Abstract
Chronic, non-communicable diseases present a major barrier to living a long and healthy life. In many cases, early diagnosis can facilitate prevention, monitoring, and treatment efforts, improving patient outcomes. There is therefore a critical need to make screening techniques as accessible, unintimidating, and cost-effective as possible. The association between ocular biomarkers and systemic health and disease (oculomics) presents an attractive opportunity for detection of systemic diseases, as ophthalmic techniques are often relatively low-cost, fast, and non-invasive. In this review, we highlight the key associations between structural biomarkers in the eye and the four globally leading causes of morbidity and mortality: cardiovascular disease, cancer, neurodegenerative disease, and metabolic disease. We observe that neurodegenerative disease is a particularly promising target for oculomics, with biomarkers detected in multiple ocular structures. Cardiovascular disease biomarkers are present in the choroid, retinal vasculature, and retinal nerve fiber layer, and metabolic disease biomarkers are present in the eyelid, tear fluid, lens, and retinal vasculature. In contrast, only the tear fluid emerged as a promising ocular target for the detection of cancer. The retina is a rich source of oculomics data, the analysis of which has been enhanced by artificial intelligence-based tools. Although not all biomarkers are disease-specific, limiting their current diagnostic utility, future oculomics research will likely benefit from combining data from various structures to improve specificity, as well as active design, development, and optimization of instruments that target specific disease signatures, thus facilitating differential diagnoses.
Plain Language Summary
Long-term diseases can stop people living long and healthy lives. In many cases, early diagnosis can help to prevent, monitor, and treat disease, which can improve patients’ health. In order to diagnose disease, we need tools that are easy for patients to access, painless, and low-cost. The eye may provide the solution. In this review, we discuss the link between changes in the eye and four types of long-term disease that, together, kill most of the population: (1) Cardiovascular disease (affecting the heart and/or blood). (2) Cancer (abnormal growth of cells). (3) Neurodegenerative disease (affecting the brain and/or nervous system). (4) Metabolic disease (problems storing, accessing, and using the body’s fuel). We show that neurodegenerative disease leaves tell-tale signs in lots of different parts of the eye. Signs of cardiovascular and metabolic disease biomarkers are mostly found in the back of the eye, and signs of cancer can be found in the tear fluid. Although signs of disease can be seen in the eye, not all of them will tell us what the disease is. We believe that future research will help us to understand more about long-term disease and how to detect it if we combine information from different structures within the eye and develop new tools to target these specific structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The “Four Horsemen” of chronic disease (cardiovascular disease, cancer, neurodegenerative disease, and metabolic disease) kill around 80% of the population. |
Changes in retinal microvasculature can reveal cardiovascular problems, and optical coherence tomography angiography shows high potential as a screening tool for cardiovascular disease. |
Alterations to the protein composition of tear fluid is one of the few promising ocular biomarkers for cancer detection. |
Neurodegenerative disease can be detected in multiple ocular structures, owing both to neuronal loss and build-up of specific proteins. |
Metabolic disease and its biomarkers are often comorbid with other diseases, so discovery of biomarkers with greater specificity, such as those related to blood sugar, is desirable. |
Introduction
In an increasingly aging population, there is a growing emphasis not only on living longer (lifespan) but on maintaining key physical and mental functions (healthspan) across the lifespan. The predominant hurdles for longevity (both lifespan and healthspan) are chronic diseases. Within industrialized nations, there are four types of disease that kill an overwhelming majority of the population (approximately 80%). These were coined the Four Horsemen of Chronic Disease by Peter Attia [1]:
-
1.
Cardiovascular disease, accounting for approximately 19 million global deaths per year [2]
-
2.
Cancer, accounting for nearly 10 million global deaths per year [3]
-
3.
Neurodegenerative disease (e.g., Alzheimer’s disease), accounting for 9 million global deaths per year [4]
-
4.
Metabolic disease (e.g., type 2 diabetes), which contributes to negative health outcomes in multiple chronic diseases [5], making the prevalence difficult to calculate. However, diabetes alone accounts for approximately 1.5 million global deaths [6]
The emergence of the Four Horsemen can, in many cases, be prevented, or their progression hindered, by lifestyle adjustments and/or treatment [7]. Regular screening for chronic disease is therefore key to identifying those at risk and diverting the deadly cavalry. For example, one study stated that “cervical screening has prevented an epidemic that would have killed about one in 65 of all British women born since 1950 and culminated in about 6000 deaths per year [in the UK]” [8]. However, using the same example, nearly a third of eligible women in the UK (approximately 4.6 million) do not attend regular cervical screening appointments, despite more than 5 million invites being sent out [9]. This highlights the critical need to make screening techniques as accessible, unintimidating, and cost-effective as possible.
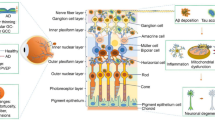
Unlike anywhere else in the human body, the eye offers a unique opportunity for direct, in vivo, and often non-invasive visualization of the neurosensory and microvascular systems. In addition to its accessibility, there are several reasons why the eye offers an attractive target for biomarker discovery for various systemic diseases.
-
The eye shares a common embryological origin with the brain [10], and the neurosensory retina and optic nerve are extensions of the brain, allowing direct visualization of the nervous system.
-
Owing to the length and continuity of the visual pathway, in conjunction with trans-synaptic degeneration mechanisms, damage to the central nervous system often manifests with inner retinal changes.
-
The blood–retina barrier mimics the blood–brain barrier [11], selectively enabling the transport of necessary substances to these metabolically active structures [12].
-
The aqueous and vitreous humors are derived from the plasma (a component of blood) [13] that allows transportation of lipid-soluble substances through diffusion, and water-soluble substances through ultrafiltration.
-
The lens is continuously growing [14], and contains molecules that build up over the lifetime, enabling mapping of molecular history.
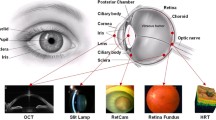
Although associations between ocular biomarkers and systemic health and disease were reported as early as the 1800s [15] the study of eye–body relationships has only recently been given its own term: “oculomics” [16]. The word is a contraction of oculus (Latin for “eye”) and omics, which is itself a contraction of the suffix “-ome”, meaning “whole of class”, and “-ics”, meaning “study of”. Recent advances in imaging techniques, such as optical coherence tomography (OCT), now enable visualization, and thus analysis, of structures on the order of microns. High-resolution images can therefore be used to identify disease features to stratify patients and facilitate a “precision medicine” approach. Furthermore, digitization of data storage and the development of analysis tools that use artificial intelligence (AI) have had a profound impact on the ability to process large datasets. For these reasons, oculomics is a burgeoning field. The purpose of this review is to evaluate oculomics’ potential to emerge as a hero in the crusade against the Four Horsemen of chronic disease.
Methods
Peer-reviewed records were identified using PubMed (titles/abstracts) and Google Scholar (titles only, as the ability to search titles and abstracts was not offered) using the following terms (March 6, 2024): “oculomics”; “ocular biomarkers” OR “ophthalmic biomarkers” AND “systemic disease” OR “chronic disease” OR “non-communicable disease” OR “cardiovascular disease” OR “cancer” OR “neurodegenerative disease” OR “metabolic disease”. Records were reviewed manually by EP using the methodology outlined in Fig. 1, yielding eight original studies, which form the basis of this review. A further 13 review articles have been used to identify additional relevant studies, providing a complementary approach. All publications were written in English and were published between 2015 and 2023.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
The following text is organized by disease type (Fig. 2). The goal is to provide a high-level overview of the key clinical findings from the “oculomics” literature in the context of the Four Horsemen of chronic disease. Our intention is to aid those who work with relevant clinical populations in understanding the possibilities and pitfalls of ocular biomarkers in screening and diagnosis. As such, discussion of methods and other technical details has been reined in, although we include a summary of the methods used in the eight original studies (Fig. 3). Table 1 shows a summary of the diseases that are relevant to biomarkers in each of the ocular structures discussed across all original and review articles, to aid those who seek further disease/structure-specific information.
