Abstract
Introduction
To analyze the correlation between orbital compliance and retinal vessel density (VD) based on dynamic Scheimpflug analyzer (Corvis ST) and optical coherence tomographic angiography (OCT-A).
Methods
In this prospective observational study, 65 eyes of 44 patients with thyroid-associated ophthalmopathy (TAO) in quiescent stage were included (15 males and 29 females). The whole eye movement (WEM) was detected by Corvis ST. The superficial capillary plexus VD (SCP-VD) and deep capillary plexus VD (DCP-VD) were obtained by scanning the 3 × 3 mm area around the fovea using OCT-A, while the peripapillary vessel density (ppVD) was obtained by scanning the 4.5 × 4.5 mm area around the optic disk. Covariances including biomechanically corrected intraocular pressure (bIOP), axial length, age and gender were adjusted during data analysis.
Results
The mean WEM of the participants was 0.235 ± 0.066 mm. The mean SCP-VD and DCP-VD in whole image were 46.20% ± 3.77% and 50.51% ± 3.96%; the mean whole pp-VD was 49.75% ± 2.01%. WEM was positively correlated with SCP-VD (r = 0.327, p = 0.01) and the whole pp-VD (r = 0.394, p < 0.01) after adjusting by gender, axial length (AL), age and bIOP, but it was not significantly correlated with DCP-VD (r = 0.072 p = 0.581).
Conclusion
Increase in orbital pressure might reduce retinal microvascular perfusion. Our data suggest orbital mechanical compression may be an important cause of retinal VD changes in quiescent patients with TAO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Retinal microvascular perfusion often decreases in patients with thyroid-associated ophthalmopathy (TAO), but the mechanism of this phenomena remains unclear. |
Mechanical compression from lowered orbital compliance may contributed to this process. |
The whole eye movement (WEM) is a reliable parameter for evaluating intraorbital pressure or orbital compliance. |
What was learned from this study? |
In this study, we first demonstrated a significant positive correlation between WEM and retinal vessel density (VD) in quiescent patients with TAO, namely a lower retinal VD with a stiffer orbit. |
This study could, to some degree, help to understand the causes of retinal VD changes in patients with TAO and the pathophysiological mechanism of TAO. |
Introduction
Thyroid-associated ophthalmopathy (TAO) has a high incidence in adult orbital diseases, which is considered as an autoimmune disease. It can occur in the setting of hyperthyroidism, hypothyroidism or even euthyroidism. It characterizes multiple manifestations, such as chronic inflammatory infiltration of orbital fat and extraocular muscles, eyelid and conjunctive congestion, eyelid retraction, restrictive strabismus and optic neuropathy. Enzmann et al. showed that approximately 70% of patients with TAO have clinical or subclinical extraocular muscle hypertrophy [1]. In most cases of TAO, intraorbital pressure is obviouslyelevated because of the hypertrophy of intraorbital soft tissue in a restricted bone compartment. Meanwhile, there were significant differences in retinal vessel density (VD) between patients with TAO and healthy individuals. Most studies agreed that retinal VD in patients with TAO was significantly lower than that in healthy people [2,3,4,5,6]. The degree of retinal VD change was related to the visual quality of the patient [7]. However, the reason for VD changes in TAO remained unclear. In this study, we aimed to evaluate whether decreased orbital compliance contributed to those retinal changes, which might expand our knowledge about pathogenesis of retinal microvascular perfusion.
Hyperplastic extraocular muscles and fat hypertrophy can lead to reduced orbital compliance. Measurement of intraorbital compliance is helpful for early diagnosis and assessment of disease severity and stability [8]. In the past, clinical evaluation of orbital compliance only relied on eyeball compression by palpation with both hands. The rough judgment was based on the degree of retraction of the eyeball back into the orbital socket. However, the direct measurement of compliance in the orbit was invasive and mainly used in experimental research, which also had many defects and was inappropriate to be generalized [9].
