recent insights from OCT angiography. Ophthalmol Retin. 2020;4(7):651–61. https://doi.org/10.1016/J.ORET.2020.02.0168.
Yanagi Y, Mohla A, Lee SY, et al. Incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration. JAMA Ophthalmol. 2018;136(8):905. https://doi.org/10.1001/JAMAOPHTHALMOL.2018.21549.
Capuano V, Miere A, Querques L, et al. Treatment-naive quiescent choroidal neovascularization in geographic atrophy secondary to nonexudative age-related macular degeneration. Am J Ophthalmol. 2017;182:45–55. https://doi.org/10.1016/j.ajo.2017.07.00910.
Heiferman MJ, Fawzi AA. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS ONE. 2019. https://doi.org/10.1371/JOURNAL.PONE.021780511.
Shaimov TB, Panova IE, Shaimov RB, Shaimova VA, Shaimova TA, Fomin AV. Optical coherence tomography angiography in the diagnosis of neovascular age-related macular degeneration. Vestn oftalmol. 2015;131(5):4–13.
Usman M, Iqbal K, Ali MH, Nafees K. Features and diagnostic accuracy of optical coherence tomography angiography in neovascular age-related macular degeneration. Cureus. 2019;11(12):e6485.
Tombolini B, Crincoli E, Sacconi R, et al. Optical coherence tomography angiography: a 2023 focused update on age-related macular degeneration. Ophthalmol Ther. 2024;13:449–67.
Ouederni M, Khalifa MBH, Sassi H, Nefaa F, Ayed O, Cheour M. Quantitative analysis of microvascular network with optical coherence tomography angiography and its correlation with visual acuity in retinal vein occlusion. J Curr Ophthalmol. 2021;33:453–60. https://doi.org/10.4103/JOCO.JOCO_163_2112.
Mejía ME, Ríos HA, Rosenstiehl S, Rodríguez FJ. Optical coherence tomography angiography as predictor of visual outcomes in retinal vein occlusion treated with antiangiogenic therapy. Eur J Ophthalmol. 2022;0(0):1–7. https://doi.org/10.1177/1120672122109948713.
Tsai G, Banaee T, Conti FF, Singh RP. Optical coherence tomography angiography in eyes with retinal vein occlusion. J Ophthalmic Vis Res. 2018;13(3):315–32. https://doi.org/10.4103/jovr.jovr_264_1714.
Pradas M, Rodriguez-Merchante MP, Estébanez N, et al. Navigating the white dot syndromes with optical coherence tomography (OCT) and OCT angiography (OCT-A). Ocul Immunol Inflamm. 2022. https://doi.org/10.1080/09273948.2022.204679815.
Agarwal A, Invernizzi A. The role of optical coherence tomography and optical coherence tomography angiography in the differential diagnosis of posterior uveitis. Ocul Immunol Inflamm. 2022. https://doi.org/10.1080/09273948.2022.207174316.
Testi I, Modugno RL, Pavesio C. Multimodal imaging supporting the pathophysiology of white dot syndromes. J Ophthalmic Inflamm Infect. 2021;11(32):1–7. https://doi.org/10.1186/S12348-021-00261-317.
Pichi F, Sarraf D, Morara M, Mazumdar S, Neri P, Gupta V. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect. 2017;7(20):1–12. https://doi.org/10.1186/S12348-017-0138-Z18.
Pichi F, Srvivastava SK, Chexal S, et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new insights into pathogenesis. Retina. 2016;36:S178–88. https://doi.org/10.1097/IAE.000000000000125519.
Dingerkus VLS, Munk MR, Brinkmann MP, et al. Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J Ophthalmic Inflamm Infect. 2019;9:10.
Fang M, Strand K, Zhang J, et al. Retinal vessel density correlates with cognitive function in older adults. Exp Gerontol. 2021;152:1–6. https://doi.org/10.1016/j.exger.2021.11143320.
Wang X, Zhao Q, Tao R, et al. Decreased retinal vascular density in Alzheimer’s disease (AD) and mild cognitive impairment (MCI): an optical coherence tomography angiography (OCTA) study. Front Aging Neurosci. 2021;12:1–10. https://doi.org/10.3389/fnagi.2020.57248421.
Abraham AG, Guo X, Arsiwala LT, et al. Cognitive decline in older adults: what can we learn from optical coherence tomography (OCT)-based retinal vascular imaging? J Am Geriatr Soc. 2021;69:2524–35. https://doi.org/10.1111/JGS.1727222.
Yan Y, Wu X, Wang X, et al. The retinal vessel density can reflect cognitive function in patients with Alzheimer’s disease: evidence from optical coherence tomography angiography. J Alzheimers Dis. 2021;79:1307–16. https://doi.org/10.3233/JAD-20097123.
Wang X, Wei Q, Wu X, et al. The vessel density of the superficial retinal capillary plexus as a new biomarker in cerebral small vessel disease: an optical coherence tomography angiography study. Neurol Sci. 2021;42:3615–24. https://doi.org/10.1007/S10072-021-05038-Z24.
Lee MJ, Abraham AG, Swenor BK, Sharrett AR, Ramulu PY. Application of optical coherence tomography in the detection and classification of cognitive decline. J Curr Glaucoma Pract. 2018;12(1):10–8. https://doi.org/10.5005/JP-JOURNALS-10028-123825.
Wisely CE, Wang D, Henao R, et al. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. Br J Ophthalmol. 2022;106(3):388–95. https://doi.org/10.1136/BJOPHTHALMOL-2020-317659.
Asanad S, Mohammed I, Sadun AA, Saeedi OJ. OCTA in neurodegenerative optic neuropathies: emerging biomarkers at the eye-brain interface. Ther Adv Ophthalmol. 2020;12:2515841420950508.
Rocholz R, Corvi F, Weichsel J, Schmidt S, Staurenghi G. OCT angiography (OCTA) in retinal diagnostics. In: Bille JF, editor. High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham (CH): Springer; 2019; pp 135–160.
Baumann B, Merkle CW, Leitgeb RA, et al. Signal averaging improves signal-to-noise in OCT images: but which approach works best, and when? Biomed Opt Express. 2019;10(11):5755–75.
Szkulmowski M, Wojtkowski M. Averaging techniques for OCT imaging. Opt Express. 2013;21(8):9757–73.
Acknowledgements
Author Contributions
Siddharth Gandhi: concept and design, manuscript writing. Niveditha Pattathil: concept and design, manuscript writing. Netan Choudhry: concept and design, manuscript writing.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Netan Choudhry is a consultant with research support from Optos and Topcon, Hoffman La Roche & Bayer. Netan Choudhry is a consultant for AbbVue, Apellis, Alcon Laboratories, Bausch & Lomb, Bayer, Viatris, Biogen, Hoffman La Roche, Johnson & Johnson Vision, Topcon, Novartis, Regenxbio, & Optos PLC. Siddharth Gandhi and Niveditha Pattathil have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gandhi, S., Pattathil, N. & Choudhry, N. OCTA: Essential or Gimmick?. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-00985-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-00985-0