Abstract
Introduction
Acute leukemia often affects microcirculation perfusion. This study aimed to investigate retinal microvascular changes in patients with acute leukemia without retinopathy during clinical remission using optical coherence tomography angiography (OCTA) and to determine the correlation of these changes with systemic laboratory values.
Methods
Thirty-eight patients in remission from acute leukemia with no retinopathy (NLR group) and 36 age-matched healthy individuals (control group) were included in this cross-sectional study. OCTA parameters, including the central foveal thickness (CFT), foveal avascular zone (FAZ) area, FAZ perimeter, acircularity index (AI), foveal density (FD300), and the vessel densities (VDs) of the superficial capillary plexus (SCP), deep capillary plexus (DCP), and choriocapillaris were analyzed in a 6 × 6 mm2 macular scan. Correlation and multiple linear regression analyses were conducted to identify potential systemic characteristics associated with these OCTA metrics.
Results
AI (P = 0.034) and FD300 (P < 0.001) differed significantly between the NLR and control groups. The VD of SCP in the parafovea (P = 0.001) and of DCP in both the parafovea (P = 0.011) and perifovea (P = 0.001) were significantly lower in the NLR group than in the control group. In a multiple linear regression analysis, the reduced VD of the perifoveal DCP was significantly correlated with the increased international normalized ratio (standardized beta [STD β] = − 0.356; P = 0.047).
Conclusions
Macular microvascular changes can be observed during remission from acute leukemia antecedent to clinically visible retinal lesions. Hematological disturbances may be associated with microvascular impairments in preclinical leukemic retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Acute leukemia often involves the eye. Clinically, retinopathy is the most common ophthalmic manifestation of acute leukemia. However, little is known about the alterations in the retinal microvascular network at different layers of capillary plexus. Detection of retinal microvascular changes might shed light on the pathogenesis of leukemic retinopathy. |
We aimed to quantitatively detect microvascular impairments in patients with acute leukemia remission without retinopathy using optical coherence tomographic angiography (OCTA). We also investigated the association between systemic factors and these changes. |
What was learned from the study? |
In this cross-sectional study, patients in remission from acute leukemia with no retinopathy had significantly higher acircularity index (AI), lower foveal density (FD300), and lower vessel density (VD) than the healthy controls. Besides, the reduced VD was significantly correlated with the increased international normalized ratio in patients with acute leukemia. |
In conclusion, findings of this study demonstrate that OCTA can detect macular microvascular impairments in remission from acute leukemia. The retinal perfusion deficits may be related to the hematological abnormalities of patients. |
Introduction
Acute leukemia often involves the eye, and the ophthalmic involvement is more commonly present in acute naïve leukemic phase than in remission [1, 2]. Clinically, retinopathy is the most common ophthalmic manifestation of acute leukemia [2], with a prevalence of 28–80% during the course of the disease [3,4,5,6,7,8]. Leukemic retinopathy has a diverse range of presentations, including retinal or vitreous hemorrhage, often with Roth spots, venous tortuosity and dilation, microaneurysms, and cotton-wool spots [1, 9]. Until now, these presentations have not been described as signs of relapse or recurrence after acute leukemia remission [10]. Earlier studies have revealed that subclinical microvascular disturbances including the decreased capillary flow velocity and the increased capillary stagnation can be found in nailfold [11] and sublingual microcirculation [12] in leukemia. However, little is known about the alterations in the retinal microvascular network at different layers of capillary plexus. Detection of retinal microvascular changes might shed light on the pathogenesis of leukemic retinopathy.
The eye provides a window through which the impact of leukemia on vascular structure can be viewed directly in vivo. Optical coherence tomographic angiography (OCTA) is a noninvasive imaging technique that allows the precise visualization of the retinal microvasculature [13], and it is a promising method of detecting early retinal microvascular impairment in a variety of diseases including diabetes [14] and Alzheimer’s disease [15]. To date, there have been a few studies on revealing pathological retinal blood flow loss via OCTA in remitted chronic myelogenous leukemia (CML) [16] and active acute leukemia [8]. Nevertheless, the available literature is limited to case reports [16, 17] and one study with small sample sizes [8]. Besides, none of these articles have examined the hemodynamics of the retinal microcirculation without clinically visible retinopathy in patients with acute leukemia in clinical remission.