Pie chart to illustrate (left) the proportion of global deaths caused by each disease type, as described in the introduction (numbers in millions), and (right) the proportion of each disease mentioned by the 21 publications included in the review. Some publications focused on more than one type of disease
Horseman One: Cardiovascular Disease
Cardiovascular disease is a general term for conditions affecting the heart or blood vessels. It is often associated with a build-up of fatty deposits inside the arteries (atherosclerosis) and an increased risk of blood clots. Obstruction of blood flow to key organs, such as the brain (stroke) or heart (heart attack), often proves fatal. As cardiovascular disease is the leading cause of global mortality, accounting for more than 30% of deaths worldwide [17], there has been significant motivation to develop effective tools to identify those most at risk.
Huang et al. (2023) published an original article describing their study on integrating oculomics (using retinal fundus images) and genomics to identify biomarkers for arterial aneurisms (swelling of the arteries) [18]. They found that the number of vessels in the optic disc and the angles between arterial branches were significantly associated with genetic risks of aneurisms. There were over 172,000 aneurism-related deaths in 2019 [19], and arterial aneurisms are usually asymptomatic prior to hospitalization, highlighting the importance of identifying biomarkers that can be used to predict and prevent adverse outcomes. Their machine-learning model (algorithms that learn with supervision, by mapping outcomes to representations) was also able to successfully stratify patients at different levels of risk and predict future aneurisms more effectively than a baseline model, and as effectively as a clinical risk model, over an 8-year period. However, as the biological causes of the vascular features remain unclear, the authors conclude that further studies are warranted to confirm their clinical value in the screening and early diagnosis of arterial aneurisms.
Clausen et al. (2023) conducted an original 20-year cohort study investigating the association between retinal artery occlusion (RAO) and dementia (the results of which will be discussed later under “Horseman Three”) [20], which also yielded findings relating to cardiovascular disease. RAO is a sight-threatening disease affecting 50,000 to 100,000 people per year, characterized by blockage of blood flow to the retina. Perhaps unsurprisingly, the prevalence of cardiovascular disease, hypertension (high blood pressure), and dyslipidemia (high levels of fat in the blood) was significantly higher in those with RAO than controls. The authors conclude that cardiovascular comorbidities can be considered shared risk factors of retinal and cerebral vascular disease.
Review articles on the topic of oculomics for cardiovascular disease have focused mainly on the use of artificial intelligence-based tools for analyzing disease risk from retinal images, such as fundus photography [16, 21,22,23,24]. Deep learning methods (algorithms that learn representations of outcomes, unsupervised) are capable of predicting risk of not only cardiovascular disease [25,26,27] but also major cardiovascular events [28], such as heart attack [29], and stroke [30], and cardiovascular disease mortality [31]. Such algorithms are also capable of predicting related risk factors, such as the presence of carotid atherosclerosis, coronary artery calcium score [32, 33], central retinal artery/vein diameter [34], and self-reported dyslipidemia [35].
The “black-box” nature of deep learning methods in the aforementioned studies precludes a full understanding of the salient retinal features associated with disease prediction, which may affect their incorporation into clinical care. However, other studies have sought to identify specific ocular features that are characteristic of cardiovascular disease. Many features of retinopathy, such as cotton wool spots (swollen debris from local cells within the retinal nerve fiber layer [RNFL], named after their appearance), arteriovenous nicking (when an arteriole crosses a venule, causing venular compression and bulging on either side), wider retinal venules, and narrower arterioles are associated with higher risk of stroke [36]. Interestingly, these vascular biomarkers are associated with coronary heart disease in women but not in men [37].
Metrics based on automated detection and segmentation of vessels, such as arteriolar and venular tortuosity, fractal dimension (a measure of vascular branching complexity and density) [25, 38,39,40] (Yu et al.’s data originates from a pre-print) and bifurcation (branching metrics) [41] can be used to predict ischemic heart disease (narrowed heart arteries) [42], hypertension [27, 28, 35], stroke [42] (Ma et al.’s data originates from a pre-print), and peripheral vascular disease (poor blood circulation throughout the body) [41]. In addition, the optic disc rim, cup-to-disc ratio, peripapillary atrophy, and fovea were related to cardiovascular disease [31, 32].
Recent advances in imaging techniques include OCT angiography (OCTA), which uses motion contrast imaging to visualize volumetric blood flow [43]. OCTA has enabled identification of additional parameters that are associated with cardiovascular profile [44], cardiovascular risk factors [45], and systemic vascular events [29, 46]. Another recent advance is the use of adaptive optics (AO), alongside other imaging techniques, such as OCT or scanning laser ophthalmoscopy (SLO), to correct for the aberrations of the eye. This enables visualization of the retina with cellular resolution [47]. Using AOSLO, an association between retinal vascular structure (inner and outer diameter, parietal thickness) and hypertension has been observed (see Bakker et al., 2022 for a review [48]).
Given the size of emerging datasets and complexity of imaging techniques, AI-based methods (in conjunction with interdisciplinary expertise) will be crucial in tackling the future healthcare challenges of common chronic disorders of the body. Further development and optimization of interdisciplinary deep learning algorithms will undoubtedly improve individualized patient outcomes and medical resource allocations. However, automated diagnosis systems that provide interpretable explanations of their predictions are warranted to promote clinical understanding and trust.
Horseman Two: Cancer
Cancer is characterized by uncontrolled growth and division of abnormal cells. Only two of the publications (both reviews) retrieved through the literature search mentioned cancer. This finding alone suggests that cancer may not be a promising target for oculomics, likely owing to the fact that cancer is typically organ/tissue/cell-specific. However, in a review by Hagan et al. (2016), the authors highlighted the utility of tear fluid biomarkers in the detection of certain cancers [49]. Tear fluid is both accessible and less complex than serum or plasma, simplifying its analysis, and recent developments in the accessibility and sensitivity of protein analysis techniques show promise for non-invasive biomarker detection. One such protein is lacryglobin, which has been found to be present in the tear fluid of patients with colon (100%), prostate (100%), breast (88%), lung (83%) and ovarian (33%) cancers [50]. Interestingly, it was also present in two controls who had a family history of breast and prostate cancer. Using panels of biomarkers has further revealed differences between patients with breast cancer and healthy controls [51,52,53], with a specificity and sensitivity of 70% [51]. These studies demonstrate that proteomic tear fluid analysis could provide a valuable screening tool for a variety of cancers. Further research into additional techniques for protein detection (such as fluorescence) in a variety of cancer types is warranted.
The only other mention of cancer was in a review by Chan et al. (2023) [24]. They describe a study that used a deep learning algorithm to predict biological age (as opposed to chronological age) from retinal fundus images. They used biological age as a biomarker for stratifying risk of all-cause mortality, cardiovascular disease, and cancer [26]. Compared with the participants in the algorithm’s first quartile, those in the fourth quartile had higher risk of cancer mortality (60%) and risk of cancer events (18%), which was independent of chronological age and known aging phenotypic biomarkers. However, it is well established that cancer risk increases with age (chronological or biological)—as do many chronic diseases—so the clinical utility of these findings is limited.
Horseman Three: Neurodegenerative Disease
Neurodegenerative diseases are characterized by progressive loss of structure or function of neurons, resulting in a decline in physical and/or mental function. A major stall guard for both timely diagnosis and development of effective treatments for neurodegenerative disease is the lack of technologies for detection and monitoring. Diagnosis of many neurodegenerative diseases is problematic and, with no single test standing as the “gold standard”, relies on a combination of cognitive assessments, blood tests, and scans. Given these clinical hurdles, the eye presents an attractive target for identifying ocular biomarkers for neurodegenerative diseases, as it shares a common embryological origin with the brain [10], the blood–retina barrier mimics the blood–brain barrier [11], and both are highly active metabolically [12].
Subramanian et al. (2020) carried out an original study that assessed the potential for neurofilament light chain (NfL) as a biomarker for neurodegenerative disease [54]. NfL is a neuronal cytoplasmic protein that is highly expressed in large-caliber myelinated axons. Vitreous humor samples were extracted from patients who were scheduled to undergo vitrectomy and used to quantify NfL. The study showed that NfL is positively associated with levels of amyloid-beta (Aβ40 and Aβ42), Tau, and other select inflammatory cytokines that are associated with neurodegenerative disease. An obvious limitation of this technique is its invasive nature, precluding it as a routine screening method. The authors encourage further investigation of NfL in other eye fluids, such as aqueous humor or tear secretions, that could offer a less invasive and more accessible means of fluid collection.
