Abstract
Introduction
This study assessed the European School of Advanced Studies in Ophthalmology (ESASO) classification’s prognostic value for diabetic macular edema (DME) in predicting intravitreal therapy outcomes.
Methods
In this retrospective, multicenter study, patients aged > 50 years with type 1 or 2 diabetes and DME received intravitreal antivascular endothelial growth factor (anti-VEGF) agents (ranibizumab, bevacizumab, and aflibercept) or steroids (dexamethasone). The primary outcome was visual acuity (VA) change post-treatment, termed as functional response, measured 4–6 weeks post-third anti-VEGF or 12–16 weeks post-steroid injection, stratified by initial DME stage.
Results
Of the 560 eyes studied (62% male, mean age 66.7 years), 31% were classified as stage 1 (early), 50% stage 2 (advanced), 17% stage 3 (severe), and 2% stage 4 (atrophic). Visual acuity (VA; decimal) improved by 0.12–0.15 decimals in stages 1–2 but only 0.03 decimal in stage 3 (all p < 0.0001) and 0.01 in stage 4 (p = 0.38). Even in eyes with low baseline VA ≤ 0.3, improvements were significant only in stages 1 and 2 (0.12 and 0.17 decimals, respectively). Central subfield thickness (CST) improvement was greatest in stage 3 (−229 µm, 37.6%, p < 0.0001), but uncorrelated with VA gains, unlike stages 1 and 2 (respectively: −142 µm, 27.4%; − 5 µm, 12%; both p < 0.0001). Stage 4 showed no significant CST change. Baseline disorganization of retinal inner layers and focal damage of the ellipsoid zone/external limiting membrane did not influence VA improvement in stages 1 and 2. Treatment patterns varied, with 61% receiving anti-VEGF and 39% dexamethasone, influenced by DME stage, with no significant differences between therapeutic agents.
Conclusion
The ESASO classification, which views the retina as a neurovascular unit and integrates multiple biomarkers, surpasses single biomarkers in predicting visual outcomes. Significant functional improvement occurred only in stages 1 and 2, suggesting reversible damage, whereas stages 3 and 4 likely reflect irreversible damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
This study was performed to validate the clinical relevance of the European School of Advanced Studies in Ophthalmology (ESASO) classification system for diabetic macular edema (DME). |
It aimed to assess how different stages of DME, as defined by the ESASO classification, respond to intravitreal treatments. |
This is crucial for enhancing understanding of DME progression and, to enable better staging of the disease, with the aim of improving treatment strategies. |
What was learned from the study? |
This study examined the efficacy of the ESASO classification system in predicting treatment outcomes for DME. |
It demonstrated that early DME stages (stages 1 and 2) respond more positively to treatment, suggesting these stages represent a reversible phase of the disease, while advanced stages (stages 3 and 4) indicate established and irreversible damage. |
The study emphasized the limited predictive value of baseline visual acuity and central subfield thickness alone, thereby reinforcing the comprehensive approach of the ESASO Classification. |
Introduction
Diabetes mellitus continues to pose a significant global health challenge, with an alarming rise in the number of affected individuals. In 2019, the International Diabetes Federation reported that approximately 240 million people worldwide had diabetes, representing a prevalence of 9.6% [1]. Projections indicate that this number is expected to surge to 356 million by 2045, resulting in a global prevalence of 11.1% [1]. Among the numerous complications associated with diabetes, diabetic retinopathy (DR) and diabetic macular edema (DME) are particularly devastating as they are leading causes of blindness in the working-age population [2].
Understanding the pathogenesis of DME is crucial for the development of effective treatment strategies. Disruption of the blood–retinal barrier, local inflammation, and neurodegeneration are among the contributing factors [2, 3]. As in every other neural tissue, the progressive damage of the retina induced by these factors may lead to irreversible damage jeopardizing visual function. It is crucial to understand and monitor the progression of the disease, generating a better therapeutic approach and more reliable prognostic indications.