The dynamic Scheimpflug analyzer system (Corvis ST; Oculus, Wetzler, Germany) is applied to the cornea by an air pulse with constant parameters, and the cornea undergoes two deformation processes. The Scheimpflug camera records the corneal deformation process and calculates considerable biomechanical parameters. Slight but significant movement of the entire eyeball occurring during the measurement is detected. When the cornea is deformed and approaching maximum displacement, the eyeball shows slow linear movement in the forward and backward direction [10, 11]. The machine can automatically measure the whole eye movement (WEM) under a certain degree of the air pulse. In recent years, some scholars have considered WEM a reliable parameter for evaluating intraorbital pressure or orbital compliance. They found that WEM in patients with TAO was significantly lower than that in healthy people [12,13,14], and this difference remained even after adjusting for intraocular pressure (IOP).
Therefore, we speculated that the changes in retinal VD in patients with TAO might be related to mechanical compression caused by decreased orbital compliance or increased orbital pressure, but there was no evidence at present. Therefore, this study aimed to detect the WEM parameters of patients with TAO with Corvis ST and the changes in fundus blood flow status with optical coherence tomographic angiography (OCT-A) and to evaluate the correlation between orbital compliance and fundus blood flow status in patients with TAO.
Methods
Subjects
In this cross-sectional study, we recruited patients diagnosed with quiescent TAO in the Eye Hospital of Wenzhou Medical University (Hangzhou Branch) from December 2021 to February 2023. The clinical activity score (CAS) was used to grade the activity status of patients [15].
Recruitment Criteria
TAO was diagnosed according to the Bartley and Gorman criteria [16]. We included patients as follows: (1) age from 18 to 60 years old; (2) CAS < 3, namely in quiescent stage; (3) all patients had completed investigations including slit-lamp biomicroscopy, fundus examination, ocular biomechanical examination by Corvis ST, macular and peripapillary VD by OCT-A and axial length (AL) by IOL Master 700.
Exclusion Conditions
Exclusion criteria were as follows: (1) a history of orbital or ocular surgery or trauma; (2) corneal inflammation, thinning, ulcer, scar and other corneal lesions; (3) vitreoretinopathy, maculopathy and other intraocular diseases; (4) hypertension, diabetes, kidney disease and other diseases affecting blood circulation; (5) mixed spherical diopter was < − 6.0D and > + 3.0D; (6) pregnant and lactating women; (7) patients with IOP ≥ 21 mmHg.
Ethical Considerations
This study was conducted according with the principles of the Declaration of Helsinki. The ethnical protocol was thoroughly reviewed and approved by the Ethics Committee of Eye Hospital of Wenzhou Medical University (H2022-023-K-23-01). All clinical data obtained from participants were saved separately to prevent risk of disclosure. Written informed consent was obtained from all participants.
Investigations
Corvis ST
Corvis ST (CST, software version 1.6r2187, OCULUS Optikgeräte, Wetzlar, Germany) is a non-contact tonometer with an ultrahigh-speed Scheimpflug device that successfully captures the image of corneal deformation caused by blowing and calculates various dynamic corneal response parameters. The CST captures about 140 cross-sectional images of the cornea during the dynamic deformation induced by an air puff using its high-speed camera system. The capability to monitor response of the cornea provides essential information that can be used to detect the precise biomechanical properties of the tissue. However, some parameters are less reproducible because of unreliable eye position and body position, which requires examiner experience.
The same experienced technician carried out all the measurements. The examiner performed the Corvis ST test as follows: the eye was positioned in front of the system at a distance of 11 mm between the air tube and corneal apex. When the eye was aligned precisely and the Scheimpflug image was in focus, the air puff was automatically released and the deformation of cornea was imaged. The CST automatically assessed the quality of each measurement. Only measurements for which the quality score was shown as OK were selected. Every participant required three eligible measurements, and the interval between two measurements was 2 min. The results were averaged over the three measurements. Biomechanical parameters such as biomechanically corrected intraocular pressure (bIOP) and WEM were automatically calculated from an internal system as in previous studies [13, 14, 17]. Details of the Corvis ST are described elsewhere [11, 18].
OCT-A
Following implementation of optical coherence tomography (OCT), OCT-A is used to further detect the blood flow of the retina and choroid. It shows good repeatability in quantitative analysis of retinal blood flow in comprehensive retinal vasculopathies. The same experienced technician carried out all OCT-A measurements.