In this study, we quantitatively detected microvascular impairments not seen on clinical examination in patients with acute leukemia remission without retinopathy using OCTA. We also investigated the association between systemic factors and these changes. The study aimed to promote better understanding of the pathogenesis of leukemic retinopathy. We also aimed to preliminarily explore the retinal microcirculatory changes in patients with acute leukemia in remission, and hoped to provide some clue to the effects of treatments in the remission induction and maintenance phase on the retina.
Methods
This study was approved by the Ethical Committee of Peking University People’s Hospital (2020PHB276). All procedures were performed in accordance with the tenets of the Declaration of Helsinki of 1964 and its later amendments. All participants signed informed consent forms before research.
Participants
In this cross-sectional study, 38 patients in remission from acute leukemia without clinical signs of leukemic retinopathy (no leukemic retinopathy [NLR] group) and 36 age-matched healthy subjects (control group) were recruited from the Department of Ophthalmology, Peking University People’s Hospital, Beijing, China, between September 2020 and March 2023.
We diagnosed acute leukemia with bone marrow aspirate based on a combination of morphological, immunophenotypic, cytogenetic, and molecular genetic tests according to the French–American–British (FAB) criteria and the World Health Organization (WHO) classification [18, 19]. Acute myeloid leukemia (AML) was defined by the presence of ≥ 20% myeloid blasts in peripheral blood or bone marrow, or the presence of recurrent genetic abnormalities. Acute lymphoblastic leukemia (ALL) was defined by the presence of ≥ 20% lymphoblasts in peripheral blood or bone marrow. In this study, patients with acute leukemia were referred from Peking University Institute of Hematology for routine ocular examinations before hematopoietic stem-cell transplantation (HSCT). Medications for the patients were used in two main treatment phases: induction and maintenance of remission. For patients with AML, “7 + 3” scheme (cytarabine 100 mg/m2/day for 7 days and daunorubicin 60 mg/m2/day for 3 days) was standardly used for induction and high doses of cytarabine with 3 g/m2/12 h for six doses for maintenance. There were a variety of medications to treat ALL. For Ph−–ALL, the induction chemotherapy included regimens such as VDCLP (V, vincristine; D, daunorubicin; C, cyclophosphamide; L, l-asparaginase; P, prednisone), VDCP, VDLP or VDP; the consolidation chemotherapy mainly included CAM (cyclophosphamide, cytarabine and 6-mercaptopurine) or high doses of methotrexate (1–5 g/m2). For Ph+–ALL, imatinib plus VDCP were applied for induction; CAM, methotrexate plus 6-mercaptopurine and imatinib were for maintenance. The age-matched controls were recruited from among the healthy population that visited our department for regular ophthalmic examinations. We only enrolled the right eye of each subject in this article. All the leukemic patients included were examined in the complete remission phase, which was defined as < 5% bone-marrow blasts, no circulating blasts, and no extramedullary disease. The exclusion criteria were as follows: (1) age > 70 years; (2) patients with leukemic retinopathy on clinical examination or fundus imaging; (3) subjects with known ocular disorders such macular degeneration, optic nerve disorders, glaucoma, uveitis, or high myopia (< − 6 diopters); (4) intraocular pressure (IOP) > 21 mmHg; (5) history of ocular surgery other than cataract surgery; and (6) patients with history of systematic diseases which could impair the retina or choroid other than leukemia.
Clinical Parameters
Comprehensive ophthalmological examinations were performed in all participants. These included measurements of best-corrected visual acuity (BCVA; converted to logMAR score), slit-lamp biomicroscopy of the anterior and posterior segments, intraocular pressure (IOP) measured using a non-contact tonometer, and color fundus photography of macula. None of the patients with acute leukemia in the NLR group were found to have any sign of retinopathy according to the fundoscopic examinations and fundus photographs.
Clinical and demographic data on age, sex, body mass index (BMI), type of diagnosis, and treatment were obtained from the medical records for each participant. Besides, we also collected the following laboratory test results from peripheral blood samples: white blood cell count (WBC), red blood cell count (RBC), hemoglobin, platelet count, fibrinogen, cross-linked fibrin degradation products (d-dimer), and international normalized ratio (INR). We took blood samples of each patient on the same day as eye screening.