Demirlek et al. (2023) conducted an original study investigating choroidal structure, using OCT with enhanced depth imaging, in first-episode psychosis [55], which can be an early sign of neurodegenerative disease. They did not find statistically significant differences in central choroidal thickness, total choroidal area, luminal (dark) choroidal area, or stromal (bright) choroidal area between those with a single psychotic episode and normal controls. However, they did find a significantly higher choroidal vascularity index and luminal-to-stromal choroidal area ratio in patients with first-episode psychosis. The authors conclude that while choroidal thickness is preserved, microvascularity is abnormal in early psychosis, even during the prodromal period (i.e., before the appearance of clinical symptoms).
Wagner et al. (2023) conducted an original retrospective analysis of the association between retinal biomarkers and schizophrenia [56]. Schizophrenia is a mental disorder characterized by episodes of psychosis, including symptoms such as hallucinations or delusions. Although it is typically considered a neurodevelopmental disease, many aspects are consistent with a neurodegenerative model [57, 58]. Wagner et al. found that schizophrenia was associated with reduced ganglion cell-inner plexiform layer thickness, reduced fractal dimension, reduced vessel density, greater tortuosity, and enlarged cup-to-disc ratio. In contrast to prior literature [59], they did not find an association between schizophrenia and RNFL, comprising the axons of retinal ganglion cells, which propagate to the optic nerve and then project to the brain. As previously mentioned, schizophrenia can affect multiple physiological systems, and when the results were adjusted for hypertension and diabetes, there was no longer an association between schizophrenia and retinovascular characteristics, except using an AI-based fractal dimension estimation, although the ganglion cell-inner plexiform layer thickness and enlarged cup-to-disc associations were retained. The authors conclude that future research combining longitudinal and multimodal analysis is needed to further understand the developmental disease course of schizophrenia.
As mentioned in “Horseman One”, Clausen et al. (2023) conducted an original 20-year cohort study investigating the association between RAO and dementia [20]. Although they did not find an association between RAO and all-cause dementia or Alzheimer’s disease, they did find an increased incidence and unadjusted hazard ratio of vascular dementia in individuals with RAO, demonstrating a clinically important association between the vascular systems in the retina and cerebrum. However, when systemic comorbidity was accounted for, the association was no longer significant.
Review articles identify numerous ocular features related to neurodegenerative disease [16, 21, 49, 59,60,61,62,63,63,64,65].
Alzheimer’s Disease
Dementia is the most feared health condition, with Alzheimer’s disease being the most common cause. Early histological work by Hinton et al. (1986) [66] demonstrated a reduction in the thickness of the RNFL and the number of retinal ganglion cells in Alzheimer’s disease. It is therefore unsurprising that much of the current oculomics literature on Alzheimer’s disease revolves around OCT, which enables cross-sectional visualization of the retinal layers. Thinning of the inner retina (i.e., the nerve-containing layers), as well as the overall macular volume has been demonstrated in patients with Alzheimer’s disease [67]. Thinning of the RNFL has emerged as a consistent feature of Alzheimer’s disease [68, 69], and is associated with lower cognitive testing scores [70], as well as increased risk of developing dementia [71]. Interestingly, RNFL thickness is similar between those with mild cognitive impairment and those with established Alzheimer’s disease, suggesting that axonal loss occurs early in the disease process. Combined, this evidence suggests that RNFL thickness may serve as an early predictive biomarker for Alzheimer’s disease.
In addition to RNFL thinning and ganglion cell loss, Alzheimer’s disease is also associated with reduced optic nerve hemoglobin, causing increased pallor and reduced thickness (and greater cup-to-disc ratio) [63, 72, 73]. Choroidal thinning [63, 74] and reduced blood flow are also associated with Alzheimer’s disease [63, 75], and the latter may occur prior to neurodegeneration [75].
Alzheimer’s disease is characterized biologically by deposition of amyloid-beta plaques and Tau protein in the brain, and these proteins have also been shown to accumulate in the retina [63, 76, 77]. Furthermore, amyloid-beta and amyloid precursor proteins have been detected in the ocular lens [63, 78], with comparable concentrations to those in the brain [79], and are associated with supranuclear cataracts [79]. Similarly, amyloid-beta has been found in the aqueous humor with comparable concentrations to the cerebrospinal fluid [79], and in the vitreous humor [80]. It has been suggested that amyloid-beta and precursor protein derivatives may be produced in the retina and transported into the aqueous via the vitreous humor [81]. Given the heightened accessibility of these anterior structures, such biomarkers are a promising target for minimally/non-invasive tools.
Fluorescence measurements have demonstrated particular utility in Alzheimer’s disease. SLO can be used to visualize curcumin, an amyloid-beta plaque-labeling fluorochrome [77], and fluorescence lifetime imaging can be used to detect endogenous fluorophores in the retina [82]. Polarization-sensitive OCT has also been used to identify Alzheimer’s-associated birefringence (propagation and polarization of light) from microtubule damage [83]. Frontotemporal dementia is frequently misdiagnosed as Alzheimer’s disease; however, outer retinal thinning in the former (as opposed to inner retinal thinning in the latter) may facilitate differential diagnosis [84, 85].
Alzheimer’s disease-related changes in the anterior eye include greater pupil dilation [63, 86], reduced corneal sensitivity [87], altered density and morphology of both corneal dendritic cells (involved in the immune response) [63, 88] and corneal nerve fibers [89, 90], as well as changes in the composition of tear fluid [91,92,93,94] (Gijs et al.’s data originates from a conference abstract).
Parkinson’s Disease
Parkinson's disease is associated with tremors, stiffness, and slow movements. Like Alzheimer’s disease, Parkinson’s disease has been associated with thinning of several retinal layers [95–97], as well as damage to retinal microvasculature [98,99,100]. Like Alzheimer’s, Parkinson’s disease is associated with aggregation of specific proteins. Fluorescence measurements have detected alpha-synuclein aggregates in the retina of patients [66]. In addition, tumor necrosis factor alpha levels in the tear fluid have been shown to be significantly higher in patients with Parkinson’s disease than in controls, although levels were not linked to disease duration or severity [101], suggesting that accumulation of this protein may occur early in the disease process.
Huntington’s Disease
Huntington’s disease is a rare genetic disorder, characterized by the progressive degeneration of neurons, leading to cognitive, motor, and behavioral impairments. Choroidal thinning has been associated with this condition, suggesting vascular involvement [102, 103].
Ultimately, there are various other forms of dementia (in addition to Alzheimer’s, Parkinson’s, and Huntington’s disease), such as vascular dementia, Lewy body dementia, and Down syndrome, which can share important common underlying molecular pathogenesis and symptoms. However, a combined oculomic approach to assess relative levels of proteins (either through proteomic analysis or fluorescence measurements) may provide an opportunity not only for screening but also for differential diagnosis.
Multiple Sclerosis
Multiple sclerosis is a condition in which the immune system attacks the myelin sheath (the protective layer that surrounds nerve fibers), which can progressively impede signaling between the brain and the body. The RNFL is thinner in patients with multiple sclerosis when compared to normal healthy eyes [104,105,106,107], and can even be used to predict long-term disability [107]. Ganglion cell layer and whole retinal thicknesses have been found to be the most discriminative retinal features for diagnosing multiple sclerosis [108].
Multiple sclerosis has been consistently linked with the presence of IgG oligoclonal (immunoglobin) bands, present in cerebrospinal fluid (CSF); the retrieval of CSF is achieved through lumbar puncture, which is a highly invasive (and painful) procedure. However, tear fluid may offer a minimally invasive alternative for detection of IgG using proteomics [109]. The literature is mixed, with some studies finding significant differences in IgG levels between patients with multiple sclerosis and normal controls [109,110,111,112], and others finding either no significant differences [113] or a lack of disease-specificity (with IgG being associated with other neurological disorders [111], central nervous system infections [113], systemic immune disorders [113], and radiologically isolated syndrome [114]). However, proteomic analysis has revealed differential expression of multiple tear fluid proteins, most notably, alpha-1-antichymotrypsin (an acute inflammatory protein) [115], warranting further investigation into the sensitivity and specificity of the various proteins present in the tear fluid (and perhaps elsewhere in the eye).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is characterized by a gradual weakening of muscles, ultimately leading to death from respiratory failure. The condition has been associated with inner nuclear layer thinning [116], as well as increased choroidal and outer wall thickness of retinal vessels [117].
