Abstract
Many important abnormalities of the vitreous, retina and choroid are predominantly located in the peripheral retina. In some retinal diseases with both central and peripheral manifestations, pathological structural or vascular changes can be apparent in the periphery before they are detectable in the central retina. Conventional optical coherence tomography (OCT) and optical coherence tomography angiography (OCT-A) imaging only cover the most posterior 30° of the retina. Wide-field OCT (WF-OCT), though offering detailed cross-sectional imaging of the peripheral retina, is not yet systematically used in clinical practice. This narrative review provides a presentation of the utilisation of WF-OCT and WF-OCT-A in the diagnosis and monitoring of a variety of ophthalmological diseases and discusses the advantages and limitations of the technology. With the rapidly developing technology, multiple WF-OCT and WF-OCT-A devices are now commercially available and enable the clinician to obtain scans within a field of view up to 200°. As detailed in this review, several studies have shown promising results in the application of WF-OCT and WF-OCT-A in diseases of the retina, choroid and vitreous, such as retinal vein occlusion, diabetic retinopathy, ocular oncology, paediatric ophthalmology, uveitis and lesions of the vitreo–retinal interface. In conclusion, WF-OCT and WF-OCT-A can reliably produce high-quality, non-invasive images of the vitreous, retinal, and choroidal structures and vascularity covering the posterior pole as well as the mid and far periphery. These methods can be a valuable part of a multimodal imaging approach in the management of a variety of ocular conditions. Future studies are warranted to investigate the patient outcome benefits of implementation of WF-OCT and WF-OCT-A imaging in a real-life clinical setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many retinal pathologies have predominantly peripheral lesions or affect a larger area than covered by conventional 30° optical coherence tomography (OCT) imaging. |
Commercially available wide-field (WF) OCT and optical coherence tomography angiography (OCT-A) now have a field of view up to 200°. |
WF-OCT and WF-OCT-A have been shown useful in the clinical management of many different retinal disease entities. |
WF-OCT enables clinicians to learn more about peripheral pathology and has the potential to improve patient outcomes. |
Introduction
Optical coherence tomography (OCT) was first introduced by Huang et al. in 1991 and since then, the technology has revolutionised diagnostics in ophthalmology and improved the understanding of both normal and pathologic retinal features [1]. OCT is a non-invasive imaging modality able to produce high-resolution cross-sectional and en face images of biological tissue and is particularly well suited for imaging of the retinal layers. Conventional OCT is standard equipment in ophthalmological practice and is essential to the management of diseases such as age-related macular degeneration (AMD), diabetic macular oedema (DME) and glaucoma.
The technology has continually been refined on multiple parameters and, in recent years, the interest in utilising OCT for imaging of a wider area of the retina has emerged. The field of view (FOV) of conventional OCT equipment is generally limited to segments of the most posterior 30° of the retina, focusing primarily on the macula and/or the optic nerve head. Until recently, the far periphery of the retina was unreachable by OCT, but this technical limitation has been overcome by several new commercially available devices with ultra-wide-field capabilities.
Many important abnormalities of the vitreous, retina and choroid are located in the retinal periphery, identifiable on skilled peripheral retinal examination with fundus ophthalmoscopy or using ultra-wide-field (UWF) fundus photography. Wide-field OCT (WF-OCT), though offering detailed cross-sectional imaging of the periphery, is not yet systematically used in clinical practice.
Many researchers have conducted promising studies investigating the utility of WF-OCT and WF-OCT angiography (WF-OCT-A) in the peripheral retina. Implementation of the WF-OCT and OCT-A imaging techniques in clinical practice may further improve the detection of peripheral pathologies and assist in the management and monitoring of diseases of the vitreous, retina and choroid. These novel imaging modalities have the potential to fill the unmet need of a detailed, cross-sectional imaging of the peripheral retinal structures. Implementation of routine imaging of the retinal periphery may improve clinical decision making and ultimately improve visual outcomes for patients.
Objectives
This review investigates the usage of wide-field OCT and OCT-A in the diagnosis and monitoring of a variety of ophthalmological diseases and discusses the advantages and limitations of the technology. It also presents examples of the usage of WF-OCT and WF-OCT-A in non-ophthalmological medical specialties. Further, it gives a brief overview of the currently available wide-field OCT and OCT-A devices.
Methods
Information sources: We searched databases PubMed, Cochrane, Web of Science and Ovid for publications on wide-field OCT and wide-field OCT-A on 3 October 2023. References were reviewed and relevant publications were selected for the narrative structure of this review. Only articles written in English were considered eligible. We selected 37 published manuscripts as our references. This narrative review is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definitions
Definitions of WF-OCT/OCT-A and UWF-OCT/OCT-A vary significantly between research groups and scientific papers. In 2019, the International Widefield Imaging Study Group presented their definitions of wide-field imaging, classifying wide field as a field of view of 60°–100°, covering the mid-periphery of the retina up to the posterior edge of the vortex vein ampulla. Ultra-wide-field was defined as a FOV of 110°–220°, imaging the far periphery of the retina, from the anterior edge of the vortex vein ampulla and beyond to ora serrata [2]. In this review, we adhere to the above-mentioned definitions, though we chose to include scanning areas beyond the conventional 30°–50° FOV in the wide-field category, as OCT (and OCT-A in particular) have a more limited FOV than other imaging modalities.