-
1.
Patients' macular and optic disc were scanned by OCT-A (Optovue Inc., Fremont, CA, USA). The patients were measured in a sitting position. The macular retinal angiography images were obtained by dynamic correction calculation of red blood cell movement after repeated scanning. The system's built-in software (version 2018,1,1,63) was used to display the distribution diagram of superficial and deep retinal capillaries and the distribution diagram of the radial capillary network of the optic disc in layers. The images obtained were confirmed correctly stratified and clear, with image quality > 5/10.
-
2.
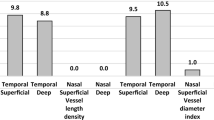
Macular microvascular status analysis: (a) stratification and zoning. The 3 mm × 3 mm flow imaging scanning mode was selected. Superficial capillary macular layer was obtained using 3–15 um signals under the inner limiting membrane (ILM), while the deep capillary layer was obtained using 15–70 um signals under the ILM. Parafoveal zone was defined as the area in the middle circular region that was beyond a diameter of 1 mm (representing the foveal avascular zone) but less than 3 mm. (b) Image analysis index: VD was defined as proportion of vessel area with blood flow over the toral area measured. The observation range includes the data of the whole circumference, upper half, lower half, temporal side, upper part, nasal side and lower part obtained by the ratio of the area of capillary distribution to the total area (Fig. 1).
-
3.
Analysis of the microvascular status of optic disc: optic disc HD 4.5 mm × 4.5 mm; blood flow imaging scanning mode was selected, and the center point of optic disc of the inspected eye was taken as a 2 mm ring area with a center width (inner and outer ring diameters of 2 mm and 4 mm, respectively) as the measurement area beside the optic disc. Peripapillary VD (ppVD) between the ILM and nerve fiber layer was automatically measured to obtain whole peripapillary, temporal, superior, inferior, superior-nasal (SN), superior-temporal (ST), inferior-nasal (IN), inferior-temporal (IT), nasal-superior (NS), nasal-inferior (NI), temporal-superior (TS) and temporal-inferior (TI) data (Fig. 1).
SCP-VD, DCP-VD and ppVD in OCT-A scanning. An example of OCT-A scanning in a right eye. The SCP-VD and DCP-VD were detected in a scanning area of 3 mm × 3 mm. The inner and outer rings in this protocol were set at a diameter of 1 mm and 3 mm, respectively. Parafoveal zone, defined as an area between the two rings, was examined. The pp-VDs were detected in the 2-mm ring area with a center width (inner and outer ring diameters of 2 mm and 4 mm, respectively) around the papillary disk in the 4.5 mm × 4.5 mm protocol. SCP-VD superficial capillary plexus vessel density, SCP-VD superficial capillary plexus vessel density, DCP-VD deep capillary plexus vessel density, ppVD peripapillary vessel density, OCT-A optical coherence tomographic angiography, S superior, T temporal, N nasal, I inferior, NS nasal-superior, NI nasal-inferior, IN inferior-nasal, IT inferior-temporal, TI temporal-inferior, TS temporal-superior, ST superior-temporal, SN superior-nasal
Statistical Analyses
SPSS version 26.0 for Windows (SPSS Inc., IBM Corp., Chicago, IL, USA) was used for the data analyses. Continuous variables were expressed as mean ± SD and categorical variables as percentage. Spearman's and Pearson's correlations were used to examine the relationship between continuous variables with normal and non-normal distribution. Age, gender, AL and bIOP were used as control variables to conduct a partial correlation analysis between WEM and macular VD and ppVD. P < 0.05 was considered a significant correlation.
Results
Demographic and Clinical Characteristics
Sixty-five eyes of 44 patients (15 males and 29 females) with thyroid-related eye disease were included. The mean age was 38.3 ± 11.6 (range 23 to 65) years. AL was 24.08 ± 1.25 mm, and bIOP was 16.32 ± 2.03 mmHg. The WEM was 0.235 ± 0.066 mm (Table 1).