OCTA Examination
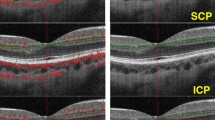
All OCTA examinations with the AngioVue OCTA system (ver. 2017.1.0.151; RTVue-XR Avanti; Optovue, Fremont, CA, USA) were performed after pupillary dilation in a dark room with split-spectrum amplitude decorrelation angiography (SSADA) algorithm. This system operates at 70,000 A-scans/s in a 6 × 6 mm2 square scan around the fovea. The central foveal thickness (CFT), which was defined as the average thickness of the retina within a foveal ring 1 mm in diameter, was collected. Vessel density (VD) represented the proportion of the region occupied by blood vessels in the area of interest. Superficial capillary plexus (SCP) and deep capillary plexus (DCP) were automatically segmented with the built-in software segmentation algorithm (Fig. 1) [20]. The SCP slab was located between the internal limiting membrane (ILM) and 10 µm above the inner plexiform layer (IPL), whereas the DCP slab comprised the blood vessels from 10 µm above the IPL to 10 µm below the outer plexiform layer (OPL). The foveal region was defined as the area within a central circle with a 1 mm diameter; the parafoveal area was an annulus around the fovea with 1 and 3 mm inner and outer ring diameters, respectively; and the perifovea represented the region of the outer annulus beyond the parafovea with a 6-mm diameter. The VD of the choriocapillaris was calculated as the ratio of the flow area within a circle of radius 1 mm around the fovea [21, 22].
Optical coherence tomographic angiography (OCTA) images (6 × 6 mm2) of the right eye of a 42-year-old woman in remission from acute myeloid leukemia (AML) with no clinically detectable retinopathy. En face OCTA images of the superficial capillary plexus (SCP; A) and the deep capillary plexus (DCP; B) were generated. The cross-sectional B-scan with flow C shows the segmentation boundaries of SCP (from the red line to the green line) and DCP (from the green line to the blue line). The internal limiting membrane (ILM) is a thin film between the neural retina and the vitreous body. The inner plexiform layer (IPL) is where retinal ganglion cells synapse with amacrine and bipolar cells. The outer plexiform layer (OPL) is where the synaptic terminals of photoreceptors synapse with horizontal and bipolar cells
The foveal avascular zone (FAZ) was defined as the avascular area in the center of the fovea. FAZ area and perimeter were also evaluated based on the retinal slab, with automated detection of the FAZ boundary using AngioVue software. The acircularity index (AI) of FAZ was the ratio of the FAZ perimeter divided by the perimeter of a circle with the area which was equal to FAZ area [23]. The foveal density (FD300) was the VD within a 300-µm region surrounding FAZ [24]. All OCTA measurements were made three times, then the best-quality measurement was used for final analyses. The projection artifacts were removed by the built-in algorithm of AngioVue system.
Statistical Analysis
All data analyses were performed with SPSS 25.0. The Shapiro–Wilk test was applied to evaluate the normal distribution of the quantitative parameters. The differences in the baseline characteristics and structural parameters were compared between the NLR and control groups with an independent-samples t test (parametric)/Mann–Whitney U test (nonparametric) for continuous variables and a χ2 test for categorical variables. Spearman’s correlation was used to examine the correlation between the OCTA metrics and serum parameters. Further multiple linear regression analyses were also conducted using the OCTA parameters of the macular microvasculature to model the potential associations between the ocular and systemic factors. In all analyses, P values < 0.05 were considered statistically significant.
Results
Thirty-eight patients with acute leukemia and 36 healthy controls were included in the study. The demographic and clinical features of the NLR and control groups are given in Table 1. Compared with the control group, the levels of WBC, RBC and hemoglobin in the NLR group were significantly decreased (all P < 0.001). No significant difference in age, sex, BMI, BCVA, IOP, platelet count, INR, fibrinogen level, or d-dimer level was observed between two groups (all P > 0.05). In the NLR group, 14 patients were diagnosed with ALL and another 24 patients with AML. Morphologically, all the 24 AML cases were categorized as M1 (n = 2), M2 (n = 11), M4 (n = 6), and M5 (n = 5). Fourteen ALL cases were categorized as L1 (n = 8) and L2 (n = 6). Most patients were in their first complete remission (CR1, n = 34). For patients in CR1, the median time from diagnosis to remission was 79 days (range 32–158 days). The median time between patients entering CR1 and being included in the study was 115 days (range 70–168 days).
