Abstract
Introduction
Herpes zoster ophthalmicus (HZO) results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. The inflammation caused by VZV involves multiple tissues in the eyes. Our goal is to evaluate pattern electroretinogram (PERG) changes and their relationship with corneal sub-basal nerve changes in patients with HZO.
Methods
Twenty-two patients with herpes zoster keratitis or conjunctivitis and 20 healthy volunteers were recruited for this cross-sectional study. A PERG test was performed on both eyes of HZO patients and one eye of the healthy controls. In vivo confocal microscopy (IVCM) was also performed on both eyes of the HZO patients to detect corneal nerve damage.
Results
Our results showed changes in the PERG parameters in both eyes of HZO patients compared to the healthy controls. Affected eyes showed delayed N95 peak time and decreased P50 and N95 amplitude compared to the unaffected eyes (p < 0.05, respectively). Both affected and unaffected eyes in HZO patients showed delayed P50 peak time and decreased N95 amplitude (p < 0.05, respectively) compared to controls. In HZO patients, no significant differences in each PERG parameter were found between eyes with and without corneal lesions or between eyes with and without increased Langham’s cells in the corneal epithelial sub-basal layer. The IVCM images showed decreased total nerve length and number at the sub-basal layer of the epithelial cornea in affected eyes compared to unaffected eyes (p < 0.05). No significant correlation was found between total nerve length and PERG changes.
Conclusions
Our results showed that VZV-affected eyes without central cornea involvement displayed reduced N95 amplitude and prolonged P50 peak time in bilateral eyes compared to the healthy controls. Larger studies are needed to further explore the effect of HZO on the electrophysiological response of the eye and the posterior segment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Herpes zoster keratitis/conjunctivitis results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. |
Pattern electroretinogram (PERG) serves as an objective tool in evaluating the function of macular and retinal ganglion cells. |
What was learned from the study? |
P50 peak time and N95 amplitude in PERG showed significant changes in bilateral eyes of mild herpes zoster keratitis/conjunctivitis patients compared to healthy controls. |
Introduction
Herpes zoster ophthalmicus (HZO) is secondary to reactivation of varicella zoster virus (VZV) in the ophthalmic division of the trigeminal nerve, which accounts for 10–20% of all herpes zoster cases [15, 19]. As VZV replicates, the inflammation along the ophthalmic nerve branch encompasses the cornea, uvea, iris, sclera, optic nerve, and retina [1]. Almost two-thirds of HZO patients develop corneal sensation loss due to corneal nerve damage, and there are recent publications about corneal nerve involvement in the fellow unaffected eyes [9, 14]. It has been suggested that reciprocal interaction between the nervous and immune systems underlies the mechanism of bilateral involvement [5].
The incidence rate of posterior segment involvement in HZO is relatively low, but the consequences can be devastating. VZV-related uveitis involving the posterior segment varies from acute retinal necrosis (ARN) to relatively milder non-necrotizing uveitis [4, 7, 11, 21]. To date, there is limited evidence reporting posterior segment involvement in herpes zoster keratitis or conjunctivitis. Considering the fact that the inflammation caused by VZV involves multiple tissues in the eyes, it is necessary to evaluate whether retinal layers are involved and the severity of retinal involvement. Pattern electroretinogram (PERG) serves as an objective and valuable tool for evaluating the function of macular and retinal ganglion cells (RGCs) [12]. Several studies have proved its usefulness in detecting glaucomatous changes and its relationship with other glaucoma-related findings, such as visual field and average thickness of retinal nerve fiber layer, as measured by optical coherence tomography [3, 6, 20].
The aim of this study is to explore whether electro-functional changes exist in the involved and fellow eye of patients with HZO and their association with corneal sub-basal nerve changes, as measured by an in vivo confocal microscope (IVCM). We obtained PERG responses, which were recorded for both eyes of HZO patients without central cornea involvement as well as the eyes of healthy controls.