OCT Structural Biomarkers Predicting Treatment Outcomes
Substantial research efforts have been directed toward identifying structural biomarkers that can predict treatment outcomes and provide insights into long-term visual prognosis, and optical coherence tomography (OCT), a high-resolution imaging technique, has proven invaluable in this regard [4,5,6,7]. OCT allows for the detection of intra- and subretinal fluid and precise measurement of retinal thickness. Several OCT parameters, including disruption of outer retinal layers such as the ellipsoid zone (EZ) and the external limiting membrane (ELM), disorganization of the inner retinal layers (DRIL), morphology and location of intraretinal cysts, as well as the presence of subretinal fluid and vitreoretinal interface abnormalities, have been associated with poorer vision and limited gains following treatment [4]. Nonetheless, previous investigations have evaluated these biomarkers mostly in isolation [8,9,10,11,12,13], necessitating a comprehensive approach to gain a deeper understanding of their combined impact.
The ESASO Classification of DME Stage
In 2020, the European School for Advanced Studies in Ophthalmology (ESASO) introduced a comprehensive OCT classification system for DME [4]. The ESASO Classification describes DME progression through the combination of five specific OCT parameters: central subfield retinal thickness, intraretinal cysts, inner retinal layers, EZ, and ELM. Four stages of progression are defined based on the combined progressive alteration of these parameters: early DME (stage 1), advanced DME (stage 2), severe DME (stage 3), and atrophic maculopathy (stage 4). The clinical use of the ESASO Classification demonstrated a high inter-observer concordance rate (94%) in a recent validation process [5]. While for scientific purposes a detailed numerical score may be used to define the stage, for clinical use the stages are defined according to standard OCT images where all parameters are fully described (Tables 1, 2, Fig. 1).
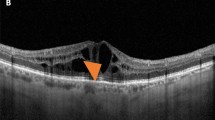
Illustrations of ESASO DME classification stages. Early diabetic maculopathy (stage 1) includes: a Small cystoid spaces near the fovea, with all retinal layers intact and normal central subfoveal thickness and macular volume. TCED-HFV: T = 1; C = 1; E = 0; D = 0; H = 0; F = 0; V = 0. b Multiple perifoveal cystoid spaces with mild macular thickening and incomplete vitreous detachment; over 30 hyperreflective foci. TCED-HFV: T = 1; C = 2; E = 0; D = 0; H = 1; F = 0; V = 1. c Mild macular edema with intact retinal profile, cystoid spaces, and initial epiretinal membrane; over 30 hyperreflective foci. TCED-HFV: T = 1; C = 2; E = 0; D = 0; H = 1; F = 1; V = 4. Advanced diabetic maculopathy (stage 2) includes: d cystoid spaces with retinal thickening and central macrocyst; damaged subfoveal ellipsoid zone. TCED-HFV: T = 2; C = 3; E = 1; D = 0; H = 1; F = 0; V = 0. e Intermediate cystoid spaces with disrupted external limiting membrane and visible internal retinal layers; no adhesion or traction. TCED-HFV: T = 2; C = 2; E = 1; D = 0; H = 0; F = 1; V = 0. f Large pseudocyst in the fovea with parafoveal cystoid spaces; damaged subfoveal external limiting membrane and partial vitreous detachment. TCED-HFV: T = 2; C = 3; E = 1; D = 1; H = 0; F = 0; V = 1. g Large cystoid spaces with shallow subfoveal detachment; non-gradable ellipsoid zone and discontinuous external limiting membrane; visible inner retinal segmentation and slightly elevated vitreous. TCED-HFV: T = 2; C = 3; E = 1; D = 0; H = 1; F = 1; V = 1. Severe diabetic maculopathy (stage 3) includes: h multiple central macrocysts with inner retinal layer disorganization; absent subfoveal external limiting membrane and ellipsoid zone. TCED-HFV: T = 2; C = 3; E = 2; D = 1; H = 0; F = 0; V = 1. i Central macrocyst with large surrounding cystoid spaces, severe DRIL, and incomplete vitreous detachment; absent subfoveal external limiting membrane and ellipsoid zone. TCED-HFV: T = 2; C = 3; E = 2; D = 1; H = 0; F = 0; V = 1. j Central macrocyst and multiple large cysts with a few hyperreflective foci; damaged but visible retinal inner layers and normal vitreoretinal relationship. Atrophic diabetic maculopathy (stage 4) includes: k Central retinal thinning with DRIL, atrophic retinal pigment epithelium, and below-normal thickness and volume; some parafoveal microcysts and peripheral cystoid spaces. TCED-HFV: T = 0; C = 1; E = 2; D = 1; H = 1; F = 0; V = 0. l Central thinning with DRIL, irregular and focally atrophic retinal pigment epithelium, and below-normal thickness and volume, but with some moderate intraretinal cysts. TCED-HFV: T = 0; C = 1; E = 2; D = 1; H = 0; F = 0; V = 0. Adapted from reference [4]. DME diabetic macular edema, DRIL disorganization of the inner retinal layers, ESASO European School of Advanced Studies in Ophthalmology, TCED-HDF Thickening–cysts–ellipsoid–DRIL–hyperreflective foci–fluid (subretinal)–vitreoretinal relationship
