Abstract
Introduction
The aim of this work is to evaluate the real-world outcomes of the reinforced treat-and-extend (RTE) protocol for the treatment of exudative age-related macular degeneration with intravitreal injections of aflibercept or ranibizumab (anti-vascular endothelial growth factor therapies).
Methods
This was a retrospective review of patients from two tertiary ophthalmology centers in France initiating the RTE protocol between February 2018 and June 2021. The primary outcome was change in best-corrected visual acuity (BCVA) after 24 months. Secondary outcomes were change in central retinal thickness (CRT), recurrence, and management-related factors (injection interval, number of injections/consultations). Outcomes were additionally evaluated after protocol changes (strict versus modified RTE protocol groups).
Results
Sixty-eight patients (72 eyes) were included (68% females; mean age 82.2 ± 7.8 years). After 24 months, mean BCVA significantly improved (65.22 ± 14 vs. 71.96 ± 13 Early Treatment Diabetic Retinopathy Study letters; p < 0.001) and CRT significantly decreased (388.6 ± 104 vs. 278.8 ± 51 μM; p < 0.001) with 21% of eyes showing signs of exudation. Over the 24 months, a mean total of 14.9 ± 4.0 injections and 8.6 ± 1.4 consultations were performed. Mean 24-month injection interval was 7.9 ± 2.3 weeks. Initial and 24-month ophthalmic outcomes for eyes in the strict (47%) versus modified (53%) groups were not significantly different, but mean time interval to first recurrence of disease activity was significantly shorter for the modified group (7.3 ± 2.4 vs. 9.9 ± 2.5 weeks; p < 0.001). Patients in the strict RTE group received significantly less injections (13.9 ± 3.6 vs. 16.5 ± 3.9; p = 0.006) and mean 24-month injection interval was significantly longer (9.5 ± 2.7 vs. 6.5 ± 2.1 weeks; p < 0.001). Consultation number was similar (8.5 ± 1.9 vs. 8.8 ± 1.6; p = 0.93). Treatment with aflibercept versus ranibizumab did not influence ophthalmic or management outcomes.
Conclusions
The RTE protocol, even when modified, reduced consultations but improved ophthalmic outcomes. The RTE protocol could reduce hospital visits and overall burden while also encouraging better patient compliance.
Video Abstract available for this article.
VIDEO ABSTRACT: Vincent Soler and François-Philippe Roubelat summarize the Reinforced Treat-and-Extend Protocol and main results (MP4 225022 KB)
Similar content being viewed by others
Why carry out this study? |
Protocols with reduced frequency of intravitreal anti-vascular endothelial growth factor injections are sought for patients with age-related macular degeneration in light of reduced therapeutic burden and better compliance. |
We assessed the real-world 2-year outcomes of the new reinforced treat-and-extend protocol that is expected to optimize and personalize treatment, while at the same time reducing number of patient visits. |
What was learned from the study? |
The reinforced treat-and-extend protocol maintained the total number of injections and reduced consultation number, but patient functional and anatomical outcomes were improved similarly to other protocols already reported in the literature. |
Investigation of this protocol use in different settings and with different drugs should confirm a reduced therapeutic burden via a decrease in hospital visits, which should in turn encourage better patient compliance. |
Digital Features
This article is published with digital features, including a Video Abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25398610.
Introduction
The management and prognosis of patients with exudative age-related macular degeneration (AMD) has radically improved since the advent of anti-vascular endothelial growth factor (VEGF) therapy. Indeed, a study conducted in Denmark [1] has shown a 50% decrease in the incidence of legal blindness caused by AMD following the introduction of anti-VEGF therapy. This reduction has since been further confirmed by Skaat et al. [2].
The initial key phase III ANCHOR [3] and MARINA [4] studies demonstrated the efficacy of fixed monthly intravitreal (IVT) injections of ranibizumab. Other studies aiming at reducing the number of injections (i.e., the PIER [5] and EXCITE [6] studies) have proposed fixed quarterly ranibizumab IVT injections after an induction phase of three monthly injections. The best compromise at the time was considered the protocol from the VIEW [7] study evaluating the efficacy of aflibercept with IVT injections at 2-month intervals following the induction phase. Indeed, the outcomes were similar to those from studies evaluating ranibizumab with fixed monthly injections. In parallel, a personalized (when required) protocol, known as ProReNata (PRN) [8, 9], was developed to avoid over-treatment; injections were only received in the event of recurrence (after an induction phase similar to those previously described). However, this required strict monthly monitoring to detect signs of recurrence at an early stage. The major disadvantages of the PRN protocol are thus the retroactive nature with the time-consuming follow-up; the latter remaining a challenge in real-life for both patients and ophthalmologists.
In light of progressively increasing IVT injection intervals and reducing the number of hospital visits, Spaide [10] proposed a treat-and-extend (T&E) protocol while sustaining maintenance dose for disease control. Here, each IVT injection is preceded by a consultation and so the patient is treated proactively; i.e., before recurrence. The T&E protocol gave clinically comparable results when compared to fixed monthly injections [11,12,13,14]. Numerous studies have since confirmed the efficacy of this protocol [15,16,17,18,19], leading to its consideration as the reference for the management of neovascular AMD [20]. However, despite a reduction in the number of visits over time with this T&E protocol, there remains a heavy therapeutic burden given each visit requires macular assessment and IVT injection with scheduling of the next injection interval. A study conducted in France [21] illustrated these difficulties in a real-world setting, with substantial protocol inaccuracies due to absences or delays.
At the same time, a study by Mantel et al. [22] put forward yet another protocol called Observe and Plan (O&P). This protocol has an induction phase comprising three initial monthly IVT injections followed by monthly monitoring until first recurrence (same as the PRN protocol). An injection series is then scheduled with intervals equal to the recurrence interval reduced by 2 weeks. When the patient has completed this series of injections, a consultation is scheduled at the same interval as the last injection to then adjust the interval of the next injections.