Methods
Subjects
The present retrospective cross-sectional study was performed according to the considerations of the tenets of the Declaration of Helsinki with approval by the institutional review board of Peking University Third Hospital (S2019341). All the participants signed the informed consent. A total of 22 HZO patients and 20 healthy controls were enrolled in this study. Inclusion criteria were clinical diagnosis of herpes zoster with facial or forehead skin involvement and herpes zoster keratitis or conjunctivitis and patients aged between 25 and 80 years old. Exclusion criteria were as follows: (1) central cornea involvement (within 6-mm diameter) that occluded the pupillary area; (2) the existence of other ophthalmic complications caused by VZV, such as iridocyclitis, scleritis, neuritis, retinitis, or secondary glaucoma; (3) the presence of any other retinopathy (for example, age-related macular degeneration, diabetic retinopathy) and glaucoma; (4) refractive media opacity like central cortical or posterior subcapsular cataracts; and (5) systematic diseases that may have an effect on the outcome of PERG, such as diabetes mellitus, Parkinson’s disease, or multiple sclerosis. Based on the existence of corneal lesions in affected eyes, we further divided HZO patients into lesion (n = 14) and non-lesion groups (n = 8), and corneal lesion was defined as the presence of VZV-related peripheral corneal infiltration or corneal nebula. According to the IVCM images, we divided HZO patients into the Langham group (eyes with activated Langham’s cells in the sub-basal layer, n = 11) and the non-Langham group (eyes without activated Langham’s cells in the sub-basal layer, n = 11). The images were observed independently by two ophthalmologists. If there was a disagreement, then it was defined by another senior doctor with more than 10 years of experience. The healthy controls were 20 volunteers aged 25–80 years who came to the ophthalmic outpatient clinic for a regular eye examination with no serious eye diseases or other systematic problems. All HZO patients underwent comprehensive eye examination performed by one ophthalmologist, including best-corrected visual acuity (BCVA) using LogMAR visual chart, non-mydriatic subjective refraction at a distance and near, intraocular pressure (IOP) with a non-contact tonometer, dilated fundus examination, posterior segment optical coherence tomography (OCT), and IVCM. A detailed medical history and ocular history were recorded. The control group did not receive the IVCM test.
PERG
PERG was recorded in accordance with the guidelines of the International Society for Clinical Electrophysiology of Vision (ISCEV) using the RETiscan System (Roland Consult, Wiesbaden, Germany) [2]. Optimal correction of each participant’s refraction was acquired before starting. The reference electrodes and ground electrodes were respectively placed on the skin of the ipsilateral outer canthus and the skin of the forehead, and the active electrodes (Dawson-Trick-Litzkow, DTL) were placed in the lower conjunctiva sac after one drop of topical anesthesia (benoxyl, 0.5% procaine hydrochloride). A black-and-white flat screen monitor (LCD color monitor; Roland Consult) was put from 1-m distance in front of each participant in a soundproof and semi-dark room. The visual angle was 23° (horizontal) × 17° (vertical). The checkerboard’s mean luminance was 80 cd/m2 and the contrast was 97%. The reversal rate of the transient PERG measurement was 4.3 reversals per second (2.15 Hz), and at least 200 artifact-free reversals were recorded and averaged for each trial. Both eyes of every participant were recorded for at least two trials without dilation of the pupils. The P50 peak time, N95 peak time, P50 amplitude, N95 amplitude, and the N95/P50 amplitude ratios were measured.