While the ESASO Classification has already provided valuable descriptive insights on DME [5, 6, 14], its clinical relevance required further validation. To address this knowledge gap, the objective of this retrospective multicenter study was to assess the therapeutic response to intravitreal agents in eyes at different stages of DME progression. Specifically, we aimed to explore the clinical relevance of this classification in forecasting treatment outcomes in comparison with single OCT biomarkers.
Methods
Study Design
This is a retrospective, multicenter observational study. The study was conducted across multiple centers to ensure a diverse patient population and enhance the generalizability of the findings. Participants in this study were selected from the ophthalmology clinics of the participating centers. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. According to the Italian Medicines Agency (AIFA) requirements, retrospective studies and the communication of pooled (aggregated) data to the Coordinating Center only necessitate communication to the Ethics Committee and do not require explicit approval. To ensure patient confidentiality and data protection, stringent measures were implemented throughout the study.
Primary Outcome Measure
Visual acuity improvement after the loading therapeutic phase according to the stage of the disease.
Eligible Eyes at the Retrospective Review
Inclusion Criteria
-
1.
Age of 50 years or older with demographic information and medical history
-
2.
Diagnosis of type 1 or type 2 diabetes and grade of diabetic retinopathy
-
3.
Evidence of DME
-
4.
Clear view of retinal structure
-
5.
Treatment with a loading dose of one of the three antivascular endothelial growth factor (anti-VEGF) injections available at the time of data collection (ranibizumab, aflibercept, and bevacizumab) or a single intravitreal dexamethasone (DEX) injection
-
6.
Naïve eyes or those already treated but without any therapeutic intervention for the previous 10 months
-
7.
To be included in this retrospective study the following parameters were necessary:
-
a.
Step 1 (baseline): VA (decimal) and OCT scans (radial or raster lines) obtained within 1 month prior to the first injection
-
b.
Step 2 (follow-up): Visual acuity (decimal) and OCT scans obtained at 4–6 weeks after the loading phase with anti-VEGF treatment or at 12–16 weeks after the single intravitreal steroid injection. To be eligible, the follow-up OCT scan had to be acquired with the same instrument and the same pattern as the baseline OCT scan
Exclusion Criteria
-
1.
The presence of any other ocular or macular pathology possibly causing macular edema or confounding the analysis
-
2.
History of intravitreal or laser treatment within the past 10 months
-
3.
History of cataract or other ocular surgeries within the past 8 months
Each participating center was required to contribute with a minimum of 30 cases. In cases of eligibility of both eyes from the same patient, the eye with the worst DME stage was included.
Methods of OCT Data Collection
High-resolution scans (length ≥ 9 mm) with the modalities “raster scan” and/or “radial scan” centered to the foveola were taken. The quantitative measurements of central subfield thickness (CST) and macular volume were automatically provided by the instrument and registered. In step 1, the scan demonstrating the worst retinal morphology according to ESASO classification was selected, and the scan with the same orientation (or the worst scan in case of discrepancy) was selected in step 2.
Staging of DME and Data Analysis
Participating centers sent their database and OCT images (steps 1 and 2) to the reading center at ESASO headquarters. Two authors (G.P. and G.D.M.) classified the OCT images at step 1 according to ESASO Classification [4] and added the selected stage to the database for statistical analysis.