Overall, both the aforementioned T&E and O&P protocols have highlighted the need of protocol adjustment to the therapeutic demand of each patient, and thus a more personalized approach to injection rate that is not possible with a fixed schedule. Indeed, a recent review has already discussed reducing IVT injection frequency for patients with AMD in light of better compliance and reduced therapeutic burden [23]. In this light, in 2018 we started using the reinforced treat-and-extend (RTE) protocol detailed in Fig. 1. The reinforced nature of this protocol lies in the intense proactive phase used for determining the injection interval of the maintenance phase. Briefly, the patient enters the maintenance phase according to the time interval to the first recurrence minus 2 weeks, with the patient receiving several IVT injections between scheduled consultations. This way, the patient receives several IVT injections at the same interval, but then the interval is adjusted every 4–6 months. Hence, we expect the total number of follow-up consultations to be reduced over time. The overall aim of this RTE protocol was thus to optimize and personalize treatment, while at the same time to reduce the number of patient visits and thus the time spent at the ophthalmology center.
Our reinforced treat-and-extend protocol for treatment of age-related macular degeneration with intravitreal anti-vascular endothelial growth factor injections. The induction phase consists of three monthly IVT injections, then injection number 4 is coupled with a follow-up consultation at 6 weeks after the third injection. During this same visit, if the patient shows no signs of exudation, then a new consultation combined with IVT injection is scheduled at 8 weeks later instead of 6 weeks later; thus the classical proactive T&E approach is adopted. At the first signs of exudative reappearance (recurrence of disease activity), the patient then enters the maintenance phase. This consists of a series of IVT injections with intervals corresponding to: time interval to recurrence of disease activity (in weeks) minus 2 weeks. Both the number of injections scheduled and injection intervals depend automatically on the recurrence interval, with exception of recurrence at 4–6 weeks: four IVT injections are scheduled with intervals of 4 weeks. Thereafter, four IVT injections are scheduled with intervals of 6 weeks for recurrence at 8 weeks, three IVT injections are scheduled with intervals of 8 weeks for recurrence at 10 weeks, etc. The patient only undergoes follow-up consultation for the last IVT injection in the series. Here, the decision is made to adjust the interval (extend or reduce by 2 weeks) according to the presence or absence of exudative recurrence. Discontinuation of the RTE protocol is considered after reaching no exudation after two IVT injections at a 12-week interval. AMD age-related macular degeneration, IVT intravitreal, RTE reinforced treat-and-extend, T&E treat-and-extend
Here, we assessed for the first time the real-world effectiveness of this RTE protocol for treatment-naive exudative AMD among patients from two tertiary ophthalmology centers in France. We evaluated first if the RTE protocol resulted in improved functional and anatomical outcomes after 2 years. Secondly, we evaluated how the RTE protocol influenced the global management of AMD (i.e., total injections/consultation numbers) compared to reports in the literature to determine whether over-treatment and hospital visits could be limited. We additionally investigated whether there were different outcomes when the RTE protocol was not strictly followed in our real-world setting.
Methods
Study Design
We conducted a non-randomized, multicentric, retrospective, cohort study of medical records from patients diagnosed with treatment-naive AMD and initiating treatment with anti-VEGF therapy according to the RTE protocol between February 1, 2018 and June 30, 2021. Patients were included from two tertiary ophthalmology centers in the South of France [Toulouse University Hospital and Clinique Honoré Cave (Montauban)]. The entire cohort is named total cohort. From here, we then created groups of patients: those that underwent rigorous application of the RTE protocol (strict RTE protocol) versus those that underwent an RTE protocol with some changes (modified RTE protocol). We also divided the total cohort into patients treated with ranibizumab versus aflibercept.
All procedures performed were part of routine care, and both in accordance with institutional guidelines and with the principles and regulations of the Helsinki Declaration of 1964 and its later amendments. An official waiver of ethical approval was granted from Toulouse University Hospital (the master ethics committee, study reference: 2023-139) in accordance with Clinique Honoré Cave. This is given the retrospective and non-interventional nature of the study as asserted by the French Jardé Ethical and Regulatory law. The study additionally complies with French MR-004 methodology (CNIL 2206723 v 0) covering data protection for both centers. Informed patient consent was obtained from all participants before inclusion and all data were anonymized for publication.
Patients
Patients included were > 50 years old and diagnosed with treatment-naive exudative AMD. AMD was detected by small, focal retinal hemorrhages and/or other features of exudation in fundus images or by optical coherence tomography (OCT). OCT features included fibrovascular or serous pigment epithelial detachment (PED) and the presence of sub- and/or intra-retinal fluid (intra-retinal cysts). Patients included underwent anti-VEGF therapy according to the RTE protocol initiated within a maximum of 4 days from diagnosis of AMD. Only patients with 24 months of follow-up from diagnosis of AMD were included.
Exclusion criteria were pre-existing retinal or macular pathology, the presence of an ophthalmic pathology related with a loss in visual acuity for which no treatment could be indicated, absence of light perception, and macular hemorrhage. Patients were also only included if they were affiliated to the French social security system (or an individual beneficiary scheme), not participating in any other clinical research studies, and not under legal safeguarding/guardianship/curatorship.