In Vivo Confocal Microscopy
The sub-basal nerve plexus and epithelial Langham’s cells in the central cornea of bilateral eyes were imaged using in vivo confocal microscopy (Rostock Cornea Module/HRT II, Heidelberg, Germany). After one drop of topical anesthesia (0.5% proparacaine) applied to both eyes, 0.3% hypromellose was placed on the tip of the objective lens as a coupling medium. The operator manually moved the lens forward until it touched the surface of the central cornea. After a full-thickness confocal scan, 350 images were obtained at a speed of 25 frames per second. The scanning area of each image was 460 × 350 μm with a 500× magnification of and a lateral resolution of 1 μm. The sub-basal nerve plexus was analyzed using NeuronJ, a plug-in semiautomated tracing program of ImageJ, which is free online software distributed by the National Institutes of Health. The total nerve length and total nerve number were calculated after analyzing three representative images of the sub-basal nerve plexus. Three representative images at the level of basal epithelial layers were chosen to analyze Langham’s cells, which were morphologically identified as individual dendriform structures, easily differentiated from the nerve plexus. Images featuring increasing Langham’s cell density were identified manually by two observers. The IVCM test was performed in both eyes of every HZO patients.
Statistical Analysis
Statistical analysis was performed using SPSS version 26.0 (Statistical Package for Social Sciences, Chicago, IL, USA). The Anderson–Darling test confirmed the normality of the distribution. All quantitative variables were expressed by the mean and SEM (standard error of mean). Student’s t test and Fisher’s exact test were used to assess the differences in age and gender between the HZO and control groups, respectively. An analysis of variance (ANOVA) was performed to compare statistically the PERG parameters among the three groups. Student’s t test was used to assess the differences in corneal sub-basal nerve damage in bilateral eyes of HZO patients. Pearson’s correlation analysis was used to address the relationship between PERG parameters and corneal sub-basal nerve changes. Differences were considered statistically significant for p < 0.05.
Results
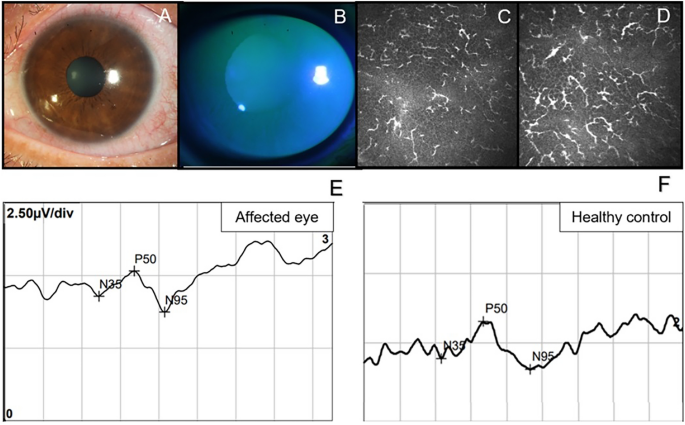
The mean age of the HZO patients enrolled in this study was 54.36 ± 13.14 years (range, 29–76 years, n = 22), and the mean age of the healthy controls was 47.70 ± 10.07 years (range, 32–65 years, n = 20; p = 0.075). A total of 13 males and nine females were in the HZO group, and eight males and 12 females were in the healthy control group (p = 0.217). Mean duration of HZO patients is 3.4 ± 2.1 months. Representative images of slit-lamp photographs, IVCM images, OCTA images, and PERG traces of a single patient with HZO are presented in Fig. 1.