Correlation Between Ocular Biomechanical Parameters and Macular Blood Flow
After adjusting with bIOP, AL, age and gender, the SCP-VD of the whole parafoveal region was significantly correlated with WEM (r = 0.362, p = 0.00). Parafoveal area was divided into four regions, superior, inferior, nasal and temporal, and the VDs in the four regions were significantly correlated with WEM: superior (r = 0.353, p = 0.01), inferior (r = 0.379, p = 0.00), nasal (r = 0.306, p = 0.02) and temporal (r = 0.326 p = 0.01). However, the DCP-VD of the whole parafoveal region did not significantly correlate with WEM (r = 0.092 p = 0.512). The superior, inferior, nasal and temporal regions also had no significant correlation with WEM. (p = 0.63, 0.39, 0.44, 0.83, respectively) (Table 2, Figs. 2, 4).
Correlation among WEM, SCP-VD and DCP-VD in different groups of patients with TAO. The red region represents a significant correlation between VD and WEM, while the blue area represents no considerable correlation between VD and WEM. *The numbers in the figure represent the correlation coefficients of WEM and VD in this region. WEM whole eye movement, SCP-VD superficial capillary plexus vessel density, DCP-VD deep capillary plexus vessel density, TAO thyroid-associated ophthalmopathy, VD retinal vessel density, S superior, T temporal, N nasal, I inferior, EDTRS Early Treatment Diabetic Retinopathy Study, TSNI temporal-superior-nasal-inferior
Correlation Between Ocular Biomechanical Parameters and Optic Disc Blood Flow
After adjusting with bIOP, AL, age and gender, there was a significant correlation between the whole pp-VD and WEM (r = 0.306, p = 0.02). The peripapillary area was divided into eight regions. WEM was significantly correlated with IN (r = 0.279, p = 0.030), IT (r = 0.290, p = 0.024), ST (r = 0.274, p = 0.033) and SN (r = 0.298, p = 0.020) regions after adjusting, but NS, SI, TI and TS regions were not significantly correlated with it after adjusting (p = 0.690, p = 0.677, p = 0.421, p = 0.424, respectively) (Table 3, Figs. 3, 4).
Correlation between WEM and ppVD in different groups of patients with TAO. The red region represents a significant correlation between VD and WEM, while the blue area represents no considerable correlation between VD and WEM. *The numbers in the figure represent the correlation coefficients of WEM and VD in this region. TSNIT temporal-superior-nasal-inferior-temporal, WEM whole eye movement, ppVD peripapillary vessel density, TAO thyroid-associated ophthalmopathy, VD retinal vessel density, NS nasal-superior, NI nasal-inferior, IN inferior-nasal, IT inferior-temporal, TI temporal-inferior, TS temporal-superior, ST superior-temporal, SN superior-nasal
Correlation between WEM and retinal microvascular perfusion (SCP-VD, DCP-VD and ppVD) in patients with TAO. Dots in blue show correlation between WEM and SCP-VD (r = 0.362, p = 0.00); squares in red show correlation between WEM and DCP-VD (r = 0.092, p = 0.512); triangles in green show correlation between WEM and ppVD (r = 0.306, p = 0.02). WEM whole eye movement, SCP-VD superficial capillary plexus vessel density, DCP-VD deep capillary plexus vessel density, ppVD peripapillary vessel density, TAO thyroid-associated ophthalmopathy
Discussion
TAO is an autoimmune disease characterized by chronic inflammatory infiltration of orbital fat and extraocular muscle, resulting in increased orbital pressure and mechanical compression of the posterior pole of the eyeball. The velocity of the superior ophthalmic vein in patients with TAO decreases, and the blood flow in different fundus areas changes to varying degrees. As early as 20 years ago, using Doppler ultrasound, some scholars showed that the level of the resistive index (RI) and pulsatility index (PI) of the branch of central retinal artery in patients with TAO changed significantly compared with normal people [19, 20]. With the wide application of OCT-A, the changes in retinal blood flow status in patients with TAO have been revealed by more and more studies. Jamshidian et al. revealed that the VD in the parafoveal area of patients with active TAO was significantly lower than that of healthy people [21], but YE et al. found that the density of the microvasculature in the deep and superficial macular area of patients with active TAO was significantly higher than that of healthy people [22]. However, other studies showed no significant difference in macular VD in the parafoveal area between patients with active TAO and healthy people [23]. In contrast, the nasal and temporal VD in the parafoveal area of patients with inactive TAO was significantly increased compared with the normal group [24]. In general, the results of major studies agreed that TAO had a reduced retinal VD [2,3,4,5,6]; the disparity might result from different activity degrees in the previous studies.