Schizophrenia
Schizophrenia is increasingly recognized as a systemic disease and associated with dysregulation in multiple physiological systems, such as cardiovascular, immune, and endocrine. Notably, cardiovascular disease accounts for approximately 40% of deaths in those with schizophrenia [118]. Both OCT and OCTA have revealed changes in retinal thickness and microvasculature respectively. Thinning has been observed in the macula and RNFL [119], ganglion cell layer [120], photoreceptor layer [121], and choroid [122], and evidence from magnetic resonance imaging suggests that retinal changes in schizophrenia are linked to structural changes in the brain and cognitive impairment [123]. Specific layers of the retina have shown both decreased and increased microvascular density (using OCTA) [124], which may help to stratify patients in terms of risk.
The cornea also shows schizophrenia-related changes, such as reduced volume and thickness [125], reduced corneal nerve fiber density, length, and branching [89], as well as elevated temperature, which may be linked to dopaminergic transmission [126]. Schizophrenia is also associated with reduced volume and depth of the anterior chamber (containing the aqueous humor) and increased lens thickness [125], which (again) may be attributable to the emmetropization effect of dopamine, although genetic factors cannot be ruled out.
Traumatic Brain Injury (TBI)
Although TBI is acquired through external force, the ensuing pathophysiological changes can lead to chronic neurodegeneration. The most frequent victims of TBI are infants (< 4 years), young adults (15–25 years), and the elderly (65+ years). There are a variety of emerging diagnostic techniques for TBI, one of the most relevant being OCT [61]. TBI is associated with retinal ganglion cell loss [127], as well as reduced retinal vasculature [128]. There are also proteins present within biofluids that increase in concentration in the chronic phase of TBI, such as P-Tau, T-Tau, amyloid-beta, and TAR DNA-binding protein 43 [129], which could offer opportunities for minimally invasive monitoring.
One consistent feature shared across different neurodegenerative diseases is retinal thickness. Although this could provide a valuable biomarker for screening, the lack of specificity limits its use as a diagnostic tool. Protein accumulation, on the other hand, shows greater specificity and, consequently, greater promise as a target for oculomics. In addition, the presence of changes in numerous ocular structures holds immense potential for differential diagnosis. The use of multimodal imaging, combined with AI analysis techniques, is therefore likely to prove transformative for early detection of neurodegenerative disease.
Horseman Four: Metabolic Disease
Metabolic disease refers to any condition that affects the normal functioning of the body’s metabolism (processing, storing, and accessing the body’s fuel). Although metabolic disease is often not a direct cause of death, this Horseman acts as a willing stablemate for the three other Horsemen (as well as other systemic conditions). Perhaps the most conspicuous condition in this category is diabetes, which is the focus of most metabolic oculomic research.
Alé-Chilet et al. (2021) published an original report on features of OCTA that are associated with diabetic kidney disease [130]—one of the many complications of diabetes. Diabetic kidney disease usually develops 5 years after type 1 diabetes diagnosis and occurs in 20–40% of patients with diabetes. The authors demonstrated significantly decreased vessel density and foveal avascular zone circularity in patients with diabetic kidney disease when compared to non-diabetic controls, with the latter metric showing potential as a biomarker for diabetic kidney disease risk and progression.
In another original report by the same group [131], Bernal-Morales et al. (2021) describe the association between OCT/OCTA and glycated hemoglobin (HbA1c), which has been the key measure of glycemic (blood sugar) control in patients with diabetes for the last 20 years. Patients with type 1 diabetes were classified by both HbA1C level (control < 6.5%, and two levels of diabetes: 6.5–7.5% and > 7.5%) and presence/absence of diabetic retinopathy—another of the many complications of diabetes. For patients with diabetic retinopathy, OCT revealed that increasing HbA1c levels were associated with greater macular central thickness, which may be a precursor to diabetic macular edema [132]. For patients without diabetic retinopathy, OCTA revealed that increasing HbA1c levels were associated with lower vessel density. When taking the group of patients with diabetes but no retinopathy as a whole, there was a significant correlation between HbA1c level and foveal avascular zone circularity. The authors suggest that OCTA may be a more effective tool for monitoring type 1 diabetes in early stages and OCT may have greater utility in later stages of disease.
Kim et al. (2022) published an original article describing a deep learning approach for prediction of sarcopenia [133]. Sarcopenia is linked to glucose metabolism and characterized by a gradual loss and dysfunction of skeletal muscle mass, which ultimately leads to metabolic and endocrine abnormalities [134]. The authors included various ophthalmologic examinations, including both anterior and posterior ocular features, and found that two measures of upper eyelid position (marginal reflex distance and levator muscle function) and the presence of cataracts were significant predictors of sarcopenia risk; the latter has implications for the pathophysiology of sarcopenia. However, these ocular measurements only accounted for 10–15% of the overall prediction.
Clausen et al.’s (2023) study on RAO (already discussed in “Horseman One” and “Horseman Two”) found that individuals with RAO had an increased likelihood of diabetes, demonstrating the comorbid nature of metabolic disease [20].
Review articles on the topic of oculomics in metabolic disease have focused primarily on deep learning for the detection of retinal features, as well as fluid biomarkers for hyperglycemia (high levels of sugar in the blood) and diabetes [21, 24, 49]. Deep learning models have demonstrated mixed performance in estimating hyperglycemia from retinal fundus images [28, 33, 135]. However, Zhang et al. were able to discriminate patients with self-reported hyperglycemia from normal controls [35]. Deep learning has been shown to predict type 2 diabetes before any apparent clinical manifestation of diabetic retinopathy, as well as quantify glycemic levels, using just retinal fundus images [136]. In addition, the algorithm was able to predict type 2 diabetes from images captured through smartphones. Such work provides promise for the future of home-monitoring for those at risk of developing diabetes.
Tear fluid is another potential source of biomarkers for diabetes, with evidence of alterations in both the sugar profile [137] and protein expression, notably lipocalin-1, heat shock protein 27, and beta-2 microglobulin [138], in diabetes. Levels of nerve growth factor have also been shown to correlate positively with blood glucose and glycated hemoglobin levels, duration of diabetes, and diabetic kidney disease [139]. This is somewhat counterintuitive, given that broader research has found a positive association between diabetes, neuropathy, and nerve growth factor deficiency [140]. However, differences in methodologies, disease stage, demographics, and comorbidities may have a confounding effect on fluid composition, warranting further research to ascertain its clinical utility for diabetes detection in tears (or indeed elsewhere in the eye).
Caveats and Challenges
Firstly, this review used relatively simple search criteria, and some relevant studies on ophthalmic biomarkers and the Four Horsemen in question will likely have been omitted as a result. However, the inclusion of review articles to identify additional relevant studies has facilitated broader capture of the literature. As such, although the search terms were used to identify literature to include as a necessity (and to summarize in Table 1), additional studies cited within the literature have been included where they were deemed to add further value. Similarly, the review has been limited purposely to the Four Horsemen of chronic disease. It must be noted that there are other chronic diseases that may be addressed through oculomics, such as sickle cell disease [141]. Moreover, oculomics is likely to have utility in detecting and monitoring other systemic conditions that are not classed as chronic, such as sepsis [142], as well as the effects of certain treatments [143].