Wide-Field OCT
OCT is an imaging technique based on interferometry. The device measures the interference spectrum of reflected light from biological tissue and transforms it into information about the depth and reflectivity of the tissue structures, thereby obtaining detailed two- and three-dimensional scanning images of the retina.
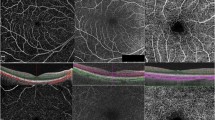
Since its invention, OCT technology has rapidly evolved, with better sensitivity, increased scanning depth and higher resolution. With the advancement of imaging technology, it is now possible to obtain OCT images of up to 200° [3]. The first and, to date, most common method for wide-field imaging is the montage method in which multiple scans are combined in a mosaic to create one wide-field image. This can be obtained either through a steerable laser head or guided fixation of the patient’s gaze in multiple directions [3]. Montaging can be done automatically and has the clear advantage of a larger FOV, but the method is susceptible to motion and other image artefacts, which may require manual montage correction. Single-capture wide-field OCT is faster and requires no montage but is susceptible to peripheral image distortion due to the retinal curvature, which impacts the image quality. Progress in imaging devices and image processing software continually improves on both methods to produce excellent resolution wide-field OCT and OCT-A scans. An example of a wide-field OCT scan in degenerative senile retinoschisis can be seen in Fig. 1 adapted from Choudhry et al. [3].
UWF montage of typical degenerative senile retinoschisis with outer retinal hole. Adapted from Choudhry et al. [3]. A UWF colour image of typical degenerative senile retinoschisis. The junction of non-schitic and schitic retina is outlined by the white inset. B A montaged UWF spectral domain (SD)-OCT cross-section from nasal periphery, through the optic nerve and fovea, to the schitic temporal periphery is registered by the green arrow on the corresponding UWF colour image (A). Schisis of the retina at the inner nuclear and outer plexiform layers is seen posteriorly in the temporal macula (B, white arrow) and proceeds all the way to the periphery, with the degree of intraretinal splitting progressively widening posteriorly (white arrowhead) to anteriorly (B, black arrow). C Colour and D, F NIR-SLO images with overlaid raster scans (yellow and green arrows) register the position of additional peripheral SD-OCT cross-sections (E, G). An inferotemporal portion of the retina (A, inset; C, D, F, arrowheads) is also analysed by peripheral SD-OCT, which reveals splitting along the inner nuclear and outer plexiform layers (E, G, white asterisks) and highlights the vitreous interface (G, black asterisk)
Commercially available WF-OCT and WF-OCT-A devices are presented in Table 1.
Wide-Field OCT Angiography
Optical coherence tomography angiography is a functional extension of OCT utilising the intrinsic motion within the layers of the retina to directly visualise retinal blood flow. The technology uses multiple sequential scans of laser light reflectance from the surface of moving red blood cells within the retinal and choroidal vessels to differentiate functional blood vessels from the surrounding static tissue [4]. En face OCT-A images of the retinal microvasculature can be generated by automated (or manually adjusted) segmentation of the retinal layers post-imaging, thus providing three-dimensional information about the retinal vascular structures.
Conventional OCT-A devices are limited to scan sizes of 3 × 3 mm or 6 × 6 mm. Montaging of these images can provide a wider field of view. Montage protocols that combine multiple smaller scans can preserve a high resolution while still imaging a wider area of the retina [5]. However, image acquisition with montaging can be difficult and time consuming in clinical practice. WF-swept-source-OCT-A (SS-OCT-A) devices provide a larger field of view with less total scans while preserving capillary level resolution. The newer devices can capture single-shot images of up to 15 × 15 mm. With a montage strategy that uses these larger base scans, WF-OCT-A images capturing the retinal periphery to the equator can be obtained [6]. WF-OCT-A scans can undergo additional image processing, allowing for the quantitative assessment of vascular parameters such as vessel density (VD) and choroidal vascularity index (CVI), which might also prove useful in the peripheral retina [6].
Application in Ophthalmology
Retinal Vein Occlusion
Retinal vein occlusion (RVO) is a common retinal vascular disorder and a relatively common cause of vision loss in otherwise healthy adults. The monitoring and treatment of RVO depends on the identification of associated lesions such as neovascularisation (NV), macular oedema and retinal non-perfusion. WF-OCT-A provides a detailed visualisation of structural vascular alterations secondary to RVO and ischaemic non-perfusion [7].