Patients with active TAO are quite different from quiescent ones. In these cases, higher intraorbital pressure may to some degree cause decreased retinal perfusion, but severe inflammation in the orbit would obviously increase the retinal or choroidal vascular perfusion.
To reduce the bias from different degrees of activity in this study, active patients with TAO with CAS ≥ 3 were excluded. Patients with bIOP ≥ 21 mmHg were also excluded because WEM was significantly affected by bIOP [10]. The accuracy of WEM measurements in patients with ocular hypertension cannot be guaranteed in those cases.
The retinal blood flow changes in patients with TAO are a dynamic processes affected by multiple factors. Circulatory and ocular factors can lead to retinal blood flow changes in patients with TAO. Theoretically, systemic hypertension can increase retinal VD in patients with TAO [25], and pulse pressure is related to retinal VD [6]. In addition, TAO orbital inflammatory changes also lead to increased blood flow velocity in most patients with TAO, but in the advanced stage of TAO, the compression caused by the denaturation and fibrosis of orbital muscle and adipose tissue can reduce retinal blood flow density [26].
Many previous studies focused on the effect of disease activity and severity on retinal VD in patients with TAO. However, most of them ignored the factor of mechanical compression. Even though some studies had considered the protuberance of the eyeball [23], it was not a satisfactory quantitative parameter for the compliance of retrobulbar soft tissue. Moreover, the blood pressure, thyroid function and other factors that might affect the systemic circulation status of patients with TAO included in each study were not uniform, which might be part of the reasons for the inconsistent results of previous studies.
In this study, we used OCTA to assess the macular VD and ppVD and corvis ST to assess the intraorbital pressure in quiescent patients with TAO. The WEM measured by the corvis ST reflects the global displacement of the eyeball during air pulse application. WEM had good repeatability [27] but was mainly affected by IOP and age [10]. Some studies also proved that WEM was positively correlated with age and had a specific relationship with AL [28, 29], so we considered those factors as covariates during data analysis.
Our study showed a significant positive correlation between WEM and VD in the superficial macular layer and part of the peripapillary area in quiescent patients with TAO. To our knowledge, this was the first study that used WEM as a parameter to quantify intraorbital pressure and analyze its correlation to retinal VD. A shorter WEM means a lower orbital compliance, a stiffer intraorbital compartment or higher intraorbital pressure. Given the different biomechanical characteristics of each part of the orbit (intraconal, extraconal, subperiosteal space, etc.), the intraorbital pressure distribution is quite variable and unlikely to test accurately in patients clinically. The direct compressive effect on the eyeball, ciliary arteries or central retinal artery could not be detected. We inferred that the retinal microvascular changes in TAO probably resulted from the central retinal artery compression based on the anatomical structures. Both macular SCP and DCP are dominated by the central retinal artery, which enters the optic nerve from the inferior direction about 1 cm away from the globe. The inferior rectus muscle is probably affected in patients with TAO. So, given the increased orbital pressure, compression of the central retinal artery might occur in the earlier stage. We also speculated that anatomical and image processing differences might be the reason for the significant correlation between macular SCP and WEM but not DCP. Though both SCP and DCP receive their own arteriolar supply from superficial retinal arterioles and respectively drain vertically to superficial venules, the DCP also connects to other venules and arterioles through radially orientated vessels [30]. Meanwhile, in the recent commercial OCT-A imaging process, the projection artifacts from superficial vessels could interfere with the DCP visualization and imaging accuracy, which could be improved by projection resolved OCT-A [31]. Both anatomical and imaging factors may play a role in this disparity. However, the specific mechanism remains unclear. In most of the Chinese population, the central retinal artery is divided into superior and inferior branches at the level of the optic disc or before entering the optic disc, and the superior and inferior ppVDs are more extensive, which may explain the significant correlation between the superior and inferior ppVD and WEM in this study.