Secondly, it should be noted that a given “Horseman” does not always ride alone, and is often accompanied by one or more of the other Four Horseman. The fourth, in particular—metabolic disease—appears to enjoy the company of his fellow riders. As stated by Barriada and Masip [23], one of the most successful predictors of cardiopathies is the presence and degree of diabetic retinopathy. So, although this review is organized by disease type for clarity, the reality of chronic disease is more convoluted. Likewise, many of the biomarkers themselves (particularly observations relating to retinal thickness) are not disease-specific (Table 1). For example, increased cup-to-disc ratio has been associated with both cardiovascular disease and Alzheimer’s disease [24, 60, 64], as well being a hallmark of glaucoma. Indeed, many of the biomarkers discussed here in relation to systemic disease may also be indicative of retinal disease, such as RNFL thinning in glaucoma and optic neuropathy [60], so caution is warranted when attempting to draw diagnostic conclusions based on a single biomarker. Despite this, the efficiency and non-invasive nature of ophthalmic techniques make them an invaluable screening tool for personalized and precision medicine by identifying those who would benefit from further investigation.
Thirdly, a recurring problem introduced by deep learning algorithms is their “black-box” nature. From a research perspective, simply characterizing an image as “diseased” does not improve scientific understanding of disease mechanisms. Furthermore, from a clinical perspective, the lack of verification that the algorithm’s output is based on identifiable and explainable image characteristics precludes scrutiny and reduces trust. Improvements in model visualization methods may help to promote clinical adoption [144]. Marked heterogeneity in vendor-specific devices (and their corresponding proprietary file formats) presents another hurdle for image comparison [145], impacting the generalizability of AI models. Lack of standardization of protocols (e.g., pupil dilation, scaling according to axial length) and varying image quality can further impact feature detection, introducing additional challenges for conducting meta-analyses on retinal indices.
Finally, for brevity, this review has not (with a few relevant exceptions) attempted to cover risk factors for disease, such as age, sex, smoking status, body mass index, etc. [16, 21, 24]. However, such factors can significantly improve predictive outcomes, particularly when integrated with AI and large datasets. The scope of this review is also limited to structural (rather than functional) biomarkers. Functional biomarkers are likely to have most utility in diagnosis and monitoring of neurodegenerative conditions [60], but may be relevant to other systemic diseases. Despite striving for simplicity in this review, it is necessary to acknowledge that an holistic approach may provide the most effective defense against the Four Horsemen, with oculomics being one weapon in the armory.
Insights
Despite the limitations of the current review, it has highlighted some of the major recent findings in oculomics research in relation to chronic disease. In particular, neurodegenerative disease is a highly promising target for oculomics, as biomarkers have been shown both to be present in several ocular structures (Table 1) and to have higher specificity than biomarkers for other disease types. Cardiovascular and metabolic disease are also promising targets, although oculomics may be better placed as a screening and monitoring tool, in conjunction with other clinical data that can assist with a differential diagnosis. In contrast, cancer appears to be a less promising target as, although it is classed as a systemic disease, it is typically organ/tissue/cell-specific, and therefore likely to have fewer ocular manifestations.
Oculomics has benefits over many other, more-invasive, diagnosis and monitoring techniques for systemic diseases, thus lending itself to rapid screening efforts. Although many ocular features are common across diseases, others, such as specific proteins that characterize neurodegenerative diseases [60], may provide more attractive alternatives for identifying patients and those at risk of developing disease. Future elucidation of the features used by deep learning algorithms may identify further features that can be used to predict disease risk, such as glycemic levels [35], the measurement of which is currently performed routinely and invasively.
Oculomics is likely to continue at a galloping pace in the screening, diagnosis, and monitoring arena; not least because it offers a less invasive, and thus more patient-friendly, alternative to many other techniques. Given the significant cost of many current in vivo techniques for detection of biomarkers, diagnosis using oculomic techniques is likely be more cost-efficient in many cases. Prices in the USA vary widely between locations, providers, and insurance status. However, as an example, positron emission tomography computed tomography (PET-CT), used for both cancer and dementia diagnosis, costs the UK National Health Service approximately £800 per scan (according to the NHS National Tariff, November 2022). An OCT scan, in comparison, can be conducted privately by a high street optician in the UK for as little as £10. Given that sight is the most valued sense (in the UK [146]), it may be leveraged to engage with those who would otherwise choose not to attend regular screenings for systemic disease with their healthcare provider. Similarly, greater accessibility to ocular imaging tools provides opportunities for new patient pathways, such as high street opticians, or specialty departments, such as neurology. With the development of non-invasive, handheld imaging tools with high patient acceptance, national screening programs become a more viable and cost-effective option; such programs could be conducted in schools, nursing homes, libraries, and other community settings, thereby increasing accessibility and reducing inequality.
Chronic disease is a particularly worthy focus for oculomics, as it is often associated with known risk factors (such as age and body mass index), enabling targeted (and thereby cost-effective) screening efforts in at-risk populations. Moreover, being chronic by definition, such diseases require frequent and long-term monitoring. The benefits to patients and wider society of effective, non-invasive, and cost-efficient oculomic techniques are thereby compounded over the course of disease.
Tellingly, the majority of publications (12/21) featured the use of AI. Its broad use in recent work illustrates its power and utility in analyzing large datasets. Widespread adoption of electronic health records connected to digital platforms for imaging and other ophthalmic data has increased the accessibility of data to researchers [147]. This vastly reduces the burden on researchers to acquire data—instead they can leap straight to the analysis stage using biobank data. Given ongoing advances in imaging techniques, offering higher resolution, AI tools are likely to become increasingly reliable, facilitating discovery of novel ophthalmic biomarkers of systemic diseases.
A major advantage of AI is that it can be used to identify qualitative ocular features of systemic disease, such as the microaneurysms of diabetes, the hemorrhages of hypertensive retinopathy, the sea-fans of sickle cell, to name a few. Moreover, for risk stratification, there is a need to detect more subtle features, such as caliber of blood vessels and thickness of the ganglion cell-inner plexiform layer. Measurement of these indices is challenging and best achieved with deep learning methods, in which measurement error is significantly reduced. AI also offers more rapid interpretation and deployment, providing patients/clinicians with an immediate actionable output.
Finally, deep learning can highlight features not previously considered by humans. One example is that excellent discrimination between males and females can be achieved through deep learning [28]. Deep learning has also revealed features of external eye photos, such as conjunctival vessel changes, that are indicative of diabetes [148], demonstrating the strength of AI over manual assessment.
All but two of the publications discussed here mentioned the use of retinal images. This likely reflects the significance of the investment in large databases and AI tools to assist with analysis of retinal images, as well as the rich and varied information that the retina holds. What is interesting, however, is the lack of any mention of the iris or ciliary muscles. Having a muscular component, it is not unreasonable to speculate that the ciliary muscles or iris may serve as a potential target for neurodegenerative disease biomarkers. Seven publications mentioned the use of biofluids (tear fluid, aqueous and vitreous humors), within which protein composition may offer a more disease-specific biomarker than many retinal features. Ocular biofluids were discussed in relation to cancer, neurodegenerative disease, and metabolic disease, but they may also have utility in detecting cardiovascular disease. Given that the aqueous and vitreous humors are derived from plasma that allows transportation of lipid-soluble substances through diffusion, it may be possible to detect high levels of fat in the blood, such as in dyslipidemia. Similarly, water-soluble substances, such as glucose, which are transported into the humors through ultrafiltration, could be used to detect biomarkers for metabolic disease, such as diabetes. Notably, however, all the biofluid-related publications included here used analysis of biological samples, requiring physically contacting (and sometimes invasive) retrieval/extraction. Alternative solutions, using non-contact optical measurement of ocular fluids, such as the aqueous humor [149], present a tantalizing opportunity for non-invasive monitoring of diseases, such as diabetes.
Although ophthalmic techniques, such as fundus photography and OCT/A, have demonstrated versatility in biomarker detection, oculomics would also benefit from active design, development, and optimization of instruments that target specific disease signatures. We believe that, in order for the next generation of ocular imaging/measurement tools to make the most impact, they should be compact, non-contacting, easy to use, and affordable. Such features will reduce the burden on clinicians, enabling deployment into the community by personnel with minimal training, thus improving accessibility. Such endeavors would facilitate earlier detection, prevention, and treatment of systemic disease, thereby improving long-term patient outcomes and reducing costs related to treatment of serious (and often lifelong) illness.