The RTE Protocol
The reinforced treat-and-extend (RTE) protocol is detailed in Fig. 1. This begins with an induction phase consisting of three monthly IVT injections [24], then injection no. 4 is coupled with a follow-up consultation at 6 weeks after the third injection. During this same visit, if the patient shows no signs of exudation, then a new consultation combined with IVT injection is scheduled at 8 weeks later instead of 6 weeks later; thus a classical T&E approach is adopted. At first signs of exudative reappearance (recurrence of disease activity), the patient then enters the maintenance phase. This consists of a series of IVT injections with intervals corresponding to the time interval to recurrence of disease activity (in weeks) minus 2 weeks. Both the number of injections scheduled and injection intervals depend automatically on the recurrence interval, with exception of recurrence at 4–6 weeks: here, four IVT injections are scheduled with intervals of 4 weeks. Thereafter, four IVT injections are scheduled with intervals of 6 weeks for recurrence at 8 weeks, three IVT injections are scheduled with intervals of 8 weeks for recurrence at 10 weeks, two IVT injections are scheduled with intervals of 10 weeks for recurrence at 12 weeks, and two IVT injections are scheduled with intervals of 12 weeks for no recurrence by 12 weeks. Note that the patient only undergoes follow-up consultation for the last IVT injection in the series. Here, the decision is made to adjust the interval (extend or reduce by 2 weeks) according to the presence or absence of exudative recurrence. Overall, Fig. 1 shows how patients are seen in consultation at a maximum of every 4 months (four IVT injections at 4-week intervals) and at least every 6 months (two IVT injections at 12-week intervals). The injection interval for both eyes of patients with bilateral AMD was based on the most severely affected eye [25]. Discontinuation of the RTE protocol is only considered after reaching no exudation after two IVT injections at a 12-week interval; i.e., the patient has been exudative-free for almost 1 year. The ophthalmologist then has two options: (1) start quarterly IVT injections (with bi-annual follow-up consultations), or (2) stop IVT injections but monitor every 3 months.
The Strict RTE Protocol
A maximum 3-week delay was tolerated in the RTE protocol for patients included in the strict RTE group given that the study period overlapped with the SARS-CoV-2 pandemic. Indeed, some patients had their IVT injections delayed by ≤ 3 weeks due to the closure of outpatient departments, making it thus impossible to carry out IVT injections and forcing us to refer some patients to different centers. This delay was only accepted once and occurred in majority to patients in their maintenance phase.
The Modified RTE Protocol
Patients included in the modified RTE protocol group initiated the same RTE protocol, but the protocol then varied from the strict protocol as a result of a change in schedule (i.e., patient choice, patient unavailability, ophthalmologist preference, the SARS-CoV-2 pandemic). Changes were either during the induction phase (with injection no. 4 given at 4 weeks rather than 6 weeks after injection no. 3) or during the maintenance phase (a different number of injections was received than expected considering the recurrence interval). Note that some patients even sustained the same injection interval instead of it being shortened or extended according to the RTE protocol. Finally, patients with delays > 3 weeks or repeated delays were included in the modified RTE protocol group. Refer to Table 1 for specific example cases of patients included in the modified RTE protocol group. Patients excluded from the modified group (and thus the entire study) were those starting maintenance phase without the prior interval determination phase, those that switched back to interval determination phase from maintenance phase, and those that underwent a switch of anti-VEGF therapy during follow-up. The indication for patients requiring a switch of therapy in both centers was to start the RTE protocol at induction phase again.
Ophthalmic Assessments
Each consultation included measurement of visual acuity, dilated fundus examination, and OCT imaging (Spectralis® OCT (Heidelberg, Germany) in Toulouse and Swept-Source (SS) DRI-OCT-Triton (Topcon Corporation, Japan) in Montauban). To convert SS-OCT data, we calculated central retinal thicknesses (CRT) using the formula SD-OCT = 33.53 + 0.994 × SS-OCT [26]. The Monoyer scale for distance visual acuity was used and then converted into logMAR and into Early Treatment Diabetic Retinopathy Study (ETDRS) letters [27].
Intravitreal Injections
IVT injections of anti-VEGF therapy were carried out in accordance with guidelines from the French Ophthalmology Society and the French Hospital Hygiene Society [28] and the anti-VEGF drug Summaries of Product Characteristics. We used ranibizumab (Lucentis® [29], Novartis Europharm Limited) and aflibercept (Eylea® [30], Bayer Healthcare). Both have marketing authorization for the treatment of neovascular AMD in France. Drug choice and criteria for retreatment during follow-up (signs of exudation by OCT imaging) were left to the ophthalmologists’ discretions.
Outcome Measures
The primary outcome was change in mean best-corrected visual acuity (BCVA) after 24 months of follow-up from diagnosis of AMD and scheduling of an RTE protocol. The secondary outcomes were change in mean CRT after 24 months of follow-up and eyes developing signs of exudation, number of eyes with signs of exudation at 24 months, mean time interval to first recurrence of disease activity, and number of eyes not developing signs of exudation over the entire 24-month follow-up period (no recurrence of disease activity). These latter patients were considered as very good responders (the “happy few” [31]) and underwent progressive injection interval prolongation followed or not by discontinuation of IVT injections. Treatment discontinuation was according to the ophthalmologists’ discretion and patients’ wishes. We additionally analyzed endpoints related to the global management of AMD: (1) mean injection interval at 24 months, (2) number of patients with ≥ 12-week injection interval at 24 months, (3) total number of IVT injections over the 24 months, and (4) total number of consultations over the 24 months. Finally, complications and adverse events were recorded.
Statistical Analyzes
Continuous variables are expressed as means with standard deviations and categorical variables as frequencies/percentages. We used the non-parametric Mann–Whitney U test in the R software (BiostatTGV http://biostatgv.sentiweb.fr/ from the Pierre Louis Institute of Epidemiology and Public Health, France) to make comparisons between different patient groups. We used the non-parametric Wilcoxon signed-rank test for paired samples to compare changes in BCVA and CRT over time within patient groups. A p value of 0.05 was considered statistically significant.