Statistical Analysis
The primary outcome measure was the therapeutic response to intravitreal agents according to the stage of the disease. This was assessed by changes in VA following intravitreal injections of anti-VEGF or DEX, stratified according to the DME stage before treatment.
Descriptive statistics were used to summarize the demographic and clinical characteristics of the study participants. The association between the ESASO classification stages and treatment outcomes was analyzed using a repeated-measure linear regression model, where the VA and the CST were the dependent variables, the visit (baseline versus post-treatment) was the main covariate, and the eye identification number was the random effect. The effect of DME stage was evaluated as the interaction term in these models. Additionally, subgroup analyses were conducted to investigate the impact of individual OCT parameters on treatment response.
Results
Baseline Characteristics
A total of 608 eyes were initially included from 18 centers, with an average contribution of 34 eyes per center. A total number of 48 eyes were excluded due to poor quality of the spectral-domain (SD)–OCT scan, differing SD-OCT scan orientation, extrafoveal scans, absence of OCT scan at follow-up, or presence of macular comorbidities. The study ultimately comprised 560 eyes from 560 patients, with 62% males and an average age of 66.7 ± 9.69 years.
DME Staging at Baseline
Demographic and clinical characteristics are detailed in Table 3. Among the eyes studied, 31% (n = 173) were classified as DME stage 1, 50% (n = 280) as stage 2, 17% (n = 96) as stage 3, and 2% (n = 11) as stage 4. Visual acuity at baseline was 0.53 ± 0.24 decimal at stage 1, 0.41 ± 0.21 decimal at stage 2, 0.18 ± 0.13 decimal at stage 3, and 0.14 ± 0.13 decimal at stage 4. The mean preinjection CST was 370 ± 58.9 µm at stage 1, 517 ± 113 µm at stage 2, 609 ± 156 µm at stage 3, and 242 ± 67 µm at stage 4, and the differences between each and all of these stages were statistically different (p < 0.001). Patients with stage 4 DME were older (p = 0.01), and a higher proportion of male patients was observed in stage 2 (p = 0.03). The types of diabetes, duration of diabetes, and history of prior treatments were comparable across all stages.
Treatment Patterns
In this study, treatment patterns varied across different ESASO stages of DME. Of the 560 eyes evaluated, 60.7% (340 eyes) received anti-VEGF treatments, while 39.3% (220 eyes) were treated with intravitreal DEX. Early and atrophic stages of DME were more frequently managed with anti-VEGF therapy (Fig. 2). Eyes with advanced (58.9% anti-VEGF versus 41.1% DEX, p = 0.03) and severe DME (46.9% anti-VEGF versus 53.1% DEX, p = 0.002) were more likely to receive DEX. Additional factors influencing the use of DEX included older age [odds ratio (OR) 1.61 for every 10-year increase, 95% confidence interval (CI) 1.33–1.97, p < 0.001] and previous treatment with other intravitreal agents (OR 1.66, compared with treatment-naïve eyes, 95% CI 1.16–2.37, p = 0.01).
VA Improvement and Its Correlation with DME Stage
We observed a correlation between DME stage and VA improvement, irrespective of the treatment modality employed. Notably, following the loading phase of treatment, stage 1 and stage 2 eyes demonstrated a significant and clinically meaningful improvement in mean VA, 0.12 and 0.15 decimal, respectively. Conversely, stage 3 eyes showed a statistically significant yet clinically irrelevant improvement of 0.03 decimal, while stage 4 eyes did not exhibit any improvement in VA (Fig. 3a). Furthermore, the disparity in VA improvement between eyes with early (stages 1 and 2) and advanced (stages 3 and 4) DME was statistically significant and substantial (p < 0.0001). Moreover, the study reveals that baseline VA was less predictive of treatment outcomes compared to the stage of DME (Fig. 4). This observation was further reinforced by the finding that the subgroup of eyes with identical low baseline VA ≤ 0.3 decimal improved only in stages 1 and 2 but not in stage 3.