Six representative images from A to D are all from the same patient with herpes zoster ophthalmicus (HZO). A Slit-lamp photograph of his cornea and B shows the picture under cobalt blue light after corneal fluorescein staining. C, D Two consecutive pictures of in vivo confocal microscopy (IVCM) in the sub-basal layer of the epithelial cornea, showing a mass of activated Langham’s cells. E The pattern electroretinogram (PERG) trace of his affected eye. F A PERG trace of a healthy control
PERG Parameters
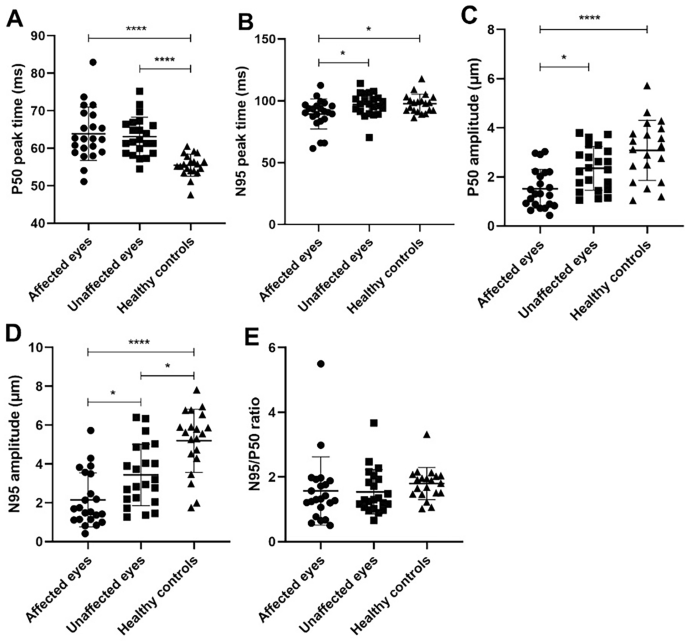
As shown in Fig. 2, a statistically significant difference was found in the P50 peak time between HZO affected eyes and healthy controls as well as between unaffected eyes and healthy controls (p < 0.0001). Both affected eyes and contralateral eyes showed delayed P50 compared to the normal controls. The P50 amplitude was significantly decreased in HZO affected eyes as compared to healthy controls (p < 0.0001) and as compared to contralateral unaffected eyes (p = 0.019). When comparing of affected eyes and healthy controls as well as contralateral eyes and healthy controls, the N95 amplitude for both showed a statistically significant decrease (p < 0.0001, and p = 0.013, respectively). Additionally, the N95 amplitude also showed a decrease in contralateral unaffected eyes compared to affected eyes (p = 0.021). To our surprise, the N95 peak time in affected eyes was significantly shorter than that in contralateral eyes and that in healthy controls (p = 0.039 and 0.027 respectively). No significant differences among the groups were found for the N95/P50 ratio. All the PERG parameters are listed in Table 1.
Table 2 shows the differences in PERG parameters between eyes with or without corneal lesions. All the five parameters showed no statistically significant differences between these two groups, though eyes with corneal lesions had a tendency toward prolonged P50 and N95 peak time and decreased P50 and N95 amplitude.
Table 3 illustrates the differences in PERG parameters between eyes with and without increased Langham cells. Similarly, no statistically significant differences were found between the two group.
IVCM Parameters
A significant decrease in sub-basal nerve plexus was found in HZO eyes compared to unaffected eyes, including total nerve length (903.11 ± 977.08 vs. 2545.96 ± 1066.98 μm/frame, p < 0.005), and total nerve number (4.45 ± 3.70 vs. 10.53 ± 3.89, p < 0.005). The healthy control group did not receive the IVCM test.
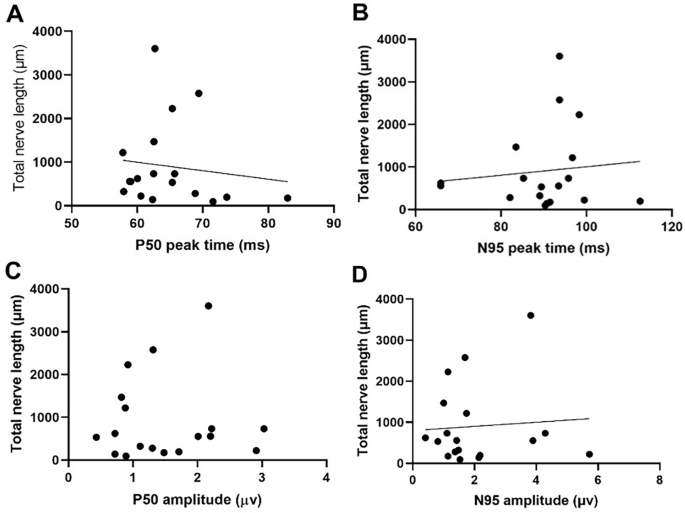
Correlation Between Corneal Sub-basal Nerve and PERG Parameters
We performed Pearson’s correlation to analyze the relationship between PERG parameters and total corneal nerve length, and the results are given in Fig. 3. There appears to be a trend in which total nerve length decreases as P50 peak time delays, though it is not statistically significant (Fig. 3). The associations between total nerve length and other PERG parameters (P50 peak time, N95 peak time, and N95 amplitude) were not statistically significant neither (Fig. 3).