Dong et al. conducted a study of WF-OCT-A detection of non-perfusion areas in 48 eyes with branch (BRVO) and central retinal vein occlusion (CRVO) [8]. Study participants underwent indirect ophthalmoscopy, fluorescein angiography (FA) and WF-OCT-A imaging with a 24 × 20 mm scanning mode. Comparing the detection rate of WF-OCT-A with that of FA, the study reports a sensitivity and specificity of WF-OCT-A in detecting non-perfusion areas in RVO of 83.3% and 100%, respectively. In a cross-sectional study of 43 eyes with RVO, Glacet-Bernard et al. compared WF-OCT-A with UWF-FA in the assessment of non-perfusion [9]. Using the PLEX Elite, they obtained five 12 × 12 mm OCT-A scans and compared the montage image with the 200° FOV FA images. For the detection of non-perfusion areas (in this study defined as ischaemic index ≥ 25% on FA), WF-OCT-A was found to have a sensitivity of 100% and a specificity of 64.9% [9]. Similar results were achieved by Shiraki et al., who used a montage protocol of five 12 × 12 mm OCT-A scans to characterise features of retinal non-perfusion in BRVO and compare the findings with those of wide-field FA [10]. They found a high correlation between the non-perfusion areas detected with WF-OCT-A and FA (r2 = 0.94, p < 0.0001). The findings of these studies suggest that WF-OCT-A could potentially replace FA as the first choice of assessing non-perfusion status in RVO.
Kreminger et al. set out to examine the retinal vascular changes in eyes with retinal non-perfusion due to RVO using single-capture 65° WF-OCT-A images [11]. The study found presence of vascular alterations in all included eyes, with tortuous vessels and capillary loss being the two most common, both found in 92.3% of eyes. Using WF-SS-OCT-A with a montaged 100° FOV, Zhang et al. showed the clinical usefulness of WF-OCT-A in imaging BRVO and CRVO patients [7]. When compared with FA, WF-OCT-A images provided more detailed and clear visualisations of the vascular features, such as tortuous vessels and dilated vessels in mild RVO. The scans also provided a detailed visualisation of other vascular abnormalities, such as microaneurysms, intraretinal microvascular abnormalities (IRMA) and NVs [7].
In summary, information about the extent of vascular disruption, non-perfusion and alterations in capillary flow are valuable characteristics in the management and treatment of RVO. WF-OCT-A constitutes a practical and non-invasive method to obtain detailed information about microvascular structures and function in the peripheral retina.
Diabetic Retinopathy
To date, the detection of vascular manifestations in diabetic retinopathy (DR) such as proliferative disease and capillary non-perfusion is largely based on WF-fluorescein angiography and WF-colour fundus photography. The advent of wide-field SS-OCT-A presents a sensitive, less invasive and more efficient method to image and characterise the retinal vascular changes in DR [6].
Hirano et al. examined the feasibility of WF-SS-OCT-A with extended field imaging for the evaluation of the retinal vasculature in diabetic retinopathy and compared it with fluorescein angiography [12]. They found that WF-SS-OCT-A detected non-perfusion areas and NVs with a sensitivity of 96% and 79%, respectively, and specificity of 100% and 96%, respectively. They also found that the WF-SS-OCT-A method was significantly more comfortable for the patients.
A prospective study by Zhu et al. compared the detection rate of NVs in multiple OCT-A scan protocols and found that a protocol with 12 × 12 mm2 centred on the fovea and optic disc provided the highest detection rate [13]. Russel et al. performed WF-SS-OCT-A and UWF-FA on 20 eyes with treatment-naïve proliferative diabetic retinopathy (PDR) and compared the detection and visualisation of NVs by the two imaging modalities before and after panretinal photocoagulation treatment (PRP) [14]. All areas of NV detected by UWF-FA were also captured on the WF-OCT-A montage scans both at baseline and the 3 month follow-up. Additionally, WF-OCT-A imaging provided a more detailed visualisation of the vascular changes, both in cases of regression and progression of NVs. As OCT-A scanning provides simultaneous corresponding OCT B-scans that can be used to detect and monitor DME, Russel et al. propose that WF-SS-OCTA may be the only imaging modality needed in both diagnosis and follow-up of PDR.
Capillary non-perfusion is one of the main characteristics of diabetic retinopathy. WF-OCT-A provides a detailed and quantifiable visualisation of peripheral areas of non-perfusion. Areas of non-perfusion disproportionately affect the retinal periphery before reaching the posterior pole and thus can serve as a warning sign for early diabetic retinopathy [15]. Wang et al. used WF-OCT-A with a 100° FOV to measure retinal non-perfusion [16]. They found that the most peripheral sector of the widefield image (50°–100°) showed a higher degree of non-perfusion compared with the central retinal areas and that this parameter was useful in determining the DR severity (i.e. distinguishing non-PDR from diabetes without retinopathy and non-PDR from PDR). Furthermore, larger areas of non-perfusion on WF-OCT-A were associated with more severe diabetic disease, as graded by clinical examination and fundus photography based on the Early Treatment Diabetic Retinopathy Study (ETDRS) definitions [16].
In summary, WF-OCT-A has been shown to provide a highly reliable and practical method for detection and monitoring of NVs and capillary non-perfusion in diabetic retinopathy.