CST as a Predictive Indicator
While baseline CST inversely correlates with baseline VA (Fig. 5), the mean CST reduction in each stage did not consistently predict functional VA improvement. Specifically, stage 3 retinas displayed substantial CST improvement (mean CST decreased by 138 µm, from 457 µm to 319 µm, p < 0.0001) without significant VA enhancement, which improved only by 0.03 decimal. In contrast, stage 2 retinas showed a modest CST improvement (mean CST decreased by 116 µm, from 456 µm to 340 µm, p < 0.0001) and a more meaningful VA improvement of 0.15 decimal. These findings indicate that DME stage more reliably predicts VA outcomes than CST changes. Stage 1 retinas were excluded from this comparison due to inherently lower baseline CST values.
Functional Biomarkers: DRIL and EZ/ELM
The study further investigated the prognostic utility of single retinal biomarkers, specifically the disorganization of retinal inner layers (DRIL) and the integrity of the EZ/ELM. These biomarkers, however, exhibited less statistical significance in predicting postoperative VA when compared to the stage of DME.
DRIL was not consistently linked with postoperative VA outcomes across all DME stages (Fig. 6a). On the contrary, the presence of DRIL at baseline did not limit a significant VA improvement in stage 2 (an increase of 1.5 lines; Fig. 6b), suggesting that DRIL is not a negative prognostic indicator at this stage. In contrast, stage 3 showed only limited VA recovery associated with DRIL.
Absolute variation in VA in DRIL at baseline and in DRIL at the end of the treatment period (a), stage 2 and in stage 3 (b), VA in EZ/ELM Grade1 (n = 224), in EZ/ELM grade 2 (c), and VA in stage 2 and stage 3. DRIL disorganization of the inner retinal layers, EZ ellipsoid zone, ELM external limiting membrane, VA visual acuity
Regarding the impact of EZ/ELM damage on VA recovery, Fig. 6c illustrates that initial damage (grade 1/focal damage) did not preclude a satisfactory VA improvement. Conversely, more extensive damage (grade 2) was linked to poorer VA outcomes. Specifically, stage 2 patients with altered EZ/ELM at baseline experienced better outcomes compared with those in stage 3, as shown in Fig. 6d. Moreover, the absence of outer retinal damage (EZ/ELM grade 0) was not predictive of VA recovery in stage 3, reinforcing the complex relationship between these biomarkers and visual prognosis.
Influence of Previous Ophthalmic Treatments
We found no significant difference in VA outcomes between treatment-naïve eyes and those that had received prior treatments (Fig. 7).
Comparative Analysis of Different Therapeutic Approaches
Although this retrospective study was not primarily designed to compare the efficacy of the different therapeutic agents used to treat DME in the study population, the extensive dataset allowed for some observations regarding treatment choices across various DME stages.
Despite being considered as a second-line therapy, steroids were frequently used as a primary treatment in 40% of cases, irrespective of whether patients were treatment-naïve or had received prior treatments. Interestingly, although steroids were administered also in early-stage DME (stage 1), their usage was more common in later stages (41% in stage 2 and 53% in stage 3). This pattern may reflect a clinical perception of a stronger efficacy of steroids in more severe disease. Furthermore, comparisons between anti-VEGF therapies and steroids revealed no significant differences in outcomes, with steroids achieving comparable results to anti-VEGF after a single injection versus a loading phase. This finding was consistent across various anti-VEGF agents.
Discussion
The concept of the neurovascular unit (NVU) is crucial in diabetic retinopathy and DME, recognizing that damage within any NVU component can impact the entire retinal structure and affect the overall function of the retina. Conventional clinical trial endpoints like visual acuity (VA) and central subfield thickness (CST) may not fully capture the early functional changes or the disease’s later progression to irreversible anatomical decline, and this highlights a gap in current clinical assessment methods.
The ESASO classification system, by integrating multiple biomarkers, provides a comprehensive view of the NVU, and enables the categorization of DME progression into four distinct stages. Our study reinforces the prognostic value of this staged approach over isolated biomarkers, and this underscores the need to reevaluate the over-reliance on single parameters for visual prognosis. The study results show that low baseline VA is not, in isolation, predictive of poor outcomes. Instead, it shows that the predictive value of baseline VA depends on the ESASO stage: similar VA values at baseline were associated with significantly different outcomes in eyes in stages 2 and 3. Similarly, the presence of DRIL was not statistically associated with a worse functional outcome, although this finding may have been due to OCT segmentation errors or the complexities inherent in interpreting OCT images of inner retinal segmentation. Similarly, focal areas of loss of EZ/ELM integrity did not influence the visual outcome in stage 2 eyes but only in stage 3 eyes.