Discussion
The current index study was designed to investigate the presence of PERG abnormalities in eyes affected by HZO and their seemingly unaffected counterparts. Additionally, we sought to establish a correlation between these PERG abnormalities and corneal subbasal nerve damage. Our primary goal was to delineate the interplay between ocular surface inflammation and ocular fundus pathology in the context of HZO. The findings from this study uncovered significant alterations in PERG parameters not only in the eyes directly affected by HZO but also in the contralateral unaffected eyes. Specifically, we observed notable changes in the P50 amplitude, N95 amplitude, and P50 peak time. As a result of these observations, we hypothesize a potential association between even mild cases of herpes zoster keratitis or conjunctivitis and retinal electrofunctional changes, as indicated by the detected PERG alterations. These findings offer a glimpse into the intricate connections between superficial ocular afflictions and underlying retinal responses in the context of HZO.
To our knowledge, this is the first study to describe electroretinographic changes in HZO patients. The reduction of the amplitude in PERG N95 reflects the abnormality of RGCs, which may be due to the lack of activities of dead RGCs or reduced activity of viable RGCs [8]. Many studies have confirmed that the dysfunction of RGCs can precede structural changes. Ventura et al. [18] conducted a cross-sectional observational study on patients with glaucoma suspect (GS) and early manifest glaucoma (EMG) and found the PERG was abnormal in 52% of GS patients and 69% of EMG patients. The research demonstrated that abnormal PERG can serve as a predictive technique for monitoring the initial onset of glaucoma by detecting RGC dysfunction. Moreover, in inflammatory diseases such as Fuchs’ heterochromic cyclitis (FHC), changes in RGCs may be among the earliest detectable signs. Murray et al. [13] found subclinical retinal damage in eyes with FHC, as their results suggested reduced amplitude and delayed peak time of both the P50 and N95 components of PERG in FHC eyes with good vision compared to normal eyes. In particular, the reduction and delay of the P50 component indicated preganglionic retinal changes in the macular area. Therefore, we hypothesize that the macular RGCs are involved and damaged in mild HZO patients. Further study is needed to explore full-scale electroretinographic changes using different measurement instruments such as multi-focal ERG and full-field ERG.
Furthermore, our results suggest that unaffected eyes also exhibit PERG abnormalities. This is consistent with previous literature. Hamrah et al. [9] demonstrated corneal nerve diminishment in HZO-affected and unaffected eyes, suggesting bilateral changes in clinically unilateral disease. Cavalcanti et al. [5] found a bilateral increase of dendritic cell (DC) density in unilateral HZO patients, and that the DC density was negatively correlated with all corneal nerve parameters, including total nerve length, the total number of nerves, and the number of nerve branches measured by IVCM. The authors postulated that a coordinated interaction between the nervous system and the immune system occurred in clinically unilateral diseases. The bilateral elevation of pro-inflammatory cytokines was confirmed in patients with unilateral bacterial keratitis and its strong correlation with nerve alteration [22]. Our finding, related to sub-basal nerve changes in bilateral eyes, is consistent with the existing literature. Thus, it is plausible to postulate that bilateral PERG abnormalities can be induced by the synergic interaction between the nervous system and immune response and that retinal ganglion cells may be involved in this process. The involvement of the posterior segment is uncommonly seen in HZO patients, and there are limited data concerning the retinal pathological changes with corneal inflammation. Roberts et al. [16] once reported two cases of herpes zoster chorioretinopathy in the retinal pigment epithelium and choroid after 8 and 5 months of the first onset. The retinal lesions of both patients remained unchanged and they ended up with good vision. Acute retinal necrosis (ARN) syndrome presents a distinctive clinical process characterized by occlusive retinal arteritis, necrotizing retinitis, and uveitis [17], whereas the clinical characteristics of patients with or without immune suppression differ greatly [10]. Thus, it is imperative to establish diagnostic tests of early retinopathy of VZV infection and to improve the visual prognosis of ARN patients.