Ocular Oncology
Benign and malignant tumours can present anywhere in the extension of the retina and choroid, from the macula to ora serrata. Wide-field fundus photography already plays an important role for documentation and diagnosis in ocular oncology clinics. The addition of WF-OCT can contribute a cross-sectional view and three-dimensional (3D)-imaging of a suspected malignant lesion and detailed characterisation of its vascularisation.
Retinoblastomas occur in young children, are often multifocal and can present anywhere in the retina [17]. Soliman et al. investigated the role of a handheld OCT device with wide-field capabilities in guiding management decisions during diagnosis, treatment and follow-up of eyes affected by retinoblastoma [18]. The study included 63 eyes that underwent a total of 339 OCT scanning sessions during the clinical management of the disease. Each OCT session was scored on multiple parameters according to its impact on diagnosis, treatment decisions and follow-up plan. Fifty-eight per cent of OCT scans were deemed to have directed treatment decisions, 16% had contributed to diagnoses and 26% influenced the modification of follow-up regimens. Overall, the handheld WF-OCT device was found to significantly improve the accuracy of the clinical evaluation of retinoblastoma [18].
Tanimukai et al. report a case of a primary vasoproliferative retinal tumour evaluated with wide-field SS-OCT-A [19]. They obtained high-resolution OCT-A images of the superficial layers of the peripherally located retinal tumour. The images depicted distinctive vascular structures on the tumour surface pre-operatively, very similar to indocyanine green angiography (ICGA) images only more clearly demarcated. Post-operatively, flow signals of the tumour vessels disappeared in the OCT-A images, indicating a successful transscleral cryopexy treatment. The authors recommend wide-field OCT-A for both examination and evaluation of treatment effect for peripheral retinal tumours as WF-OCT-A was shown to identify features that are not readily apparent on clinical examination [19].
Using multimodal imaging including SS-OCT and SS-OCT-A, Xuan et al. used the new UWF Toward Pi BMizar device to examine the morphologic features of choroidal osteomas in 23 eyes [20]. With a 120° FOV, they were able to image the tumours and characterise their features and associated NVs. On WF-OCT-A en face images, the tumour boundaries could be clearly demarcated, and in combination with the corresponding B-scans, the anterior and posterior surfaces of the tumour were delineated. This detailed and easily reproducible tumour delineation could prove very useful in monitoring tumour development and deciding a course of treatment.
To date, the gold standard for evaluation of choroidal and retinal tumours remains the clinical examination [21]. With continued technological improvement, WF-OCT has the potential to play an important role in initial documentation, surgical planning, determining the relationship of the tumour to adjacent structures, following the tumour size after treatment and monitoring for recurrence.
Uveitis
The widest possible FOV is of great importance in imaging of uveitis because the onset and progression of disease often involves the mid and far periphery [22]. Many uveitis entities involve not only the retina and choriocapillaris but also the deeper choroidal layers, making SS-OCT technology a very valuable tool due to its deeper tissue penetration [22].
Tian et al. evaluated retinal and choroidal vascular changes in intermediate uveitis with and without concomitant retinal vasculitis using WF-SS-OCT-A acquired by montage and covering 80°–90° FOV [23]. The findings of WF-OCT-A were compared with central 3 × 3 mm OCT-A scans. Interestingly, the quantitative changes of the vascular density in the 3 × 3 mm scan were not correlated to the degree of peripheral non-perfusion found on WF-OCT-A. This indicates that central retinal changes are not necessarily indicative of the status of the peripheral retina in uveitis patients and highlights the need for wide-field imaging in these patients.
Posterior uveitis is a heterogeneous group of diseases with inflammation of the choroid and/or retina, which may result in severe vision loss if not treated [24]. Tian et al. compared ICGA with SS-WF-OCT-A for the evaluation of patients with various types of posterior uveitis (idiopathic posterior uveitis, birdshot chorioretinitis, Vogt–Koyanagi–Harada chorioretinitis, ocular sarcoidosis) [25]. They used WF-OCT-A with a 70°–90° FOV and UWF-ICGA with 150° FOV and recorded flow deficits on OCT-A and presence of hypofluorescent lesions on ICGA. In several cases, the lesion found by UWF-ICGA was not detected by WF-OCT-A, but in the majority of these cases, the lesion was located outside the FOV of OCT-A – suggesting the need for availability and clinical implementation of UWF-OCT-A devices. Further, the study found that the mean lesion size was significantly larger on ICGA than on WF-OCT-A.
Invernizzi et al. investigated the utility of WF-OCT imaging in differentiating causes of infectious necrotising retinitis (NIR) [26]. WF-OCT scans of eyes suffering from active NIR due to herpesvirus or toxoplasmosis were characterised and compared. The study found a significant correlation between WF-OCT features such as hypo-reflectivity of the choroid and disruption of choroidal architecture in a clinically and microbiologically confirmed diagnosis of NIR due to toxoplasmosis. Likewise, intraretinal oedema was significantly associated with NIR due to herpesvirus. Differential diagnosis of NIR can be challenging, and WF-OCT may be a useful tool in identification of the causative agent so specific treatment can be promptly initiated.