Results
Initial Demographic and Clinical Characteristics
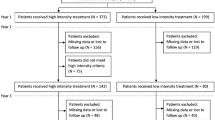
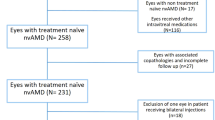
A total of 202 patients (206 eyes, four patients with bilateral AMD) were diagnosed with treatment-naive AMD and were scheduled to initiate an RTE protocol between February 1, 2018 and June 30, 2021 in both centers combined. Then, a total of ten practicing ophthalmologists initiated an RTE protocol within a maximum of 4 days from diagnosis of AMD. Among these 202 patients, exclusions were: 56 (28%) patients due to < 24 months of follow-up, 34 (17%) patients due to loss to follow-up, 42 (21%) patients that underwent an injection schedule deviating too far from even the modified RTE protocol, and two (1%) patients that voluntarily stopped IVT injections. Overall, 68 patients (72 eyes) were included for study with 24 months of follow-up (referred to as total). Among these, approximately half of the patients underwent each RTE protocol type: 33 (49%) patients [34 (47%) eyes] underwent a strict RTE protocol and 35 (51%) patients [38 (53%) eyes] underwent a modified RTE protocol. Finally, slightly more eyes received aflibercept in the total cohort [n = 42 (58%) eyes received aflibercept versus n = 30 (42%) eyes received ranibizumab]. Please refer to Fig. 2 for the study flowchart.
The demographic and initial characteristics of our total cohort and protocol groups are summarized in Table 2. Briefly, there was a female predominance (0.68) and the mean age was 82.2 ± 7.8 years. The right eye was affected in 58% of cases. The mean initial BCVA was 65.22 ± 14 ETDRS letters and the mean CRT was 388.6 ± 104 µm. Overall, we found no differences when comparing these characteristics between the strict and modified RTE protocol groups. Again, we found a trend for slightly more eyes receiving aflibercept in both protocol groups (56% received aflibercept in the strict and 61% received aflibercept in the modified RTE protocol groups; p = 0.7).
24-Month Ophthalmic Outcomes
Figure 3 shows the significant improvement in mean BCVA over the 24 months of follow-up compared to before treatment in the total cohort (initial 65.22 ± 14 vs. 71.96 ± 13 ETDRS letters after 24 months; p < 0.001). Mean BCVA also significantly improved in both protocol groups: strict RTE group (initial 66.41 ± 13 vs. 73.99 ± 10 ETDRS letters after 24 months; p < 0.001) and modified RTE group (initial 64.35 ± 15 vs. 70.24 ± 15 ETDRS letters after 24 months; p = 0.005). Mean BCVA after 24 months was comparable between the strict and modified RTE protocol groups (73.99 ± 10 vs. 70.24 ± 15 ETDRS letters; p = 0.713). We observed an almost identical increase in mean BCVA after 3 months, but this was followed by a sharper increase from 3 months to 1 year for the modified RTE group compared to a more progressive increase in the strict RTE group. The mean BCVA gain at 24 months was + 6.74 ± 12 ETDRS letters for the total cohort, with no difference between the two protocol groups (strict + 7.57 ± 13 vs. modified + 5.89 ± 11 ETDRS letters; p = 0.552) (data summarized in Table 3).
Change in mean BCVA over time from diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 72 eyes) as well as the strict (n = 34 eyes) and modified (n = 38 eyes) RTE protocol groups. The non-parametric Wilcoxon signed-rank test for paired samples was performed to compare mean BCVA from before treatment to after 24 months in each group. **p ≤ 0.01; ***p ≤ 0.001. BCVA best-corrected visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, RTE reinforced treat-and-extend
Figure 4 highlights the significant decrease in mean CRT over the 24 months of follow-up compared to before treatment for the total cohort (initial 388.6 ± 104 vs. 278.8 ± 51 μM after 24 months; p < 0.001). Mean CRT also significantly decreased in both protocol groups: strict RTE group (initial 380 ± 93 vs. 276.4 ± 46 μM after 24 months; p < 0.001) and modified RTE group (initial 396.4 ± 114 vs. 281.0 ± 54 μM after 24 months; p < 0.001). The mean reduction in CRT after 24 months for the total cohort was − 109.8 ± 88 µm, and again showed no difference (p = 0.61) between the strict RTE group (− 103.6 ± 69 µm) versus modified RTE group (− 115.4 ± 125 µm) (data summarized in Table 3). Mean CRT after 24 months was 278.8 ± 51 µm for the total cohort, and showed no difference (p = 0.10) between the strict RTE group (276.4 ± 46 µm) compared to the modified RTE group (281.0 ± 54 µm). Note that by after 3 months, CRT had decreased by − 87.0 ± 83 µm in the total cohort, and by − 74.9 ± 65 µm in the strict RTE group and by − 98.3 ± 96 µm in the modified RTE group. This reduction was similar in both protocol groups (p = 0.554) (data not shown). This means that mean reduction in CRT had already reached 80% of its total 24-month reduction by after 3 months for the total cohort; this was 73% for the strict RTE group and 85% for the modified RTE group.
Change in mean CRT over time from diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 72 eyes) as well as the strict (n = 34 eyes) and modified (n = 38 eyes) RTE protocol groups. The non-parametric Wilcoxon signed-rank test for paired samples was performed to compare mean CRT from before treatment to after 24 months in each group. ***p ≤ 0.001. CRT central retinal thickness, RTE reinforced treat-and-extend
Signs of exudation were detected in 21% of eyes in the total cohort at 24 months with a similar proportion of eyes showing signs of exudation between both protocol groups (21% in both groups; p = 0.97). The mean time interval to first recurrence of disease activity was 8.4 ± 2.7 weeks for the total cohort; this was significantly shorter for the modified RTE group than the strict RTE group (7.3 ± 2.4 vs. 9.9 ± 2.5 weeks; p < 0.001). In line with these findings, significantly more eyes never developed signs of disease activity over the 24-month follow-up period in the strict RTE group than in the modified RTE group: no recurrence among 26.5% of eyes in the strict RTE group vs. 2.6% of eyes in the modified RTE group (p = 0.005) (data summarized in Table 3). Finally, there were no serious adverse events or complications during follow-up.