As we mentioned before, higher intraorbital pressure could compress many areas of the soft tissues in the orbit quite variably. In this study we only evaluated the retinal vascular prefusion which was supplied from the central retinal artery. The perfusion changes in choroidal vessels supplied from ciliary arteries were ignored. So, in the future both should be studied simultaneously to assess the intraorbital circulation redistribution status, which would help to better understand mechanical compression on ocular perfusions.
The main limitation of this study was that VD data from the more extensive perifoveal area, which had a different vascular distribution from the parafoveal area, were not included. Including perifoveal data might help to better understand the retinal VD in patients with TAO. Second, this study was cross-sectional and could not render a definite result for the orbital compressive effect on retinal microvascular structures. However, this study could to some degree help to understand the causes of retinal VD changes in patients with TAO and the pathophysiological mechanism of TAO.
Conclusion
In conclusion, we demonstrated a significant positive correlation between WEM and retinal VD in quiescent patients with TAO, which meant an increase in orbital pressure might reduce retinal microvascular perfusion. The mechanical pressure of the eyeball in patients with TAO might be one of the important reasons leading to the change of VD in patients with TAO. However, further longitudinal studies are still needed to establish causality conclusively.
Data Availability
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
References
Enzmann D, Marshal WH Jr, Rosenthal AR, Kriss JP. Computed tomography in Graves’ ophthalmopathy. Radiology. 1976;118(3):615–20.
Wu Y, Tu Y, Wu C, et al. Reduced macular inner retinal thickness and microvascular density in the early stage of patients with dysthyroid optic neuropathy. Eye Vis (Lond). 2020;7:16.
Wu Y, Tu Y, Bao L, et al. Reduced retinal microvascular density related to activity status and serum antibodies in patients with graves’ ophthalmopathy. Curr Eye Res. 2020;45(5):576–84.
Yang X, Huang D, Ai S, Liang X, Zhao J, Fang L. Retinal vessel oxygen saturation and vessel diameter in inactive graves ophthalmopathy. Ophthalm Plast Reconstr Surg. 2017;33(6):459–65.
Wu Y, Yang Q, Ding L, et al. Peripapillary structural and microvascular alterations in early dysthyroid optic neuropathy. Eye Vis (Lond). 2022;9(1):30.
Ye J, Liu W, Hu X, et al. Elevated pulse pressure correlated with reduced retinal peripapillary capillary in thyroid-associated ophthalmology with visual field defect. Front Endocrinol (Lausanne). 2022;13: 941051.
Feng J, Yang X, Xu M, et al. Association of microvasculature and macular sensitivity in idiopathic macular epiretinal membrane: using OCT angiography and microperimetry. Front Med (Lausanne). 2021;8: 655013.
Means JH, Stanbury JB. Clinical orbitonometry. Am J Med Sci. 1950;220(4):357–61.
Riemann CD, Foster JA, Kosmorsky GS. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology. 1999;106(7):1296–302.
Vinciguerra R, Elsheikh A, Roberts CJ, et al. Influence of pachymetry and intraocular pressure on dynamic corneal response parameters in healthy patients. J Refract Surg. 2016;32(8):550–61.
Vinciguerra R, Ambrosio R Jr, Elsheikh A, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32(12):803–10.
Hwang HS, Kim EC, Kim MS, Yang SW. A novel method for quantifying the biomechanical parameters of orbital soft tissue using a corneal dynamic scheimpflug analyser: a retrospective study. BMC Ophthalmol. 2019;19(1):53.
Leszczynska A, Moehler K, Spoerl E, et al. Measurement of orbital biomechanical properties in patients with thyroid orbitopathy using the dynamic Scheimpflug analyzer (Corvis ST). Curr Eye Res. 2018;43(3):289–92.
Li HX, Zhao XH, Song Y, et al. Changes in ocular biomechanics after treatment for active Graves’ orbitopathy. J Endocrinol Investig. 2021;44(3):453–8.
Menconi F, Profilo MA, Leo M, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: Rundle’s curve revisited. Thyroid. 2014;24(1):60–6.
Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119(6):792–5.