In summary, WF-OCT and WF-OCT-A provide and can be a valuable part of a multimodal imaging approach to uveitis, characterising and quantifying the extent of uveitis lesions in the retinal periphery.
Vitreo–Retinal Interface
The cross-sectional nature of WF-OCT scans is well suited for imaging of lesions in the vitreo–retinal interface but is rarely used in clinical practice for lesion characterisation and surgical planning, potentially contributing to an omission of vital anatomical features [27].
In a prospective case series of 125 eyes referred for various retinal pathologies, Sodhi et al. evaluated the feasibility of WF-OCT imaging of the retinal periphery [27]. Using the Optos Silverstone SS-OCT, they acquired 6–23 mm line scans of retinal lesions within a 200° FOV, along with standard clinical examination. In 97.6% of examined eyes, the lesions were successfully captured by UWF-OCT imaging. Exclusively peripheral retinal lesions (most frequently retinal tears, retinal holes and retinoschisis), that would not be detected by standard OCT devices with a 50° FOV, were found in 69% of study eyes. The study found peripheral OCT particularly useful in surgical cases, where the UWF-OCT images confirmed the diagnosis made by clinical examination and were evaluated before deciding on surgical intervention.
Ripa et al. compared the findings and diagnostic abilities of the Xephilio S1 WF-OCT with WF and UWF colour fundus photography [28]. The study cohort included eyes with vitreous abnormalities, peripheral retinal lesions, retinal detachment and lesions of the choroid. WF-OCT was found to be a more reliable method than UWF colour fundus photography (CFP) for diagnosis of peripheral lesions, as 39% of examined eyes presented significant peripheral lesions on UWF-OCT that were neither visible with split lamp biomicroscopy nor UWF-CFP. Posterior vitreous detachments were detected on WF-OCT that could not be visualised on colour fundus photography. Further, WF-OCT was found effective in revealing the location and extent of retinal detachments and location of retinal tears. In lesions in the peripheral retina, WF-OCT detected lesions that was overlooked on both UWF-CFP and standard OCT [28].
Peripheral retinal lesions such as retinal holes, retinoschisis and retinal tears, even if they are treated or spontaneously scarred, have a risk of progression to vision-threatening retinal detachment. Thus, continuous monitoring of structural characteristics over time, e.g. change in the amount or distribution of subretinal fluid, may inform clinical decision making and facilitate early intervention in a progressing lesion.
Paediatric Ophthalmology
Recently, advances in OCT equipment such as handheld devices and more dynamically oriented imaging systems have made it possible to obtain OCT images in infants and young children not able to cooperate to conventional imaging methods. In imaging of the infant eye, a short acquisition time and some resistance to motion artefacts are crucial in obtaining an image of sufficient quality to aid in clinical diagnosis and decision making. Many paediatric retinal diseases have complicated vitreo–retinal interface abnormalities and vascular malformations, often primarily in the periphery [29]. These are lesions for which WF-OCT and WF-OCT-A imaging could prove very valuable and contribute to the findings of a clinical examination with indirect ophthalmoscopy. However, until recently, practically feasible wide-field OCT and OCT-A imaging for paediatric patients was unavailable to the clinician [30].
Campbell et al. used a prototype hand-held OCT device to obtain ultra-wide-field OCT images in four neonates with retinopathy of prematurity (ROP) [31]. Their imaging system was able to obtain non-contact en face OCT images and horizontal line scans with approximately 40° field of view and up to 100° field of view using a contact lens-based approach. The WF-OCT scans were able to show various degrees of foveal development and choroidal thickness and confirmed the clinical finding of pre-retinal fibrotic membranes in areas of previous extra-retinal NV.
In a case series of paediatric patients aged 2 months to 16 years, Nguyen et al. demonstrated the broad utility of WF-OCT and WF-OCT-A [29]. Their portable handheld SS-OCT wide-field system had a modular lens system providing a 105° FOV for structural OCT and 55° FOV for high-resolution OCT-A with concurrent OCT. Imaging of infants took place in the operating room in general anaesthesia. Diagnoses and lesions included ROP, tractional retinal detachments secondary to ROP, rhegmatogenous retinal detachment, chronic exudative retinopathy, coats disease, x-linked retinoschisis, chorioretinal scarring with retinal traction secondary to non-accidental trauma, persistent foetal vasculature and incontinenta pigmenti with peripheral avascular retina and NVs. For all these disease entities and lesions, Nguyen et al. demonstrated successful hand-held WF-OCT imaging and presented high-resolution scanning images. Additionally, they obtained detailed 3D imaging of haemangioblastomas and scans of retinoblastoma during and after treatment, and a case of retinoblastoma with vitreous seeding and multifocal tumours. Intraocular tumours, both benign and malignant, often occur outside of the central retina and may be associated with exudation, subretinal and intraretinal fluid, and vitreoretinal traction. These are lesions for which WF-OCT imaging is very well suited [29].