24-Month Management Outcomes
Patients in the total cohort received a total of 14.9 ± 4.0 IVT injections over the 24 months of follow-up. Patients in the strict RTE group received significantly less IVT injections compared to the modified RTE group at both after 24 months (13.9 ± 3.6 vs. 16.5 ± 3.9; p = 0.006) and after 12 months (8.1 ± 1.4 vs. 9.3 ± 1.4; p < 0.001) (Fig. 5 and Table 3). The mean injection interval at 24 months was 7.9 ± 2.3 weeks for the total cohort, with 18 (25%) patients having reached a ≥ 12-week injection interval. Among these 18 patients, we decided to stop IVT injections for six patients and sustain a 12-week injection interval for the other 12 patients. Mean injection interval at 24 months was significantly longer for the strict RTE group compared to the modified RTE group (9.5 ± 2.7 vs. 6.5 ± 2.1 weeks; p < 0.001). The six patients for whom we had already decided to stop IVT injections at 24 months were not included in this analysis. Significantly more eyes reached a ≥ 12-week injection interval at 24 months in the strict RTE protocol group than in the modified RTE group (47% vs. 5%: p < 0.001). This analysis included patients for whom we had already decided to stop IVT injections (Table 3). We found the same trend for eyes reaching a ≥ 10-week injection interval after 24 months: 56% of eyes in the strict RTE group versus 11% in the modified RTE group (p < 0.001) (data summarized in Fig. 6).
Mean number of intravitreal injections at 12 months and 24 months after diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 72 eyes) as well as the strict (n = 34 eyes) and modified (n = 38 eyes) RTE protocol groups. The non-parametric Mann–Whitney U test was used to compare between strict and modified groups at each time point. **p ≤ 0.01; ***p ≤ 0.001. RTE reinforced treat-and-extend
Injection interval at 24 months after diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 72 eyes) as well as the strict (n = 34 eyes) and modified (n = 38 eyes) RTE protocol groups. The non-parametric Mann–Whitney U test was used to compare between strict and modified groups at each time interval. ***p ≤ 0.001. RTE reinforced treat-and-extend
Patients in the total cohort underwent a mean total of 8.6 ± 1.4 consultations over the 24 months of follow-up and we found no difference between the strict and modified RTE protocol groups at both after 24 months (8.5 ± 1.9 vs. 8.8 ± 1.6; p = 0.93) and after 12 months (5.9 ± 1.3 vs. 5.5 ± 1.4; p = 0.15) (Fig. 7 and Table 3).
Mean number of consultations 12 months and 24 months after diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 68 patients) as well as the strict (n = 33 patients) and modified (n = 35 patients) RTE protocol groups. The non-parametric Mann–Whitney U test was used to compare between groups at each time point, but no significant differences were found. RTE reinforced treat-and-extend
Outcomes with Aflibercept Versus Ranibizumab
We wanted to check that the results obtained so far were not influenced by any differences in outcomes between patients receiving ranibizumab versus aflibercept. To do this, first we compared the demographic and pre-treatment clinical characteristics of patients treated with ranibizumab versus aflibercept (Table 4). We found no difference in sex and age, as well as no difference in mean initial BCVA and mean initial CRT. Moreover, approximately 50% of patients underwent a strict RTE protocol and 50% a modified RTE protocol in both anti-VEGF therapy groups. Then, we compared outcomes between ranibizumab and aflibercept groups 24 months after diagnosis of AMD and scheduling of an RTE protocol (Table 5). Overall, we found no difference in mean BCVA and mean CRT. Mean reduction in CRT and mean BCVA gain (Fig. 8) were similar between patients receiving both therapies. In terms of management, after 24 months there were no differences in mean total number of IVT injections, mean injection interval, or mean total number of consultations.
Mean gain in BCVA over time from diagnosis of age-related macular degeneration and scheduling of an RTE protocol for the total cohort (n = 72 eyes) as well as according to anti-vascular endothelial growth factor therapy. No significant differences were found using the non-parametric Mann–Whitney U test to compare mean BCVA gains after 24 months between eyes receiving ranibizumab (n = 30) versus aflibercept (n = 42). BCVA best-corrected visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, RTE reinforced treat-and-extend
Discussion
This is the first study reporting the real-world 2-year outcomes of patients with treatment-naive AMD undergoing anti-VEGF therapy using the RTE protocol. Overall, patient functional and anatomical outcomes improved after 2 years, while both reducing the number of consultations (and hence hospital visits) but not affecting the total number of injections.
After 24 months of follow-up from scheduling of an RTE protocol, our total cohort (n = 72 eyes; 58% receiving aflibercept and 42% receiving ranibizumab) had a mean BCVA gain of 6.74 ± 12 ETDRS letters and a mean reduction in CRT of − 109.8 ± 88 μM. Over these 24 months, there was no recurrence of disease activity in 14% of eyes, and patients had received a mean total of 14.9 ± 4.0 IVT injections and undergone a mean total of 8.6 ± 1.4 follow-up consultations. At 24 months, patients had a mean injection interval of 7.9 ± 2.3 weeks, with six (9%) patients having already stopped IVT injections and 12 (17%) eyes having reached a 12-week injection interval. Finally, we detected no signs of adverse effects secondary to IVT injection of anti-VEGF therapy [32].