Conclusion
As the adage goes: “prevention is better than cure” and, arguably, never has there been a more pertinent application than chronic disease. Not only does prevention of chronic disease vastly improve an individual’s longevity (both lifespan and healthspan) but there are also significant societal benefits to keeping the Four Horsemen at bay. Chronic disease can have a devastating impact on families and friends, many of whom bear an unquantifiable financial and emotional burden to care for those who are afflicted.
As a society, we must prioritize preventative medicine, both in terms of lifestyle and primary care. Cost-effective and accessible screening and monitoring tools are fundamental to achieving this. There is an urgent need to drive policy change in the approach to healthcare beyond short-term achievements (i.e., to treat symptoms) towards long-term goals (i.e., to prevent the need for treatment). By improving the efficiency, accessibility, and usability of tools for screening, diagnosing, and monitoring patients, we move one step closer to putting the Four Horsemen of chronic disease out to pasture.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Attia P, Gifford B. Outlive: the science & art of longevity. 1st ed. New York: Harmony; 2023.
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153-639.
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–89.
Carroll WM. The global burden of neurological disorders. Lancet Neurol. 2019;18:418–9.
Madan K, Paliwal S, Sharma S, Kesar S, Chauhan N, Madan M. Metabolic syndrome: the constellation of co-morbidities, a global threat. Endocr Metab Immune Disord Drug Targets. 2023;23:1491–504.
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Healthcare access and quality index 1990–2019. Institute for Health Metrics and Evaluation (IHME); 2022. https://ghdx.healthdata.org/record/ihme-data/gbd-2019-healthcare-access-and-quality-1990-2019. Accessed 2023 Dec 20.
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46.
Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–56.
National Health Service England. NHS urges women to book a cervical screening as a third don’t take up vital offer. https://www.england.nhs.uk/2023/01/nhs-urges-women-to-book-a-cervical-screening-as-a-third-dont-take-up-vital-offer/. Accessed 2023 Dec 20.
Lovicu FJ, Robinson ML, editors. Development of the ocular lens. Cambridge, UK: Cambridge University Press; 2004.
Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood–retinal barrier: cellular basis and development. Vision Res. 2017;139:123–37.
Howard J, Blakeslee B, Laughlin SB. The intracellular pupil mechanism and photoreceptor signal: noise ratios in the fly Lucilia cuprina. Proc R Soc Lond B Biol Sci. 1987;231:415–35.
Reddy DVN, Kinsey VE. Composition of the vitreous humor in relation to that of plasma and aqueous humors. Arch Ophthalmol. 1960;63:715–20.
Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–7.
Bright R. Tabular view of the morbid appearances in 100 cases connected with albuminous urine. Guy’ Hospital reports; 1836
Wagner SK, Fu DJ, Faes L, et al. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol. 2020;9:6.
World Health Organization. Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016. Geneva, Switzerland: WHO; 2018.
Huang Y, Li C, Shi D, et al. Integrating oculomics with genomics reveals imaging biomarkers for preventive and personalized prediction of arterial aneurysms. EPMA J. 2023;14:73–86.
Huang X, Wang Z, Shen Z, et al. Projection of global burden and risk factors for aortic aneurysm—timely warning for greater emphasis on managing blood pressure. Ann Med. 2022;54:553–64.
Clausen AR, Stokholm L, Blaabjerg M, Frederiksen KH, Pedersen FN, Grauslund J. Retinal artery occlusion does not act as an independent marker of upcoming dementia: results from a Danish 20-year cohort study. Int J Retina Vitreous. 2023;9:50.
Wu J-H, Liu TYA. Application of deep learning to retinal-image-based oculomics for evaluation of systemic health: a review. J Clin Med. 2022;12:152.
Arnould L, Meriaudeau F, Guenancia C, et al. Using artificial intelligence to analyse the retinal vascular network: the future of cardiovascular risk assessment based on oculomics? A narrative review. Ophthalmol Ther. 2023;12:657–74.
Barriada RG, Masip D. An overview of deep-learning-based methods for cardiovascular risk assessment with retinal images. Diagnostics. 2022;13:68.
Chan Y, Cheng C-Y, Sabanayagam C. Eyes as the windows into cardiovascular disease in the era of big data. Taiwan J Ophthalmol. 2023;13:151.
Zekavat SM, Raghu VK, Trinder M, et al. Deep learning of the retina enables phenome- and genome-wide analyses of the microvasculature. Circulation. 2022;145:134–50.
Nusinovici S, Rim TH, Yu M, et al. Retinal photograph-based deep learning predicts biological age, and stratifies morbidity and mortality risk. Age Ageing. 2022;51:afac065.
Khan NC, Perera C, Dow ER, et al. Predicting systemic health features from retinal fundus images using transfer-learning-based artificial intelligence models. Diagnostics. 2022;12:1714.
Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2:158–64.
Diaz-Pinto A, Ravikumar N, Attar R, et al. Predicting myocardial infarction through retinal scans and minimal personal information. Nat Mach Intell. 2022;4:55–61.
Zhu Z, Hu W, Chen R, et al. Retinal age gap as a predictive biomarker of stroke risk. BMC Med. 2022;20:466.
Chang J, Ko A, Park SM, et al. Association of cardiovascular mortality and deep learning-funduscopic atherosclerosis score derived from retinal fundus images. Am J Ophthalmol. 2020;217:121–30.
Son J, Shin JY, Chun EJ, Jung K-H, Park KH, Park SJ. Predicting high coronary artery calcium score from retinal fundus images with deep learning algorithms. Transl Vis Sci Technol. 2020;9:28.
Rim TH, Lee G, Kim Y, et al. Prediction of systemic biomarkers from retinal photographs: development and validation of deep-learning algorithms. Lancet Digit Health. 2020;2:e526–36.
Cheung CY, Xu D, Cheng C-Y, et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng. 2020;5:498–508.
Zhang L, Yuan M, An Z, et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: a cross-sectional study of chronic diseases in central China. PLoS One. 2020;15:e0233166.
Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–40.
McGeechan K, Liew G, Macaskill P, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–32.
Shi D, Lin Z, Wang W, et al. A deep learning system for fully automated retinal vessel measurement in high throughput image analysis. Front Cardiovasc Med. 2022;9: 823436.
Yu F, Zhao J, Gong Y, et al. Annotation-free cardiac vessel segmentation via knowledge transfer from retinal images. arXiv. 2019. https://arxiv.org/abs/1907.11483. Accessed 2023 Dec 20.
Hoque ME, Kipli K. Deep learning in retinal image segmentation and feature extraction: a review. Int J Online Biomed Eng. 2021;17:103–18.
Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094–103.
Ma Y, Xiong J, Zhu Y, et al. Development and validation of a deep learning algorithm using fundus photographs to predict 10-year risk of ischemic cardiovascular diseases among Chinese population. Cardiovasc Med. 2021;127(9):85. https://doi.org/10.1101/2021.04.15.21255176.
De Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitr. 2015;1:5.
Arnould L, Guenancia C, Azemar A, et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59:4299.
Chua J, Chin CWL, Hong J, et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens. 2019;37:572–80.
Wang J, Jiang J, Zhang Y, Qian YW, Zhang JF, Wang ZL. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed Opt Express. 2019;10:1532–44.
Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14:2884.
Bakker E, Dikland FA, Van Bakel R, et al. Adaptive optics ophthalmoscopy: a systematic review of vascular biomarkers. Surv Ophthalmol. 2022;67:369–87.
Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15.
Evans V, Vockler C, Friedlander M, Walsh B, Willcox MD. Lacryglobin in human tears, a potential marker for cancer. Clin Exp Ophthalmol. 2001;29:161–3.
Lebrecht A, Boehm D, Schmidt M, Koelbl H, Schwirz RL, Grus FH. Diagnosis of breast cancer by tear proteomic pattern. Cancer Genomics Proteomics. 2009;6:177–82.
Lebrecht A, Boehm D, Schmidt M, Koelbl H, Grus FH. Surface-enhanced laser desorption/ionisation time-of-flight mass spectrometry to detect breast cancer markers in tears and serum. Cancer Genomics Proteomics. 2009;6:75–83.