The structural parameters in the NLR and control groups are compared in Table 2. There was no significant difference in CFT between controls and the NLR group (P = 0.650). For FAZ parameters, FAZ area (P = 0.494) and perimeter (P = 0.760) were similar in two groups, whereas AI was significantly higher in patients with acute leukemia than in controls (P = 0.034). FD300 decreased significantly in the NLR group (P < 0.001). In SCP, only the parafoveal VD showed a significant increase in the NLR group than in controls (P = 0.001). In DCP, the NLR group showed significantly lower VD in parafovea (P = 0.011) and perifovea (P = 0.001) compared with healthy controls. Reductions in VD in the other area of SCP and DCP were also observed in acute leukemia, although the differences were not statistically significant (all P > 0.05). No significant difference was observed in VD of the choriocapillaris between the NLR group and control group (P = 0.079).
Figure 2 shows the Spearman’s correlation coefficients for retinal microvasculature parameters and systemic characteristics of the NLR group. The lower parafoveal VD in SCP was significantly associated with a lower BMI (ρ = 0.385; P = 0.017), but with a higher level of INR (ρ = − 0.355; P = 0.029). VD of DCP in parafovea showed significant positive correlations with age (ρ = 0.365; P = 0.024) and d-dimer level (ρ = 0.383; P = 0.018), and a significant negative correlation with INR (ρ = − 0.425; P = 0.008). The increase in VD in perifoveal DCP correlated significantly with the increase in age (ρ = 0.338; P = 0.038) and the level of d-dimer (ρ = 0.368; P = 0.023), but the decrease in INR (ρ = − 0.507; P = 0.001).
Correlation analysis of the retinal microvascular parameters and systemic characteristics in patients in remission from acute leukemia with no retinopathy. The color scale indicates Spearman’s correlation coefficient (ρ): positive values (blue) indicate a positive correlation and negative values (red) indicate a negative correlation. The darker the shading, the stronger the correlation. *P < 0.05, **P < 0.01, ***P < 0.001 for pairwise comparisons. FAZ foveal avascular zone, AI acircularity index, FD300 foveal density in a 300-μm region around the FAZ, VD vessel density, SCP superficial capillary plexus, DCP deep vascular complex, BMI body mass index, WBC white blood cell count, INR international normalized ratio
In the multiple linear analyses of these three significantly differing OCTA parameters, only VD of the perifoveal DCP showed significant associations between systemic factors and was significantly associated with INR (standardized beta [STD β] = − 0.356; P = 0.047) (Table 3).
Discussion
In the study, we assessed the macular microcirculatory manifestations in patients with acute leukemia without retinopathy during clinical remission using OCTA. We found that patients in remission from acute leukemia with no retinopathy had significantly higher AI, lower FD300, lower VD of SCP in the parafovea, and lower VD of DCP in both the parafovea and perifovea than the healthy controls. Besides, the reduced VD of the perifoveal DCP was significantly correlated with the increased INR in patients with acute leukemia. Given that this is a cross-sectional study, the OCTA alterations may not initially occur during remission, but rather earlier in the course of the disease. Future cohort studies are needed to report the total duration of leukemia in study patients (even their baseline status before initial treatment).
Given that the dimensions of FAZ area correlate with the extent of capillary nonperfusion [25], there has also been increasing evidence indicating the deformation of the FAZ boundary to be one of the earliest features of microvascular impairments [26, 27]. In our study, although the FAZ area in the NLR group was similar to that in controls, a higher AI was observed in the NLR group, consistent with the previous study [8]. The findings support the notion that the irregularities in the FAZ margin precede FAZ enlargement in acute leukemia. Although the pathogenesis of leukemic retinopathy remains unclear, the main ophthalmic manifestations of leukemic involvement are believed to result from direct leukemic infiltration or indirect hematological abnormalities of leukemia [1]. Leukemia could influence the retina through a similar mechanism. Therefore, the possible explanations of the irregularities in the FAZ borders may involve uneven capillary dropout [23], vascular remodeling [28] and capillary closure [29] in the leukemic retina, which result from the hematological disturbances of acute leukemia such as severe anemia, thrombocytopenia and hyperviscosity [1]. In addition, a recent study found that the ratio of the inner nuclear layer (INL)-free zone area to the FAZ area could have higher accuracy in diagnosing nonproliferative diabetic retinopathy than the traditional measurements of the FAZ area [30]. The emergence of more advanced quantitative FAZ metrics may better highlight microvascular changes occurring in acute leukemia and point out possible new biomarkers.