Comparing these outcomes to those reported in the literature over time, and thus according to the implementation of different treatment protocols, our mean BCVA gain is in line with results from initial key studies on the efficacy of different doses of ranibizumab: the ANCHOR [3] (8.1 ± 16/10.7 ± 17 ETDRS letters for 21.5/21.3 injections) and MARINA [4] (5.4–6.6 ETDRS letters for 24 injections) studies. Similarly, the VIEW [7] study reported mean gains of 7.6 ± 13 ETDRS letters after 1 year for patients receiving 2 mg aflibercept every 4 or every 8 weeks. Note, however, that patients in our study received less IVT injections than those in these three studies. The PrONTO study [8], testing a personalized protocol (such as PRN), demonstrated a mean 2-year BCVA gain of 11.1 ± 12.2 ETDRS letters after an average of 9.9 IVT injections. This result is indeed better than in our study, but the disadvantage of this protocol lies in the strict monthly monitoring (requiring consultations) to detect recurrence of disease activity. This imposes a heavy burden on both patients and healthcare staff, frequently leading to poor protocol compliance [23]. Next, O&P protocols [22, 33] after 2 years have demonstrated very similar results to our findings here: a mean BCVA gain of 6.2 ETDRS letters for 15.3 IVT injections and 6.8 consultations. However, the disadvantage of this protocol lies in the retroactive monitoring for the first recurrence and therefore risk of initial under-treatment. Lastly, different studies evaluating the T&E protocol [11, 14,15,16,17,18,19, 34, 35] show BCVA gains between 2.4 and 8.7 ETDRS letters for a mean total number of IVT injections ranging from 10.4 to 18.6. Our results here are thus approximately intermediate. It is noteworthy that another recent retrospective study was conducted in France on the treatment of 136 eyes with aflibercept using a T&E protocol [36]. This study was multicentric. The authors demonstrated a lower mean BCVA gain at 2 years (2.5 vs. 6.74 ETDRS letters here) for less IVT injections (11.4 vs. 14.9 here), but a similar number of consultations (8.9 vs. 8.6 here). Finally, focusing on the most recent meta-analysis by Matonti et al. [37] comparing different IVT anti-VEGF therapies using fixed, PRN, or T&E protocols (a total of 47 different studies), the T&E protocols were found to be the most efficient. Indeed, the T&E protocols showed durable improvements in visual acuity/CRT whilst reducing IVT injection number. Very similar to our present study, the T&E protocol studies included in the meta-analysis showed a mean BCVA gain of 6.4 ETDRS letters after 2 years for 14.6 IVT injections.
Comparing our strict RTE [n = 34 (47%) eyes] and modified RTE [n = 38 (53%) eyes] protocol groups, firstly we found no difference in anti-VEGF therapy received; slightly more eyes (59%) received aflibercept in both protocol groups. Improvements in mean BCVA and mean CRT after 24 months were similar between both protocol groups, as for the mean total number of consultations. On the other hand, patients in the modified RTE protocol group received significantly more IVT injections over the 24-month period (+ 2.6 injections), and their injection intervals at 24 months were significantly shorter (by 3 weeks) with significantly less patients reaching a ≥ 12-week injection interval (5% vs. 47%). Moreover, mean time interval to first recurrence was significantly longer (+ 2.3 weeks) in the strict RTE protocol group, with significantly more patients not developing exudation (i.e. the “happy few”) over the 24-month follow-up period (n = 9 vs. 1). Note that we found no differences in visual or management outcomes according to treatment with ranibizumab or aflibercept, meaning that the therapy received could not have influenced these aforementioned differences. We cannot rule out the potential for inclusion of patients developing more severe AMD in the modified group, meaning earlier signs of exudation and thus more injections. Nonetheless, we can conclude that variations in our RTE protocol did not affect 24-month visual recovery and the proactive nature of our RTE protocol meant injections were performed at an interval preceding recurrence and therefore likely limiting under-treatment.
Figure 9 summarizes 24-month mean BCVA gain versus mean total number of IVT injections for: (1) our total cohort, (2) our strict and modified RTE protocol groups, and (3) a selection of studies from the literature reporting on different protocols in different settings. Given circle size is inversely proportional to the mean total number of consultations, we can conclude that our RTE protocol (strict or modified) resulted in the maintenance of adequate improvements in BCVA after two years, but with a reduced consultation number. Indeed, the original aim of our RTE protocol was to reduce consultation number and hence patient hospital visits; we aimed for follow-up consultation every 20 ± 4 weeks, i.e. between 4 and 6 months, with injections scheduled on the same days as consultations. This approach avoids the need for strict monthly or close follow-up examinations [19]. The burden on both patients and the ophthalmologists is thus reduced using our RTE protocol. Monitoring at a given fixed interval also gives the patient a better understanding of their treatment protocol and a clearer view of their scheduled appointments. In all, reducing the number of visits would contribute to improving adherence to treatment [38].
Twenty-four-month mean gain in BCVA versus mean total number of intravitreal anti-vascular endothelial growth factor injections for patients undergoing our RTE protocol and from a selection of other cohorts from studies in the literature reporting on different protocols. Total cohort (tRTE: grey); strict protocol group (sRTE: dark blue); modified protocol group (mRTE: light blue). Circle size is inversely proportional to the mean total number of consultations ((1/mean total consultations) × 100); i.e., the larger the circle, the less consultations over 24 months. Rectangles denote studies not indicating total number of consultations. Note that we took into account findings from the patient group under an irregular treatment protocol for the RAINBOW study [24]. ALTAIR study [18]; ANCHOR study [3]; ARIES study [19]; ARMADA study [36]; Kim et al. [43]; MARINA study [4]; Matonti et al. [37]; Mekjavic et al. [44]; Observe and Plan (O&P) study [22]; TREX-AMD study [11]; PrONTO study [8]; Vardarinos et al. [45]; VIEW study [7]. BCVA best-corrected visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, RTE reinforced treat-and-extend
A study on a T&E protocol with ranibizumab in Canada [14] (237 patients) has shown that 43% of patients reached the maximum injection interval of 12 weeks. This was 56.9% and 60.2% in the ALTAIR study using 2 mg aflibercept depending on the T&E protocol used [18]. These values are comparable to our 47% for the strict RTE protocol group. The poorer results of the modified RTE protocol group (5% reaching a 12-week interval) could be explained by determination of an overly-reduced injection interval for the maintenance phase (see example case in Table 1), rather than prolonging the interval until signs of recurrence. This hints the potential for over-treatment among some patients in our real-world setting. In other real-world studies, patients have been described as more likely to be under-treated because they fail to attend follow-up consultations and therefore miss injections [21, 39]. Our study here is thus among those that rarely describe the potential for over-treatment in the event of a protocol change, which is always preferable to under-treatment for the patient.