Our data suggest that stages 1 and 2 of the ESASO classification of DME represent a window where the pathology of the disease may still be reversible and that stages 3 and 4 indicate a disease state where irreversible anatomical damage has occurred. Although these findings need to be confirmed by prospective clinical studies, they appear to have an important clinical relevance not only for daily clinical practice but also for future clinical trials, possibly generating a better understanding of the agents under investigation, and avoid the confounding effect of a reduced treatment efficacy in the more advanced, less responsive stages of the disease.
In addition to the primary findings, this study also sheds light on treatment preferences, revealing that steroids, typically regarded as a secondary option in the literature, are frequently chosen as the primary treatment in clinical practice in Italy. Our retrospective analysis shows that steroids were used in approximately 40% of cases, with their use escalating as DME progressed, making them the dominant treatment by stage 3. This trend was observed in both treatment-naïve and previously treated patients. Notably, our data indicate no significant difference in outcomes between steroid and anti-VEGF therapies following the initial treatment phase.
This study has limitations, including its retrospective design, which may introduce selection and confirmation biases and limit causal conclusions. Data derived from electronic medical records may be incomplete or inaccurately documented. Additionally, variations in treatment application across multiple centers could affect the results.
Conclusions
The study confirms that the ESASO classification is a reliable predictor of therapeutic outcomes in DME following intravitreal drug therapy treatment. While OCT parameters offer additional insights, the direct correlation of ESASO stages with treatment response underscores its utility in clinical decision making.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
International Diabetes Federation. IDF Diabetes Atlas Ninth Edition. 2019.
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–64. https://doi.org/10.2337/diacare.26.9.2653.
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. https://doi.org/10.1038/nrdp.2016.12.
Panozzo G, Cicinelli MV, Augustin AJ, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30(1):8–18. https://doi.org/10.1177/1120672119880394.
Panozzo G, Franzolin E, Giannarelli D, et al. Validation of ESASO classification of diabetic maculopathy. Eur J Ophthalmol. 2023. https://doi.org/10.1177/11206721231186649.
Panozzo G, Mura GD, Franzolin E, et al. Early DMO: a predictor of poor outcomes following cataract surgery in diabetic patients. The DICAT-II study. Eye (Lond). 2022;36(8):1687–93. https://doi.org/10.1038/s41433-021-01718-4.
Apuzzo A, Gravina S, Panozzo G, Lattanzio R, Cicinelli MV, Bandello F. From diagnosis to prognosis: a paradigm shift for multimodal imaging in assessing diabetic macular edema. Eur J Ophthalmol. 2023. https://doi.org/10.1177/11206721231199149.
Wong WM, Chee C, Bhargava M, et al. Systemic factors associated with treatment response in diabetic macular edema. J Ophthalmol. 2020;2020:1875860. https://doi.org/10.1155/2020/1875860.
Itoh Y, Petkovsek D, Kaiser PK, Singh RP, Ehlers JP. Optical coherence tomography features in diabetic macular edema and the impact on anti-VEGF response. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):908–13. https://doi.org/10.3928/23258160-20161004-03.
Chen X, Zhang L, Sohn EH, et al. Quantification of external limiting membrane disruption caused by diabetic macular edema from SD-OCT. Investig Ophthalmol Vis Sci. 2012;53(13):8042–8. https://doi.org/10.1167/iovs.12-10083.
Campos A, Campos EJ, do Carmo A, et al. Choroidal thickness changes stratified by outcome in real-world treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1857–65. https://doi.org/10.1007/s00417-018-4072-z.
Campos A, Campos EJ, do Carmo A, et al. Evaluation of markers of outcome in real-world treatment of diabetic macular edema. Eye Vis (Lond). 2018;5:27. https://doi.org/10.1186/s40662-018-0119-9.