Moreover, our results support that damage to the anterior segment does not necessarily influence damage to the posterior segment, as no significant changes were found between eyes with and without corneal lesions. The absence of corneal inflammation does not exclude posterior retinopathy, as no significant changes between eyes with and without the activated Langham cells in the corneal sub-basal layer were detected. This can be induced by direct virus infection or the secondary immune response targeted at the retinal ganglion cells. Future research needs to be conducted to elucidate the underlying mechanism of this pathogenesis.
Our study has certain limitations. First, our sample size was small. Furthermore, fundus measurement instruments other than PERG, such as full-field ERG and multifocal ERG, are not applied in this study, thus rendering the evaluation of electroretinographic changes incomplete. We speculate that the full-field ERG may be normal, as it reflects the function of the global retina. PhNR may induce certain changes in HZO patients. Future research will benefit from performing more participants and performing a more comprehensive evaluation. Moreover, the IVCM test was not performed on the control group; therefore, we failed to analyze the association between IVCM changes and PERG parameters in the control group. Many confounding factors such as patient compliance or manual counting of the total nerve length and dendritic cells using ImageJ software, might also have caused inaccuracy. Due to the nature of the retrospective study, we failed to conduct a follow-up period recording and prospectively analyze the PERG change in different HZO duration. We will improve the study design in future research.
Conclusions
In summary, our results show that HZO patients display diminished N95 amplitude and prolonged P50 peak time in bilateral eyes. No significant connection was established between corneal nerve changes and PERG parameters changes in bilateral eyes. The research provides evidence of retinal functional abnormality in HZO keratitis and conjunctivitis and facilitates further investigation of the multi-tissue VZV infection relationship in the future.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–81. https://doi.org/10.1128/cmr.9.3.361.
Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126:1–7. https://doi.org/10.1007/s10633-012-9353-y.
Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–95. https://doi.org/10.1097/OPX.0b013e318177ebf3.
Bodaghi B, Rozenberg F, Cassoux N, Fardeau C, LeHoang P. Nonnecrotizing herpetic retinopathies masquerading as severe posterior uveitis. Ophthalmology. 2003;110:1737–43. https://doi.org/10.1016/s0161-6420(03)00580-3.
Cavalcanti BM, Cruzat A, Sahin A, Pavan-Langston D, Samayoa E, Hamrah P. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf. 2018;16:101–11. https://doi.org/10.1016/j.jtos.2017.09.004.
Elgohary AM, Elbedewy HA, Saad HA, Eid TM. Pattern electroretinogram changes in patients with primary open-angle glaucoma in correlation with visual field and optical coherence tomography changes. Eur J Ophthalmol. 2020;30:1362–9. https://doi.org/10.1177/1120672119872606.
Engstrom RE Jr, Holland GN, Margolis TP, Muccioli C, Lindley JI, Belfort R Jr, Holland SP, Johnston WH, Wolitz RA, Kreiger AE. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488–502. https://doi.org/10.1016/s0161-6420(94)31142-0.
Fiorentini A, Maffei L, Pirchio M, Spinelli D, Porciatti V. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981;21:490–3.
Hamrah P, Cruzat A, Dastjerdi MH, Prüss H, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–7. https://doi.org/10.1016/j.ophtha.2012.07.036.