There are several limitations to our RTE protocol. Firstly, we used a 6-week interval for injection no. 4 coupled with consultation in the induction phase, instead of a 4-week interval. The aim was to distinguish responders from early recurrences. Indeed, several studies have investigated different intervals for this same aim, for example Ohji et al. 2020 [18] reporting on outcomes after an 8-week interval. However, 20 (28%) eyes already showed recurrence at this follow-up in our total cohort and exudation was detected in 14 (19%) eyes at the end of the first series consisting of four IVT injections at 4-week intervals (data not shown). This RTE protocol therefore harbors a potential risk of initial under-treatment. Secondly, we did not use OCT-angiography as our study period coincides with when OCT-angiography implementation was in full expansion and thus before promising reports were available on the outcomes for detecting signs of disease activity [40, 41]. Indeed, OCT-angiography can now be incorporated at the end of our RTE protocol to guide the consideration of treatment discontinuation toward: (1) starting quarterly IVT injections (with bi-annual follow-up consultations), or (2) stopping IVT injections with monitoring every 3 months. Next, new treatment protocols have recently emerged with the advent of new drugs, such as faricimab [42]. Our protocol could thus be adapted to this new drug by for instance changing the 2-week increment to a 4-week increment.
Our study limitations owe to the retrospective design that ultimately limits patient inclusion and lacks a control group. Indeed, comparison of our current total cohort of patients that underwent the RTE protocol to patients having undergone a conventional T&E protocol would have enabled more robust comparisons instead of comparisons to reports in the literature. Finally, we opted to exclude patients undergoing a switch in anti-VEGF therapy during follow-up (n = 9, data not shown). These patients started the induction phase again of the RTE protocol after switching. While these patients were excluded to eliminate effects of therapy switch on injection schedule changes, we are aware that a selection bias could have been introduced by excluding patients who were likely less good responders. Future, larger prospective studies should include patients undergoing a switch in therapy, potentially in a subgroup for comparison, to deduce their ophthalmic outcomes and overall effects on protocol management.
Conclusions
Overall, the RTE protocol in our real-world setting maintained the total number of injections but reduced consultation number (improved management outcomes), while still improving patient functional and anatomical outcomes similarly to other protocols already used. Hence, our RTE protocol could reduce therapeutic burden by reducing hospital visits and in turn encouraging better patient compliance. Real-world protocol changes, such as incorrect injection numbers or intervals, while increasing the risk of over-treatment did not affect functional and anatomical outcomes. We should now investigate adjustments to this RTE protocol in light of further improving effectiveness, without compromising management outcomes, as well as the adaptability of the RTE protocol to new drugs and different settings.
Data Availability
The datasets generated and analyzed in this study are available from the corresponding author on reasonable request.
References
Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209-213.e2.
Skaat A, Chetrit A, Belkin M, Kinori M, Kalter-Leibovici O. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2012;153(2):214-221.e1.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. ANCHOR—ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration. Ophthalmology. 2009;116(1):57-65.e5.
Rosenfeld PJ, Kaiser PK. MARINA—ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;13:1419.
Abraham P, Yue H, Wilson L. PIER study year 2—randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration. Am J Ophthalmol. 2010;150(3):315-324.e1.
Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R, et al. EXCITE—efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration. Ophthalmology. 2011;118(5):831–9.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. VIEW 1–2—intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. PrONTO study—a variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;148(1):43-58.e1.
Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181–92.
Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679–80.
Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, et al. TREX-AMD 2 ans randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration. Ophthalmol Retina. 2017;1(4):314–21.
Haga A, Kawaji T, Ideta R, Inomata Y, Tanihara H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018;96(3):e393–8.
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J. TREND—treat-and-extend versus monthly regimen in neovascular age-related macular degeneration. Ophthalmology. 2018;125(1):57–65.
Kertes PJ, Galic IJ, Greve M, Williams G, Baker J, Lahaie M, et al. CANTREAT—efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244.
Berg K, Hadzalic E, Gjertsen I, Forsaa V, Berger LH, Kinge B, et al. LUCAS—ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the Lucentis compared to Avastin study treat-and-extend protocol. Ophthalmology. 2016;123(1):51–9.
DeCroos FC, Reed D, Adam MK, Salz D, Gupta OP, Ho AC, et al. ATLAS—treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Pecheur FL, et al. RIVAL—Macular atrophy in neovascular age-related macular degeneration. Ophthalmology. 2020;127(2):198–210.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. ALTAIR—efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings. Adv Ther. 2020;37(3):1173–87.
Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, et al. ARIES—efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration. Retina. 2021;41(9):1911–20.
Daien V, Finger RP, Talks JS, Mitchell P, Wong TY, Sakamoto T, et al. Evolution of treatment paradigms in neovascular age-related macular degeneration: a review of real-world evidence. Br J Ophthalmol. 2021;105(11):1475–9.
Souied EH, Cohen SY, de Pouvourville G, Dupeyron G, Latour E, Ponthieux A, et al. Treatment of wet age-related macular degeneration by ranibizumab in “real life” in France: treatment behaviours and associated visual outcome. Acta Ophthalmol. 2015;93(2):e179–80.
Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A. Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol. 2014;98(9):1192–6.
Ghanchi F, Bourne R, Downes SM, Gale R, Rennie C, Tapply I, et al. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye. 2022;36(6):1154–67.
Weber M, Dominguez M, Coscas F, Faure C, Baillif S, Kodjikian L, et al. RAINBOW—impact of intravitreal aflibercept dosing regimens in treatment-naïve patients with neovascular age-related macular degeneration. BMC Ophthalmol. 2020;20(1):206.
Baillif S, Creuzot-Garcher C, Dot C, Kodjikian L, Matonti F, Mrejen S, et al. Consensus d’experts français sur la mise en œuvre pratique du régime Treat-and-Extend par anti-angiogéniques chez les patients atteints de DMLA exsudative. J Français d’Ophtalmol. 2021;44(1):1–12.