Sorour OA, Levine ES, Baumal CR, et al. Persistent diabetic macular edema: definition, incidence, biomarkers, and treatment methods. Surv Ophthalmol. 2023;68(2):147–74. https://doi.org/10.1016/j.survophthal.2022.11.008.
Leite J, Ferreira A, Castro C, et al. Retinal changes after fluocinolone acetonide implant (ILUVIEN(R)) for DME: SD-OCT imaging assessment using ESASO classification. Eur J Ophthalmol. 2023. https://doi.org/10.1177/11206721231183471.
Acknowledgements
The members of ESASO Study Group: Edoardo Angelini, Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy. Pietro Airaghi, Department of Ophthalmology, Department of Ophthalmology, San Gerardo Hospital, via Gian Battista Pergolesi 33, Monza, Italy. Teresio Avitabile, Department of Ophthalmology, University of Catania, Catania, Italy. Francesco Bandello, Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Milan, Italy. Andrea Beccastrini, Azienda ULSS n. 2 Marca Trevigiana, Treviso, Veneto, Italy. Giorgia Benedetti, Ophthalmic Unit, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy. Federico Bertuzzi, Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Milan, Italy. Vincenza Maria Elena Bonfiglio, Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy. Francesco Boscia, Eye Clinic, University of Bari, Italy. Adriano Carnevali, Department of Ophthalmology, University Magna Græcia of Catanzaro, Viale Europa, Catanzaro, Germaneto, Italy. Marianna Carosielli, Department of Medicine and Health Sciences "Vincenzo Tiberio", University of Molise, Campobasso, Italy. Matteo Giuseppe Cereda, Eye Clinic, Department of Biomedical and Clinical Science “Luigi Sacco”, Sacco Hospital, University of Milan, via G.B Grassi 74, Milan, Italy. Cecilia Contardi, Ophthalmology Clinic, Department of Medicine and Science of Ageing, University G. D'Annunzio Chieti-Pescara, Chieti, Italy. Michele Coppola, Department of Ophthalmology, Department of Ophthalmology, San Gerardo Hospital, via Gian Battista Pergolesi 33, Monza, Italy. Ciro Costagliola, Department of Neurosciences, Reproductive Sciences and Dentistry, University of Naples Federico II, Naples, Italy. Riccardo Cristofolini, Ophthalmic Unit, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy. Pasquale Cucciniello, Department of Neurosciences, Reproductive Sciences and Dentistry, University of Naples Federico II, Naples, Italy. Rossella D’Aloisio, Ophthalmology Clinic, Department of Medicine and Science of Ageing, University G. D'Annunzio Chieti-Pescara, Chieti, Italy. Maddalena De Bernardo, Eye Unit, Department of Medicine Surgery and Dentistry, Scuola Medica Salernitana, University of Salerno. Alessandro De Filippis, Ophthalmology Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy. Roberto Dell’Omo, Department of Medicine and Health Sciences “Vincenzo Tiberio”, University of Molise, Campobasso, Italy. Ilenia Di Paola, Eye Unit, Department of Medicine Surgery and Dentistry, Scuola Medica Salernitana, University of Salerno. Matteo Dell’Acqua, ASST Ovest Milanese, Legnano, Italy. Alessio Franco, Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy. Maria Oliva Grassi, Eye Clinic, University of Bari, Italy. Giulia Gregori, Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Milan, Italy. Elena Gusson, Ophthalmic Unit, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy. Rosangela Lattanzio, Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Milan, Italy. Paolo Lanzetta, Department of Medicine-Ophthalmology, University of Udine, Udine, Istituto Europeo di Microchirurgia Oculare (IEMO), Udine. Antonio Longo, Department of Ophthalmology, University of Catania, Catania, Italy. Giorgio Marchini, Ophthalmic Unit, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy. Paola Marolo, Department of Surgical Sciences, Eye Clinic Section. University of Turin, Italy. Rodolfo Mastropasqua, Ophthalmology Clinic, Department of Medicine and Science of Ageing, University G. D'Annunzio Chieti-Pescara, Chieti, Italy. Giuliana Mele Bertoldo, UO Diabetologia Schio, ULSS 7 Pedemontana, Italy. Giuseppina