Continued improvements to the FOV of portable and hand-held OCT and OCT-A devices will likely improve the detection of paediatric retinal diseases with predominantly peripheral pathology.
Application in Other Fields
Optical coherence tomography was developed in the context of ophthalmology, in which it is still by far most widely used [1]. However, due to the fast acquisition time and near-microscopic resolution of OCT, interest has emerged in applying the technology for imaging in other medical fields.
Skin Cancer
Dubois et al. used a prototype line-field OCT device for real-time imaging of human skin in vivo, including carcinomas and melanomas [32]. Patients with suspicious skin lesions were clinically assessed with dermascopy before OCT imaging, where a series of about 20 B-scan images were acquired for each lesion, allowing cross-sectional visualisation of a lateral area of max. 24 mm and a penetration depth of 0.5 mm. After OCT imaging, the lesions were surgically removed and histologically examined. In total, 105 lesions were imaged. OCT images were found to correlate strongly with conventional histopathological images, and the method was found to be able to show most of the histopathological elements that allowed for lesion diagnosis [32]. The use of WF-OCT in imaging skin lesions suspected of malignancy in clinical practice could potentially improve the diagnostic accuracy and reduce the number of biopsies and unnecessary excision of benign lesions.
Breast Cancer
Schmidt et al. investigated the utility of wide-field OCT in intraoperative determination of tissue margins in lumpectomy for breast cancer [33]. The study included 185 tissue samples of 50 women with invasive or in situ carcinoma. Lumpectomy specimens and final excision margins were imaged by WF-OCT immediately prior to standard histological examination. Multiple images were captured to cover a 10 × 10 cm tissue surface and evaluated for positive/negative excision margins. Compared with histological examination, WF-OCT imaging was found to have an accuracy of 86% for main lumpectomy specimen and 96.2% for main specimen and additional re-excision specimens. The authors propose WF-OCT as a promising real-time adjunct intraoperative imaging modality for excision margin assessment, with the potential to lower re-excision rates in lumpectomy [33].
Discussion
The enhanced field of view offered by WF-OCT and WF-OCT-A has been shown to be a useful and reliable method in recognition and monitoring of peripheral retinal pathology in a variety of ocular diseases, as outlined in this review. The continued development and implementation of WF-OCT and WF-OCT-A devices is contributing to a trend towards a more widespread adoption of new imaging technology in clinical practice. WF and UWF devices are no longer exclusively research tools, and several devices with a FOV beyond the conventional 30°–50° are now commercially available.
The use of WF-OCT and WF-OCT-A imaging has not yet been widely integrated into clinical practice, though it presents multiple advantages. Most importantly, these novel imaging modalities has the potential to inform clinical decision making and improve patient outcomes, as the OCT and OCT-A examination of not only the posterior pole, but also the mid and far periphery provides a more complete clinical picture [27]. WF-OCT imaging may also be advantageous in complicated patients with unknown pathology or uncharacteristic symptoms, cases that rely on a high-resolution examination of the retina with a wide FOV able to discover marginal structural changes.
Wide-field OCT angiography allows for a detailed visualisation and functional characterisation of retinal vascular networks without the need for intravenous access and contrast dye, contributing to its convenience, speed and safety over FA. The technique can be used to image the retinal vessels without regard for kidney disease, needle aversion or pregnancy, as it is non-toxic and does not involve radiation. Even though adverse effects from fluorescein injection are very rare, the risk of nausea and anaphylaxis is eliminated with OCT-A. Thus, WF-OCT-A has the potential to replace the more time-consuming fluorescein angiography in imaging of vascular and microvascular lesions in the peripheral retina.
As presented in this review, WF and UWF OCT and OCT-A have been proven useful in many different ocular diseases and settings. However, the improvement in everyday clinical management is difficult to quantify. This depends on multiple factors, such as the specific imaging protocol used in the clinic, the skill level of the photographer, the training of the doctor in analysing the images and the other imaging modalities available in the clinic [27]. Clinicians must be trained to evaluate wide-field scans and incorporate them into their decision making. Future clinical studies will need to explore the patient outcome benefits of implementation of WF-OCT and WF-OCT-A in a real-life setting.
For research purposes, the ability of WF-OCT to acquire full-field cross-sectional images of the retina can be very valuable, deepening our structural understanding of retinal diseases and potentially identifying novel connections between peripheral and macular pathologies. As of now, the most microscopic, cross-sectional information on retinal peripheral anatomy comes from post-mortem specimens, but these do not permit dynamic comparison over time or in vivo characterisation [3].
Limitations to WF-OCT and WF-OCT-A
There are some important limitations to consider in the use of WF-OCT and WF-OCT-A. A major limitation is the high cost of the devices, restricting their availability and use in clinical practice.
Producing wider FOV images while preserving high resolution entails a longer acquisition time, even for single-capture images. If a montage protocol is implemented, the scan time is increased due to multiple scan positions. Further, the montage requires post-imaging processing time and possibly manual correction [34]. Using a steering-based technique, patients are required to fixate their gaze on a peripheral light source, which poses a challenge in patient cooperation, especially to patients with limited central vision due to maculopathy. This can limit the quality of the acquired images and be tiresome for the patients [3]. Another limitation is optical distortion due to media opacities [34].