Xiong K, Gong X, Li W, Yuting L, Meng J, Wang L, et al. Comparison of macular thickness measurements using swept-source and spectral-domain optical coherence tomography in healthy and diabetic subjects. Curr Eye Res. 2021;46(10):1567–73.
Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30(7):1046–50.
Cohen SY, Kodjikian L, Devin F, Delyfer MN, Dot C, Oubraham H, et al. Avis d’experts: actualisation des bonnes pratiques des injections intravitréennes. Recommandations de la Société française d’ophtalmologie et de la Société française d’hygiène hospitalière. J Français d’Ophtalmol. 2020;43(1):59–62.
Lucentis®, Novartis Europharm Limited, Summary of Product Characteristics for ranibizumab. https://base-donnees-publique.medicaments.gouv.fr/extrait.php?specid=64339586. 2014; Accessed 12 Dec 2023.
Eylea®, Bayer Healthcare, Summary of Product Characteristics for aflibercept. https://base-donnees-publique.medicaments.gouv.fr/extrait.php?specid=68795701. 2012; Accessed 12 Dec 2023.
Semoun O, Cohen SY, Srour M, Creuzot-Garchet C, Oubraham-Mebroukine H, Kodjikian L, et al. Prise en charge individualisée des patients atteints de DMLA exsudative, le protocole IOI: injection–observation–individualisation. J Français d’Ophtalmol. 2017;40(3):169–76.
Ghasemi Falavarjani K, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27(7):787–94.
Parvin P, Zola M, Dirani A, Ambresin A, Mantel I. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration treated with Aflibercept. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2127–34.
Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122(6):1212–9.
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration. Ophthalmol Retina. 2020;4(8):767–76.
Gascon P, Ramtohul P, Delaporte C, Kerever S, Denis D, Comet A. Aflibercept in real-life for the treatment of age-related macular degeneration using a treat and extend protocol: the Armada study. Eur J Ophthalmol. 2022;32(1):356–63.
Matonti F, Korobelnik JF, Dot C, Gualino V, Soler V, Mrejen S, et al. Comparative effectiveness of intravitreal anti-vascular endothelial growth factor therapies for managing neovascular age-related macular degeneration: a meta-analysis. JCM. 2022;11(7):1834.
Okada M, Mitchell P, Finger RP, Eldem B, Talks SJ, Hirst C, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration. Ophthalmology. 2021;128(2):234–47.
Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye. 2016;30(2):270–86.
Levine ES, Custo Greig E, Mendonça LSM, Gulati S, Despotovic IN, Alibhai AY, et al. The long-term effects of anti-vascular endothelial growth factor therapy on the optical coherence tomography angiographic appearance of neovascularization in age-related macular degeneration. Int J Retin Vitr. 2020;6(1):39.
Kim JM, Cho HJ, Kim Y, Jung SH, Lee DW, Kim JW. Responses of types 1 and 2 neovascularization in age-related macular degeneration to anti-vascular endothelial growth factor treatment: optical coherence tomography angiography analysis. Semin Ophthalmol. 2019;34(3):168–76.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–40.
Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418–31.
Jaki Mekjavić P, Gregorčič B, Oberč C, Podgoršek S. Treat-and-extend therapy using intravitreal aflibercept for neovascular age-related macular degeneration: 2-year real-world practice data from Slovenia. BMC Ophthalmol. 2018;18(1):333.
Vardarinos A, Gupta N, Janjua R, Iron A, Empeslidis T, Tsaousis KT. 24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real life setting. BMC Ophthalmol. 2017;17(1):58.
Funding
No funding was received for conducting this study. The journal’s Rapid Service Fee was funded by Toulouse University Hospital.
Author information
Authors and Affiliations
Contributions
François-Philippe Roubelat, Pierre Fournié, Vincent Gualino, and Vincent Soler contributed to study conception and design. Data collection was performed by François-Philippe Roubelat, Lisa Barioulet, Fanny Varenne, Clément Escudier, Pauline Meyer, and Clément Gomane. Data analysis and interpretation was performed by François-Philippe Roubelat, Lisa Barioulet, Fanny Varenne, Clément Escudier, Jacqueline Butterworth, Véronique Pagot-Mathis, Vincent Gualino, and Vincent Soler. François-Philippe Roubelat wrote the first draft of the manuscript and all authors contributed to later drafts. All authors have approved the final manuscript version and agree to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Conflict of Interest
Vincent Soler has received consulting honoraria from Bayer Healthcare, Novartis, Horus Pharma, Roche, Allergan-AbbVie. Vincent Gualino has received consulting honoraria from Alcon, Bayer Healthcare, Allergan-AbbVie, Bausch & Lomb, Novartis, Roche. Pierre Fournié received consulting honoraria from Horus Pharma, Théa Laboratoires, Essilor International, Allergan-AbbVie. François-Philippe Roubelat, Lisa Barioulet, Fanny Varenne, Clément Escudier, Pauline Meyer, Clément Gomane, Jacqueline Butterworth, and Véronique Pagot-Mathis have nothing to disclose.
Ethical Approval
All procedures performed were part of routine care, and both in accordance with institutional guidelines and with the principles and regulations of the Helsinki Declaration of 1964 and its later amendments. An official waiver of ethical approval was granted from Toulouse University Hospital (the master ethics committee, study reference: 2023-139) in accordance with Clinique Honoré Cave. This is given the retrospective and non-interventional nature of the study as asserted by the French Jardé Ethical and Regulatory law. The study additionally complies with French MR-004 methodology (CNIL 2206723 v 0) covering data protection for both centers. Informed patient consent was obtained from all participants before inclusion and all data has been anonymized for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Roubelat, FP., Barioulet, L., Varenne, F. et al. The Reinforced Treat-and-Extend Protocol for Exudative Age-Related Macular Degeneration: Retrospective Assessment of 24-Month Real-World Outcomes in France. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-00938-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-00938-7