Vellara HR, Hart R, Gokul A, McGhee CNJ, Patel DV. In vivo ocular biomechanical compliance in thyroid eye disease. Br J Ophthalmol. 2017;101(8):1076–9.
Joda AA, Shervin MM, Kook D, Elsheikh A. Development and validation of a correction equation for Corvis tonometry. Comput Methods Biomech Biomed Engin. 2016;19(9):943–53.
Thyroid Doppler ultrasonography and resistive index in the evaluation of the need for ablative or antithyroid drug therapy in Graves' hyperthyroidism.pdf. 2001.
Kurioka Y, Inaba M, Kawagishi T, et al. Increased retinal blood flow in patients with Graves’ disease: influence of thyroid function and ophthalmopathy. Eur J Endocrinol. 2001;144(2):99–107.
Jamshidian Tehrani M, Mahdizad Z, Kasaei A, Fard MA. Early macular and peripapillary vasculature dropout in active thyroid eye disease. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2533–40.
Ye L, Zhou SS, Yang WL, et al. Retinal microvasculature alteration in active thyroid-associated ophthalmopathy. Endocr Pract. 2018;24(7):658–67.
Yu L, Jiao Q, Cheng Y, Zhu Y, Lin Z, Shen X. Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography. BMC Ophthalmol. 2020;20(1):421.
Akpolat C, Kurt MM, Yilmaz M, Ordulu F, Evliyaoglu F. Analysis of foveal and parafoveal microvascular density and retinal vessel caliber alteration in inactive Graves’ ophthalmopathy. J Ophthalmol. 2020;2020:7643737.
Alp MN, Ozgen A, Can I, Cakar P, Gunalp I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br J Ophthalmol. 2000;84(9):1027–30.
Zhang X, Liu W, Zhang Z, Dai J, Zhang J, Lin L. Analysis of macular blood flow changes in thyroid associated ophthalmopathy. BMC Ophthalmol. 2022;22(1):501.
Vellara HR, Ali NQ, Gokul A, Turuwhenua J, Patel DV, McGhee CN. Quantitative analysis of corneal energy dissipation and corneal and orbital deformation in response to an air-pulse in healthy eyes. Investig Ophthalmol Vis Sci. 2015;56(11):6941–7.
Hon Y, Lam AK. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90(1):e1-8.
Aoki S, Murata H, Matsuura M, et al. The effect of air pulse-driven whole eye motion on the association between corneal hysteresis and glaucomatous visual field progression. Sci Rep. 2018;8(1):2969.
Nesper PL, Fawzi AA. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Investig Opthalmol Vis Sci. 2018;59(10).
Hormel TT, Jia Y, Jian Y, et al. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res. 2021;80.
Funding
This study and its publication, including the journal’s Rapid Service Fee, was supported by Wenzhou Municipal Science and Technology Bureau (no. Y20220777).
Author information
Authors and Affiliations
Contributions
Wei Fang, Fangjun Bao and Lijun Shen contributed to the study conception and design. Material preparation, data collection and analysis were performed by Wei Fang and Ziyun Zhou. The first draft of the manuscript was written by Ziyun Zhou and Wei Fang. Zhenbin Qian, Mengdi Wang and Lijun Shen comprehensively reviewed the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Wei Fang, Ziyun Zhou, Zhenbin Qian, Mengdi Wang, Fangjun Bao and Lijun Shen declare that they have no competing interests.
Ethical Approval
This study was conducted according with the principles of the Declaration of Helsinki. The ethnical protocol was thoroughly reviewed and approved by the Ethics Committee of Eye Hospital of Wenzhou Medical University (H2022-023-K-23–01). All the clinical data obtained from participants were saved separately to prevent risk of disclosure. Written informed consent was obtained from all participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fang, W., Zhou, Z., Qian, Z. et al. Effect of Intraorbital Mechanical Compression on Retinal Microvascular Perfusion in Quiescent Thyroid-Associated Ophthalmopathy Based on Ocular Biomechanics Measured by Corvis ST. Ophthalmol Ther 13, 1159–1170 (2024). https://doi.org/10.1007/s40123-024-00912-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00912-3