As well as the shape and size of FAZ, VD has also been involved in clinical practice for the quantification of capillary nonperfusion. Although CFT was similar between groups in this study, VD of SCP in parafovea and of DCP in both parafovea and perifovea were significantly reduced in acute leukemia than in controls. FD300 was also significantly decreased in acute leukemia. The reasons of the observed microvascular changes remain unclear. The possible explanation may be that the hyperviscous state secondary to the initial leukocytosis [31, 32] could lead to microvascular obstruction and hypoxic tissue [33], thus inducing microcirculation impairments. Besides, this increased viscosity could also contribute to the reduction in the blood flow velocity, which may be below the threshold of detection with OCTA and therefore undetectable by the current OCTA technology [34]. Previously reported findings have demonstrated that cytoreductive surgery could reduce the whole blood viscosity and increase the velocity of the retinal venous blood [35,36,37], which in turn suggests that increased viscosity contributes to abnormalities in the microcirculation. In this context, the negative relationship between VD and INR in the present study may also result from the hyperviscosity in patients with leukemia, because the increased viscosity state not only reduces the microvascular perfusion, but also enhances the risk of disseminated intravascular coagulation, which manifests with increased INR [38].
Recently, Cicinelli et al. evaluated the retinal microcirculation in 12 patients with acute leukemia in the active phase [8]. They found that only VD of SCP in parafovea was significantly reduced in active acute leukemia, whereas no significant differences were observed in the VD of DCP or the optic nerve in these patients. However, in the present study, the VD of DCP in parafovea and perifovea also significantly decreased in 38 patients in remission from acute leukemia. This discrepancy is hypothesized to be secondary to the difference in duration of the disease course [2]. In the previous study, 12 patients with active acute leukemia were enrolled, 11 (91.7%) of whom were newly diagnosed and had not yet been treated. However, the patients included in our study had all achieved clinical remission after chemotherapy and had experienced leukemia for a longer period. A longer period of disease may increase the duration and extent of the retinal damage caused by leukemia [39]. Moreover, the remission phase was defined as < 5% bone-marrow blasts and no circulating blasts, which does not imply hematological normalization and the complete resolution of retinal changes. Therefore, the patients in remission in the present study may have experienced more profound disturbances of the microcirculatory hemodynamics of the retina than the newly diagnosed patients in the study of Cicinelli et al. Interestingly, our study our study indicates that the reduced VD of the perifoveal DCP was significantly associated with the increased INR in remitted acute leukemia, while Cicinelli et al. found that the lower parafoveal VD of SCP was related to lower WBC and lower platelets in active acute leukemia. The medications used in remission and the abnormal liver function may cause the microcirculatory difference between the active phase and the remission state. Especially, methotrexate, often used in remission, could affect both the hemostatic system [40] and the retina [41, 42]. Besides, the serum aspartate aminotransferase level of the patients in the study reached 42.95 ± 12.83 U/l (slightly higher than the normal range: 15–40 U/l), while the abnormal liver function also has harmful effects on coagulation function and microcirculation [43]. Further longitudinal studies with larger cohorts are required to provide better understanding of the pathogenesis of the retinal microvascular changes associated with the remission of acute leukemia.
This is the first study to use OCTA to quantify the microcirculation impairment in patients with acute leukemia remission without retinopathy. The study has several limitations. Firstly, the sample size of the study is relatively small, given that acute leukemia is a relatively rare disease. Secondly, although OCTA provides detailed assessments of microvascular impairments at the posterior pole, there is a lack of evaluation of the more peripheral retina in leukemia due to the small scanning range. Besides, although some of the OCTA imaging artifacts of SCP were removed through the built-in artifact-removal algorithm, the artifacts might still possibly affect the detection of the DCP flow. Moreover, due to the device constraints, there is a lack of data regarding the thickness of macular layers to assess if OCTA changes of DCP are just a vascular flow reduction or if these changes are also associated with the structural impairment of the retina. In addition, given the existence of the detection threshold of OCTA, the nonperfusion area defined by OCTA in the study might actually be caused by slow or turbulent flow, rather than real nonperfusion [27]. Last but not least, it is not possible to explain whether OCTA changes are due to the drugs (used for induction and remission) or leukemia. To solve this problem, a further study can be planned with naive patients at the time of diagnosis of acute leukemia.