Monteleone, Eye Clinic, University of Bari, Italy. Elina Ortisi, Department of Ophthalmology, University of Catania, Catania, Italy. Guglielmo Parisi, Department of Surgical Sciences, Eye Clinic Section. University of Turin, Italy. Salvatore Parrulli, Eye Clinic, Department of Biomedical and Clinical Science “Luigi Sacco”, Sacco Hospital, University of Milan, via G.B Grassi 74, Milan, Italy. Porzia Pucci, Azienda ULSS n 2 Marca Trevigiana, Treviso, Veneto, Italy. Marco Rocco Pastore, Eye Clinic, Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy. Michele Reibaldi, Department of Surgical Sciences, Eye Clinic Section. University of Turin, Italy. Stanislao Rizzo, Ophthalmology Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy. Francesco Romano, Eye Clinic, Department of Biomedical and Clinical Science “Luigi Sacco”, Sacco Hospital, University of Milan, via G.B Grassi 74, Milan, Italy. Nicola Rosa, Eye Unit, Department of Medicine Surgery and Dentistry, Scuola Medica Salernitana, University of Salerno. Valentina Sarao, Department of Medicine-Ophthalmology, University of Udine, Udine, Istituto Europeo di Microchirurgia Oculare (IEMO), Udine. Giuseppe Scarpa, Azienda ULSS n 2 Marca Trevigiana, Treviso, Veneto, Italy. Vincenzo Scorcia, Department of Ophthalmology, University Magna Græcia of Catanzaro, Viale Europa, Catanzaro, Germaneto, Italy. Andrea Scupola, Ophthalmology Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy. Giovanni Staurenghi, Eye Clinic, Department of Biomedical and Clinical Science “Luigi Sacco”, Sacco Hospital, University of Milan, via G.B Grassi 74, Milan, Italy. Valentina Sunseri Trapani, Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy. Daniele Tognetto, Eye Clinic, Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy. Giuseppe Trabucchi, ASST Ovest Milanese, Legnano, Italy. Sabrina Vaccaro, Department of Ophthalmology, University Magna Græcia of Catanzaro, Viale Europa, Catanzaro, Germaneto, Italy. Maria Vadalà, Biomedicine, Neuroscience and Advanced Diagnostic Department, University of Palermo, Palermo, Italy. Daniele Veritti, Department of Medicine-Ophthalmology, University of Udine, Udine, Istituto Europeo di Microchirurgia Oculare (IEMO), Udine. Alex Lucia Vinciguerra, Eye Clinic, Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy. Emma Clara Zanzottera, Department of Ophthalmology, Department of Ophthalmology, San Gerardo Hospital, via Gian Battista Pergolesi 33, Monza, Italy.
Funding
No funding or sponsorship was received for this study or publication of this article. The rapid service fee was funded by the authors.
Author information
Authors and Affiliations
Consortia
Contributions
Giacomo Panozzo, Maria Vittoria Cicinelli, Giulia Dalla Mura, Maria Vadalà, Vincenza Bonfiglio, Giovanni Bellisario, and Francesco Bandello provided substantial intellectual input during both the drafting and critical revision of the manuscript. Diana Giannarelli conducted the statistical analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Neither Giacomo Panozzo nor Maria Vittoria Cicinelli, Giulia Dalla Mura, Diana Giannarelli, Maria Vadalà, Vincenza Bonfiglio, Giovanni Bellisario, nor Francesco Bandello received financial support for the research,authorship, and/or publication of this article.
Ethical approval
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. According to the Italian Medicines Agency (AIFA) requirements, retrospective studies, and the communication of pooled (aggregated) data to the Coordinating Center only necessitate communication to the Ethics Committee and do not require explicit approval.
Additional information
The members of ESASO Study Group are listed in Acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Panozzo, G., Cicinelli, M.V., Dalla Mura, G. et al. Enhancing Diabetic Macular Edema Treatment Outcomes: Exploring the ESASO Classification and Structural OCT Biomarkers. Ophthalmol Ther 13, 1383–1398 (2024). https://doi.org/10.1007/s40123-024-00925-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00925-y