Böhm D, Keller K, Pieter J, et al. Comparison of tear protein levels in breast cancer patients and healthy controls using a de novo proteomic approach. Oncol Rep. 2012;28:429–38.
Subramanian ML, Vig V, Chung J, et al. Neurofilament light chain in the vitreous humor of the eye. Alzheimers Res Ther. 2020;12:111.
Demirlek C, Atas F, Yalincetin B, et al. Choroidal structural analysis in ultra-high risk and first-episode psychosis. Eur Neuropsychopharmacol. 2023;70:72–80.
Wagner SK, Cortina-Borja M, Silverstein SM, et al. Association between retinal features from multimodal imaging and schizophrenia. JAMA Psychiat. 2023;80:478–87.
Kochunov P, Elliot Hong L. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40(4):721–728. https://doi.org/10.1093/schbul/sbu070
Li C, Yang T, Ou R, Shang H. Overlapping genetic architecture between schizophrenia and neurodegenerative disorders. Front Cell Dev Biol. 2021;9:797072. https://doi.org/10.3389/fcell.2021.797072
Silverstein SM, Choi JJ, Green KM, Bowles-Johnson KE, Ramchandran RS. Schizophrenia in translation: why the eye? Schizophr Bull. 2022;48:728–37.
Majeed A, Marwick B, Yu H, Fadavi H, Tavakoli M. Ophthalmic biomarkers for Alzheimer’s disease: a review. Front Aging Neurosci. 2021;13:720167.
Harris G, Rickard JJS, Butt G, et al. Review: emerging eye-based diagnostic technologies for traumatic brain injury. IEEE Rev Biomed Eng. 2023;16:530–59.
Suh A, Ong J, Kamran SA, et al. Retina oculomics in neurodegenerative disease. Ann Biomed Eng. 2023;51:2708–21.
Singh A, Verma S. Use of ocular biomarkers as a potential tool for early diagnosis of Alzheimer’s disease. Indian J Ophthalmol. 2020;68:555.
Jones-Odeh E, Hammond CJ. How strong is the relationship between glaucoma, the retinal nerve fibre layer, and neurodegenerative diseases such as Alzheimer’s disease and multiple sclerosis? Eye (Lond). 2015;29:1270–84.
Javaid FZ, Brenton J, Guo L, Cordeiro MF. Visual and ocular manifestations of Alzheimer’s disease and their use as biomarkers for diagnosis and progression. Front Neurol. 2016;7:55.
Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–7.
Cheung CY, Chan VTT, Mok VC, Chen C, Wong TY. Potential retinal biomarkers for dementia: what is new? Curr Opin Neurol. 2019;32:82–91.
Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst). 2015;1:136–43.
Coppola G, Di Renzo A, Ziccardi L, et al. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One. 2015;10:e0134750.
Ko F, Muthy ZA, Gallacher J, et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol. 2018;75:1198.
Mutlu U, Colijn JM, Ikram MA, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 2018;75:1256.
Tsai CS, Ritch R, Schwartz B, et al. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol. 1991;109:199–204.
Bambo MP, Garcia-Martin E, Gutierrez-Ruiz F, et al. Analysis of optic disk color changes in Alzheimer’s disease: a potential new biomarker. Clin Neurol Neurosurg. 2015;132:68–73.
Trebbastoni A, Marcelli M, Mallone F, et al. Attenuation of choroidal thickness in patients with Alzheimer disease: evidence from an Italian prospective study. Alzheimer Dis Assoc Disord. 2017;31:128–34.
Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement (Amst). 2015;1:144–51.
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54:S204–17.
Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2:e93621.
Tian T, Zhang B, Jia Y, Li Z. Promise and challenge: the lens model as a biomarker for early diagnosis of Alzheimer’s disease. Dis Markers. 2014;2014:826503.
Goldstein LE, Muffat JA, Cherny RA, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361:1258–65.
Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1–42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49:106–8.
Prakasam A, Muthuswamy A, Ablonczy Z, et al. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J Alzheimers Dis. 2010;20:1243–53.
Jentsch S, Schweitzer D, Schmidtke K-U, et al. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmol. 2015;93:e241–7.
Martins RN, Villemagne V, Sohrabi HR, et al. Alzheimer’s disease: a journey from amyloid peptides and oxidative stress, to biomarker technologies and disease prevention strategies-gains from AIBL and DIAN cohort studies. J Alzheimers Dis. 2018;62:965–92.
Kim BJ, Irwin DJ, Song D, et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology. 2017;89:1604–11.
Kim BJ, Grossman M, Song D, et al. Persistent and progressive outer retina thinning in frontotemporal degeneration. Front Neurosci. 2019;13:298.
Granholm EL, Panizzon MS, Elman JA, et al. Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J Alzheimers Dis. 2017;56:1419–28.
Örnek N, Dağ E, Örnek K. Corneal sensitivity and tear function in neurodegenerative diseases. Curr Eye Res. 2015;40:423–8.
Dehghani C, Frost S, Jayasena R, et al. Morphometric changes to corneal dendritic cells in individuals with mild cognitive impairment. Front Neurosci. 2020;14: 556137.
Ponirakis G, Al Hamad H, Sankaranarayanan A, et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann Clin Transl Neurol. 2019;6:689–97.
Al-Janahi E, Ponirakis G, Al Hamad H, et al. Corneal nerve and brain imaging in mild cognitive impairment and dementia. J Alzheimers Dis. 2020;77:1533–43.
Gijs M, Nuijts RM, Ramakers I, Verhey F, Webers CAB. Differences in tear protein biomarkers between patients with Alzheimer’s disease and controls. Invest Ophthalmol Vis Sci. 2019;60:1744–1744.
Kenny A, Jiménez-Mateos EM, Zea-Sevilla MA, et al. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci Rep. 2019;9:15437.
Wang Y-R, Chuang H-C, Tripathi A, et al. High-sensitivity and trace-amount specimen electrochemical sensors for exploring the levels of β-amyloid in human blood and tears. Anal Chem. 2021;93:8099–106.
Kalló G, Emri M, Varga Z, et al. Changes in the chemical barrier composition of tears in Alzheimer’s disease reveal potential tear diagnostic biomarkers. PLoS ONE. 2016;11: e0158000.
Huang J, Li Y, Xiao J, et al. Combination of multifocal electroretinogram and spectral-domain OCT can increase diagnostic efficacy of Parkinson’s disease. Parkinsons Dis. 2018;2018:4163239.
Stemplewitz B, Keserü M, Bittersohl D, et al. Scanning laser polarimetry and spectral domain optical coherence tomography for the detection of retinal changes in Parkinson’s disease. Acta Ophthalmol. 2015;93:e672–7.
Unlu M, Gulmez Sevim D, Gultekin M, Karaca C. Correlations among multifocal electroretinography and optical coherence tomography findings in patients with Parkinson’s disease. Neurol Sci. 2018;39:533–41.
Christou EE, Konitsiotis S, Pamporis K, et al. Inner retinal layers’ alterations of the microvasculature in early stages of Parkinson’s disease: a cross sectional study. Int Ophthalmol. 2023;43:2533–43.
Christou EE, Asproudis I, Asproudis C, Giannakis A, Stefaniotou M, Konitsiotis S. Macular microcirculation characteristics in Parkinson’s disease evaluated by OCT-angiography: a literature review. Semin Ophthalmol. 2022;37:399–407.
Kwapong WR, Ye H, Peng C, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22.
Çomoğlu SS, Güven H, Acar M, Öztürk G, Koçer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson’s disease. Neurosci Lett. 2013;553:63–7.
Andrade C, Beato J, Monteiro A, et al. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s disease. Mov Disord. 2016;31:377–83.
Di Maio LG, Montorio D, Peluso S, et al. Optical coherence tomography angiography findings in Huntington’s disease. Neurol Sci. 2021;42:995–1001.
Petzold A, De Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–32.
Britze J, Frederiksen JL. Optical coherence tomography in multiple sclerosis. Eye. 2018;32:884–8.
Paul F, Calabresi PA, Barkhof F, et al. Optical coherence tomography in multiple sclerosis: a 3-year prospective multicenter study. Ann Clin Transl Neurol. 2021;8:2235–51.