Conclusions
In conclusion, findings of this study demonstrate that OCTA can detect macular microvascular impairments in remission from acute leukemia in the absence of clinical findings on examination. The retinal perfusion deficits may be related to the hematological abnormalities of patients. We recommend patients in remission to detect retinal microvascular changes via OCTA, which may preliminarily provide some clue to the effects of the drugs (used for induction and remission) and leukemia itself on the retinal microcirculation. Further research is required to provide better understanding of the benefits and limitations of OCTA in detection of retinal microcirculation in patients with leukemia. Besides, more advanced quantitative metrics should be used in future studies.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sharma T, Grewal J, Gupta S, et al. Ophthalmic manifestations of acute leukaemias: the ophthalmologist’s role. Eye (Lond). 2004;18:663–72.
Allen RA, Straatsma BR. Ocular involvement in leukemia and allied disorders. Arch Ophthalmol. 1961;66:490–508.
Reddy SC, Jackson N, Menon BS. Ocular involvement in leukemia—a study of 288 cases. Ophthalmologica. 2003;217:441–5.
Holt JM, Gordon-Smith EC. Retinal abnormalities in diseases of the blood. Br J Ophthalmol. 1969;53:145–60.
Vishnevskia-Dai V, Sella King S, Lekach R, et al. Ocular manifestations of leukemia and results of treatment with intravitreal methotrexate. Sci Rep. 2020;10:1994.
Leonardy NJ, Rupani M, Dent G, et al. Analysis of 135 autopsy eyes for ocular involvement in leukemia. Am J Ophthalmol. 1990;109:436–44.
Sayadi J, Gouider D, Allouche Y, et al. Ophthalmic manifestations of newly diagnosed acute leukemia patients in a Tunisian cohort. Clin Ophthalmol. 2022;16:3425–35.
Cicinelli MV, Mastaglio S, Menean M, et al. Retinal microvascular changes in patients with acute leukemia. Retina. 2022;42:1762–71.
Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27:211–32.
Zhao C, Wei D, Shi X, et al. Unilateral isolated optic nerve infiltration combined with central retinal artery occlusion in a patient with acute myeloid leukemia. Am J Ophthalmol Case Rep. 2022;26: 101493.
Tooke JE, Milligan DW. Capillary flow velocity in leukaemia. Br Med J (Clin Res Ed). 1983;286:518–9.
Meinders AJ, Elbers P. Images in clinical medicine. Leukocytosis and sublingual microvascular blood flow. N Engl J Med. 2009;360:e9.
Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50.
Zhang B, Chou Y, Zhao X, et al. Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol. 2021;222:226–37.
Rifai OM, McGrory S, Robbins CB, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: a systematic review. Alzheimers Dement (Amst). 2021;13: e12149.
Pour EK, Bazvand F, Mehrabi Bahar MR, et al. Unilateral macular choroidal neovascularization; a rare manifestation in chronic myelogenous leukemia. J Curr Ophthalmol. 2020;32:103–6.
Yang X, Xu J, Yang J, et al. Unilateral macular choroidal neovascularization-a rare manifestation in acute myelocytic leukemia: Case report. Medicine (Baltimore). 2018;97: e0344.
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British Cooperative Group. Ann Intern Med. 1985;103:620–5.
Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201.
Xu M, Xu H, Li X, et al. Characteristics of macular morphology and microcirculation in diabetic macular edema patients with serous retinal detachment. BMC Ophthalmol. 2022;22:299.
Cai Y, Liu WB, Zhou M, et al. Diurnal changes of retinal microvascular circulation and RNFL thickness measured by optical coherence tomography angiography in patients with obstructive sleep apnea-hypopnea. Front Endocrinol (Lausanne). 2022;13: 947586.
Tam J, Dhamdhere KP, Tiruveedhula P, et al. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:9257–66.