Helbig H, Bornfeld N, Bechrakis NE, Kellner U, Foerster MH. Varicella zoster virus infections of the retina in patients with and without immune suppression. Klin Monbl Augenheilkd. 1994;205:103–8. https://doi.org/10.1055/s-2008-1045500.
Kim JY, Lee JH, Lee CS, Lee SC. Varicella zoster virus-associated Chorioretinitis: a case report. BMC Ophthalmol. 2018;18:28. https://doi.org/10.1186/s12886-018-0696-3.
Maffei L, Fiorentini A, Bisti S, Holländer H. Pattern ERG in the monkey after section of the optic nerve. Exp Brain Res. 1985;59:423–5. https://doi.org/10.1007/bf00230925.
Murray DC, Stavrou P, Good PA, Murray PI. Electroretinographic findings in Fuchs’ heterochromic cyclitis. Eye (Lond). 1997;11(Pt 1):102–8. https://doi.org/10.1038/eye.1997.20.
Pavan-Langston D. Herpes zoster ophthalmicus. Neurology. 1995;45:S50-51. https://doi.org/10.1212/wnl.45.12_suppl_8.s50.
Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61:310–6. https://doi.org/10.1097/00005792-198209000-00003.
Roberts TV, Francis IC, Kappagoda MB, Dick AD. Herpes zoster chorioretinopathy. Eye (Lond). 1995;9(Pt 5):594–8. https://doi.org/10.1038/eye.1995.146.
Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome. Semin Ophthalmol. 2008;23:275–83. https://doi.org/10.1080/08820530802111325.
Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK 2nd. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–9. https://doi.org/10.1016/j.ophtha.2004.07.018.
Vrcek I, Choudhury E, Durairaj V. Herpes Zoster ophthalmicus: a review for the internist. Am J Med. 2017;130(1):21–26. https://doi.org/10.1016/j.amjmed.2016.08.039
Wanger P, Persson HE. Pattern-reversal electroretinograms in unilateral glaucoma. Investig Ophthalmol Vis Sci. 1983;24:749–53.
Wensing B, de Groot-Mijnes JD, Rothova A. Necrotizing and nonnecrotizing variants of herpetic uveitis with posterior segment involvement. Arch Ophthalmol. 2011;129:403–8. https://doi.org/10.1001/archophthalmol.2010.313.
Yamaguchi T, Calvacanti BM, Cruzat A, Qazi Y, Ishikawa S, Osuka A, Lederer J, Hamrah P. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Investig Ophthalmol Vis Sci. 2014;55:7457–66. https://doi.org/10.1167/iovs.14-15411.
Acknowledgements
We thank the participants of the study.
Funding
This study was supported by National Natural Science Foundation of China Grants (No. 81700799; 82070926). The authors funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Research design: Yun Feng; Jingyi Li; Yuexin Wang; Xin Xie; data acquisition: Xin Xie; Jingyi Li; Weizhen Zeng; Data analysis and interpretation: Jingyi Li; Yun Feng; Shiying Li; Manuscript preparation: Jingyi Li; Yuexin Wang; Yun Feng; Shiying Li; Rupesh Agrawal; Yun Feng.
Corresponding authors
Ethics declarations
Conflict of interest
Jingyi Li, Yuexin Wang, Xin Xie, Weizhen Zeng, Shiying Li, Rupesh Agrawal, and Yun Feng do not have any conflicts of interest to declare.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki principles. The Human Ethics Committees of Peking University Third Hospital approved the protocol numbered S2019341. All the subjects gave their written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, J., Wang, Y., Xie, X. et al. Bilateral Pattern Electroretinogram Abnormalities in Patients with Herpes Zoster Keratitis and Conjunctivitis. Ophthalmol Ther 13, 1589–1599 (2024). https://doi.org/10.1007/s40123-024-00928-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00928-9