Montolío A, Martín-Gallego A, Cegoñino J, et al. Machine learning in diagnosis and disability prediction of multiple sclerosis using optical coherence tomography. Comput Biol Med. 2021;133:104416.
López-Dorado A, Ortiz M, Satue M, et al. Early diagnosis of multiple sclerosis using swept-source optical coherence tomography and convolutional neural networks trained with data augmentation. Sensors (Basel). 2021;22:167.
Coyle PK, Sibony PA. Tear analysis in multiple sclerosis. Neurology. 1986;36:547–50.
Coyle PK, Sibony P, Johnson C. Oligoclonal IgG in tears. Neurology. 1987;37:853–6.
Martino G, Servalli C, Filippi M, et al. Absence of oligoclonally restricted immunoglobulins in tears from multiple sclerosis patients. J Neuroimmunol. 1993;44:149–55.
Devos D, Forzy G, de Seze J, et al. Silver stained isoelectrophoresis of tears and cerebrospinal fluid in multiple sclerosis. J Neurol. 2001;248:672–5.
Mavra M, Thompson EJ, Nikolic J, et al. The occurrence of oligoclonal IgG in tears from patients with MS and systemic immune disorders. Neurology. 1990;40:1259–62.
Lebrun C, Forzy G, Collongues N, et al. Tear analysis as a tool to detect oligoclonal bands in radiologically isolated syndrome. Rev Neurol (Paris). 2015;171:390–3.
Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteomics Clin Appl. 2014;8:185–94.
Ringelstein M, Albrecht P, Südmeyer M, et al. Subtle retinal pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2014;1:290–7.
Abdelhak A, Hübers A, Böhm K, Ludolph AC, Kassubek J, Pinkhardt EH. In vivo assessment of retinal vessel pathology in amyotrophic lateral sclerosis. J Neurol. 2018;265:949–53.
Westman J, Eriksson SV, Gissler M, et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci. 2018;27:519–27.
Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn. 2015;2:46–55.
Kazakos CT, Karageorgiou V. Retinal changes in schizophrenia: a systematic review and meta-analysis based on individual participant data. Schizophr Bull. 2019. https://doi.org/10.1093/schbul/sbz106.
Samani NN, Proudlock FA, Siram V, et al. Retinal layer abnormalities as biomarkers of schizophrenia. Schizophr Bull. 2018;44:876–85.
Kurt A, Ramazan Zor K, Küçük E, Yıldırım G, Erdal EE. An optical coherence tomography study that supports the neurovascular basis of schizophrenia disease. Alpha Psychiatry. 2022;23:12–7.
Bannai D, Lizano P, Kasetty M, et al. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: a preliminary study. Psychiatry Res Neuroimaging. 2020;299:111061.
Green KM, Choi JJ, Ramchandran RS, Silverstein SM. OCT and OCT angiography offer new insights and opportunities in schizophrenia research and treatment. Front Digit Health. 2022;4:836851.
Cumurcu T, Keser S, Cumurcu BE, Gunduz A, Kartalci S. Refraction and eye anterior segment parameters in schizophrenic patients. Arq Bras Oftalmol. 2015;78:180–4.
Shiloh R, Munitz H, Portuguese S, et al. Corneal temperature in schizophrenia patients. Int J Neuropsychopharmacol. 2005;8:537.
Xu L, Nguyen JV, Lehar M, et al. Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol. 2016;275(Pt 3):436–49.
Childs C, Barker LA, Gage AM, Loosemore M. Investigating possible retinal biomarkers of head trauma in Olympic boxers using optical coherence tomography. Eye Brain. 2018;10:101–10.
Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18:165–80.
Alé-Chilet A, Bernal-Morales C, Barraso M, et al. Optical coherence tomography angiography in type 1 diabetes mellitus—report 2: diabetic kidney disease. J Clin Med. 2021;11:197.
Bernal-Morales C, Alé-Chilet A, Martín-Pinardel R, et al. Optical coherence tomography angiography in type 1 diabetes mellitus. Report 4: glycated haemoglobin. Diagnostics. 2021;11:1537.
Yeung L, Sun C-C, Ku W-C, et al. Associations between chronic glycosylated haemoglobin (HbA1c) level and macular volume in diabetes patients without macular oedema. Acta Ophthalmol. 2010;88:753–8.
Kim BR, Yoo TK, Kim HK, et al. Oculomics for sarcopenia prediction: a machine learning approach toward predictive, preventive, and personalized medicine. EPMA J. 2022;13:367–82.
Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33:737–48.
Gerrits N, Elen B, Craenendonck TV, et al. Age and sex affect deep learning prediction of cardiometabolic risk factors from retinal images. Sci Rep. 2020;10:9432.
Zhang K, Liu X, Xu J, et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat Biomed Eng. 2021;5:533–45.
Nguyen-Khuong T, Everest-Dass AV, Kautto L, Zhao Z, Willcox MDP, Packer NH. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology. 2015;25:269–83.
Kim H-J, Kim P-K, Yoo H-S, Kim C-W. Comparison of tear proteins between healthy and early diabetic retinopathy patients. Clin Biochem. 2012;45:60–7.
Park KS, Kim SS, Kim JC, et al. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am J Ophthalmol. 2008;145:432–7.
Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4:271–85.
Pinhas A, Migacz JV, Zhou DB, et al. Insights into sickle cell disease through the retinal microvasculature. Ophthalmol Sci. 2022;2:100196.
Courtie E, Veenith T, Logan A, Denniston AK, Blanch RJ. Retinal blood flow in critical illness and systemic disease: a review. Ann Intensive Care. 2020;10:152.
Pinhas A, Zhou DB, Otero-Marquez O, et al. Efficacy of CRISPR-based gene editing in a sickle cell disease patient as measured through the eye. Case Rep Hematol. 2022;2022:1–6.
Keel S, Wu J, Lee PY, Scheetz J, He M. Visualizing deep learning models for the detection of referable diabetic retinopathy and glaucoma. JAMA Ophthalmol. 2019;137:288.
Lee AY, Campbell JP, Hwang TS, Lum F, Chew EY. Recommendations for standardization of images in ophthalmology. Ophthalmology. 2021;128:969–70.
Enoch J, McDonald L, Jones L, Jones PR, Crabb DP. Evaluating whether sight is the most valued sense. JAMA Ophthalmol. 2019;137:1317–20.
Denniston AK, Kale AU, Lee WH, Mollan SP, Keane PA. Building trust in real-world data: lessons from INSIGHT, the UK’s health data research hub for eye health and oculomics. Curr Opin Ophthalmol. 2022;33:399–406.
Babenko B, Mitani A, Traynis I, et al. Detection of signs of disease in external photographs of the eyes via deep learning. Nat Biomed Eng. 2022;6:1370–83.
Di Filippo D, Sunstrum FN, Khan JU, Welsh AW. Non-invasive glucose sensing technologies and products: a comprehensive review for researchers and clinicians. Sensors (Basel). 2023;23:9130.
Funding
This research, including the journal’s Rapid Service fee, was funded by Occuity.
Author information
Authors and Affiliations
Contributions
Conceptualization: Emily Patterson, Richard Kadri-Langford; Methodology: Emily Patterson; Formal analysis and investigation: Emily Patterson; Writing—original draft preparation: Emily Patterson; Funding acquisition: Dan Daly; Resources: Dan Daly; Supervision: Dan Daly. Emily Patterson, Alistair Bounds, Siegfried Wagner, Richard Kadri-Langford, Robin Taylor, and Dan Daly read, reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Emily Patterson, Alistair Bounds, Richard Kadri-Langford, Robin Taylor and Dan Daly are employed by Occuity. Alistair Bounds and Richard Kadri-Langford are investors in Occuity. Robin Taylor and Dan Daly are investors in, co-founders and board members of, and hold patents with Occuity. Siegfried Wagner declares no conflicts of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Patterson, E.J., Bounds, A.D., Wagner, S.K. et al. Oculomics: A Crusade Against the Four Horsemen of Chronic Disease. Ophthalmol Ther 13, 1427–1451 (2024). https://doi.org/10.1007/s40123-024-00942-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00942-x