Mo S, Krawitz B, Efstathiadis E, et al. Imaging foveal microvasculature: optical coherence tomography angiography versus adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT130–40.
Bresnick GH, Condit R, Syrjala S, et al. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102:1286–93.
de Carlo TE, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70.
Inanc M, Tekin K, Kiziltoprak H, et al. Changes in retinal microcirculation precede the clinical onset of diabetic retinopathy in children with type 1 diabetes mellitus. Am J Ophthalmol. 2019;207:37–44.
Sasongko MB, Wong TY, Nguyen TT, et al. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011;54:2409–16.
Tam J, Dhamdhere KP, Tiruveedhula P, et al. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom Vis Sci. 2012;89:E692-703.
Kulikov AN, Maltsev DS, Burnasheva MA. Improved analysis of foveal avascular zone area with optical coherence tomography angiography. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:2293–9.
Frank RN, Ryan SJ Jr. Peripheral retinal neovascularization with chronic myelogenous leukemia. Arch Ophthalmol. 1972;87:585–9.
Morse PH, McCready JL. Peripheral retinal neovascularization in chronic myelocytic leukemia. Am J Ophthalmol. 1971;72:975–8.
Nobacht S, Vandoninck KF, Deutman AF, et al. Peripheral retinal nonperfusion associated with chronic myeloid leukemia. Am J Ophthalmol. 2003;135:404–6.
Cheung CMG, Teo KYC, Tun SBB, et al. Differential reperfusion patterns in retinal vascular plexuses following increase in intraocular pressure an OCT angiography study. Sci Rep. 2020;10:16505.
Willerslev A, Hansen MM, Klefter ON, et al. Non-invasive imaging of retinal blood flow in myeloproliferative neoplasms. Acta Ophthalmol. 2017;95:146–52.
Jampol LM, Goldberg MF, Busse B. Peripheral retinal microaneurysms in chronic leukemia. Am J Ophthalmol. 1975;80:242–8.
Mehta AB, Goldman JM, Kohner E. Hyperleucocytic retinopathy in chronic granulocytic leukaemia: the role of intensive leucapheresis. Br J Haematol. 1984;56:661–7.
Emerson G, Kaide CG. Rapid fire: acute blast crisis/hyperviscosity syndrome. Emerg Med Clin North Am. 2018;36:603–8.
Seraly MP, Gupta SK, Mehmi I, et al. A unique case of chronic myeloid leukemia presenting as monocular vision loss with unilateral retinopathy. Am J Ophthalmol Case Rep. 2019;14:67–9.
Fisgin T, Yarali N, Kara A, et al. Hemostatic side effects of high-dose methotrexate in childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2004;21:77–83.
Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16:1589–99.
Chahar A, Sen A, Shukla D, et al. The role of intravitreal methotrexate as an adjunct to local or systemic corticosteroids in vitrectomy for rhegmatogenous retinal detachment and choroidal detachment: a pilot study. Retina. 2024.
Wu J, Duan C, Yang Y, et al. Insights into the liver-eyes connections, from epidemiological, mechanical studies to clinical translation. J Transl Med. 2023;21:712.
Acknowledgements
We thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81970815) and the National Key Research and Development Program of China (2020YFC2008200). The study sponsors also funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Concept and design: Lvzhen Huang, Xuan Shi; methodology: Xuan Shi; data collection: Miao Zhou, Duo Wei; statistical analysis: Yi Cai; drafting the manuscript: Miao Zhou, Kinji Hashimoto; preparing tables: Duo Wei; supervision: Mingwei Zhao. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Miao Zhou, Kinji Hashimoto, Duo Wei, Yi Cai, Lvzhen Huang, Xuan Shi and Mingwei Zhao have nothing to disclose.
Ethical Approval
This study was approved by the Ethical Committee of Peking University People’s Hospital (2020PHB276). All procedures were performed in accordance with the tenets of the Declaration of Helsinki of 1964 and its later amendments. All participants signed informed consent forms before research.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhou, M., Hashimoto, K., Wei, D. et al. Detection of Retinal Microvascular Changes with Optical Coherence Tomography Angiography in Patients with Acute Leukemia Without Retinopathy. Ophthalmol Ther 13, 1145–1157 (2024). https://doi.org/10.1007/s40123-024-00904-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00904-3