Because OCT-A technology requires repeated scans in the same spatial position during data collection to characterise the vasculature, the technology is highly sensitive to motion artefacts from blinking, eye movement and loss of fixation. This can prove challenging in imaging of patients with poor central vision or non-compliant patients [6]. Also, dry eyes and eyes with severe DR has been found especially susceptible to motion artefacts [35]. Motion artefacts are more common at the periphery of the retina. Movement during the scanning process can cause misalignment and undocumented areas in the B-scans, 3D-reconstructions and OCT-A montage images, seen as dark horizontal bands across the images [28]. Possible solutions to this limitation such as eye-tracking systems and blink-detection correction systems are being developed to minimise motion artefacts [36].
Segmentation errors are a frequent cause of imaging artefacts in OCT-A, and they occur more often in the presence of NVs [37]. Conversely, an accurate segmentation of the retinal layers is crucial to the detection of NV through en face OCT-A images [6]. Other factors commonly causing segmentation errors are presence of intraretinal fluid and highly myopic eyes [28]. Errors of the automated segmentation can be corrected manually.
Despite these limitations, as the technology advances and cost decreases, WF-OCT and WF-OCT-A imaging will likely become an integral part of the high-quality clinical management of patients [28].
Conclusions
WF-OCT and WF-OCT-A can reliably produce high-quality, non-invasive images of the vitreous, retinal, and choroidal structures and vascularity covering the posterior pole as well as the mid and far periphery. Thus, these methods can be a valuable part of a multimodal imaging approach in the management of a variety of ocular conditions. Continued advancement in hardware technology and software processing will allow for an even wider field of view, faster acquisition and more accurate assessment of retinal and choroidal diseases with peripheral manifestations.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81.
Choudhry N, Duker JS, Freund KB, Kiss S, Querques G, Rosen R, et al. Classification and guidelines for widefield imaging: recommendations from the international widefield imaging study group. Ophthalmol Retina. 2019;3(10):843–9.
Choudhry N, Golding J, Manry MW, Rao RC. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123(6):1368–74.
White B, Pierce M, Nassif N, Cense B, Park B, Tearney G, et al. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography. Opt Express. 2003;11(25):3490–7.
de Carlo TE, Salz DA, Waheed NK, Baumal CR, Duker JS, Witkin AJ. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):611–6.
Wang M, Garg I, Miller JB. Wide field swept source optical coherence tomography angiography for the evaluation of proliferative diabetic retinopathy and associated lesions: a review. Semin Ophthalmol. 2021;36(4):162–7.
Zhang Q, Attaran-Rezaei K, Wang RK. Ultra-wide field optical coherence tomography angiography for evaluation of retinal venous occlusion. Investig Ophthalmol Visual Sci 2019;60(9).
Dong W, Liu S, Li J, Li M, Zhong J. Detection performance of ultra-wide field optical coherence tomography angiography on the retina non-perfusion areas in eyes with retina vein obstruction. Recent Adv Ophthalmol. 2023;43(1):40–3.
Glacet-Bernard A, Miere A, Houmane B, Tilleul J, Souied E. Nonperfusion assessment in retinal vein occlusion: comparison between ultra-widefield fluorescein angiography and widefield optical coherence tomography angiography. Retina. 2021;41(6):1202–9.
Shiraki A, Sakimoto S, Tsuboi K, Wakabayashi T, Hara C, Fukushima Y, et al. Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide-field optical coherence tomography angiography. Acta Ophthalmol. 2019;97(6):E913–8.
Kreminger J, Iby J, Stino H, Baratsits M, Niederleithner M, Schlegl T, et al. Retinal vascular alterations in eyes with nonperfused retinal vein occlusion detected on single-capture 65°-widefield OCT-angiography. Invest Ophthalmol Vis Sci. 2023;64(8):1784.
Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol. 2018;102(9):1199–203.
Zhu Y, Cui Y, Wang JC, Lu Y, Zeng R, Katz R, et al. Different scan protocols affect the detection rates of diabetic retinopathy lesions by wide-field swept-source optical coherence tomography angiography. Am J Ophthalmol. 2020;215:72–80.
Russell JF, Shi Y, Hinkle JW, Scott NL, Fan KC, Lyu C, et al. Longitudinal wide-field swept-source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Retina. 2019;3(4):350–61.
Antropoli A, Arrigo A, La Franca L, Bianco L, Barlocci E, Fusi E, et al. Peripheral and central capillary non-perfusion in diabetic retinopathy: an updated overview. Front Med (Lausanne). 2023;10:1125062.
Wang F, Saraf SS, Zhang Q, Wang RK, Rezaei KA. Ultra-widefield protocol enhances automated classification of diabetic retinopathy severity with OCT angiography. Ophthalmol Retina. 2020;4(4):415–24.
Skalet AH, Campbell JP, Jian Y. Ultrawide-field OCT for retinoblastoma. Ophthalmology. 2022;129(6):718.
Soliman SE, VandenHoven C, MacKeen LD, Héon E, Gallie BL. Optical coherence tomography-guided decisions in retinoblastoma management. Ophthalmology. 2017;124(6):859–72.
Tanimukai T, Noda K, Hirooka K, Kase S, Ishida S. Noninvasive imaging of a vasoproliferative retinal tumor treated with cryopexy. Case Reports Ophthalmol. 2022;13(2):611–6.
Xuan Y, Chang Q, Zhang Y, Ye X, Liu W, Li L, et al. Clinical observation of choroidal osteoma using swept-source optical coherence tomography and optical coherence tomography angiography. Appl Sci. 2022;12(9):4472.
Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. Int J Retina Vitreous. 2019;5(Supplement 1):49.
Grewal DS, Agarwal M, Munk MR. Wide field optical coherence tomography and optical coherence tomography angiography in uveitis. Ocular Immunol Inflamm. 2022;32(1):105–15.
Tian M, Tappeiner C, Zinkernagel MS, Wolf S, Munk MR. Swept-source optical coherence tomography angiography reveals vascular changes in intermediate uveitis. Acta Ophthalmol. 2019;97(5):e785–91.
Singh RB, Sinha S, Saini C, Elbasiony E, Thakur S, Agarwal A. Recent advances in the management of non-infectious posterior uveitis. Int Ophthalmol. 2020;40(11):3187–207.
Tian M, Zeng G, Tappeiner C, Zinkernagel MS, Wolf S, Munk MR. Comparison of indocyanine green angiography and swept-source wide-field optical coherence tomography angiography in posterior uveitis. Front Med. 2022;9: 853315.
Invernizzi A, Agarwal AK, Ravera V, Mapelli C, Riva A, Staurenghi G, et al. Comparing optical coherence tomography findings in different aetiologies of infectious necrotising retinitis. Br J Ophthalmol. 2018;102(4):433–7.
Sodhi SK, Golding J, Trimboli C, Choudhry N. Feasibility of peripheral OCT imaging using a novel integrated SLO ultra-widefield imaging swept-source OCT device. Int Ophthalmol. 2021;41(8):2805–15.
Ripa M, Motta L, Florit T, Sahyoun JY, Matello V, Parolini B. The role of widefield and ultra widefield optical coherence tomography in the diagnosis and management of vitreoretinal diseases. Diagnostics. 2022;12(9):2247.
Nguyen TP, Ni S, Liang G, Khan S, Wei X, Skalet A, et al. Widefield optical coherence tomography in pediatric retina: a case series of intraoperative applications using a prototype handheld device. Front Med (Lausanne). 2022;9: 860371.
He Y, Chen X, Tsui I, Vajzovic L, Sadda SR. Insights into the developing fovea revealed by imaging. Prog Retin Eye Res. 2022;90: 101067.
Campbell JP, Nudleman E, Yang JL, Tan O, Chan RVP, Chiang MF, et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. Jama Ophthalmology. 2017;135(9):977–81.
Dubois A, Levecq O, Azimani H, Siret D, Barut A, Suppa M, et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J Biomed Opt. 2018;23(10):1–9.
Schmidt H, Connolly C, Jaffer S, Oza T, Weltz CR, Port ER, et al. Evaluation of surgically excised breast tissue microstructure using wide-field optical coherence tomography. Breast Journal. 2020;26(5):917–23.
McNabb RP, Grewal DS, Mehta R, Schuman SG, Izatt JA, Mahmoud TH, et al. Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol. 2016;100(10):1377–82.
Cui Y, Zhu Y, Wang JC, Lu Y, Zeng R, Katz R, et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl Vis Sci Technol. 2019;8(6):18.
Wei X, Hormel TT, Guo Y, Hwang TS, Jia Y. High-resolution wide-field OCT angiography with a self-navigation method to correct microsaccades and blinks. Biomed Opt Express. 2020;11(6):3234–45.
GhasemiFalavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2017;101(5):564–8.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
The idea for the manuscript was formulated by Anne Helene Køllund Nissen and Anna Stage Vergmann. Anne Helene Køllund Nissen searched databases, assessed articles for relevance and prepared the original draft. Anna Stage Vergmann read, critically reviewed and edited the draft through multiple rounds for intellectual content. The final version of the manuscript received approval from all authors before submission. All authors contributed to the creation of the manuscript with a substantial amount of work.
Corresponding author
Ethics declarations
Conflict of Interest
Anne Helene Køllund Nissen and Anna Stage Vergmann do not have any conflicts of interest to declare. Anna Stage Vergmann is an Editorial Board member of Ophthalmology and Therapy. Anna Stage Vergmann was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nissen, A.H.K., Vergmann, A.S. Clinical Utilisation of Wide-Field Optical Coherence Tomography and Angiography: A Narrative Review. Ophthalmol Ther 13, 903–915 (2024). https://doi.org/10.1007/s40123-024-00905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00905-2