Abstract
Introduction
With the global aging population on the rise, age-related macular degeneration (AMD) poses a growing healthcare burden. Prior research hints at immune-mediated inflammatory diseases (IMIDs) potentially elevating AMD risk via diverse mechanisms. However, causality remains disputed as a result of confounding factors. Hence, our Mendelian randomization (MR) study aims to untangle this link, mitigating confounding effects to explore the IMID–AMD causal relationship. This study aims to investigate the causal relationship between IMIDs and AMD, providing new strategies for the prevention and treatment of AMD in clinical practice.
Methods
This study was registered with PROSPERO, CRD42023469815. We obtained data on IMIDs and AMD from Genome-Wide Association Studies (GWAS) summary statistics and the FinnGen consortium. Rigorous selection steps were applied to screen for eligible instrumental single nucleotide polymorphisms (SNPs). We conducted univariate Mendelian randomization, inverse variance-weighted (IVW), weighted median, Mendelian randomization-Egger (MR-Egger), and multivariate Mendelian randomization (MVMR) analyses. Various sensitivity analysis methods were employed to assess pleiotropy and heterogeneity. The aim was to explore the causal relationships between IMIDs and AMD.
Results
The MR analysis revealed that Crohn’s disease (CD) (IVW: odd ratios (OR) 1.05, 95% CI (confidence interval) 1.01–1.10, p = 0.007), rheumatoid arthritis (RA) (IVW: OR 1.09, 95% CI 1.04–1.15, p = 0.0001), and type 1 diabetes (T1D) (IVW: OR 1.05, 95% CI 1.02–1.09, p = 0.001) were correlated with an elevated risk of AMD, while multiple sclerosis (MS) (IVW: OR 2.78E−18, 95% CI 2.23E−31 to 3.48E−05, p = 0.008) appeared to be protective against AMD. These findings were supported by an array of MR analysis methodologies and the MVMR approach.
Conclusion
Our study results, based on MR, provide genetic evidence indicating a causal relationship between specific IMIDs and AMD. CD, RA, and T1D are factors increasing the risk of AMD, while MS may have a protective effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The increasing global aging population is contributing to a rising healthcare burden from age-related macular degeneration (AMD). |
Previous studies suggest that immune-mediated inflammatory diseases (IMIDs) may affect AMD risk, but causality remains debated as a result of confounding factors. This research investigates the causal link between IMIDs and AMD, revealing specific associations. |
What was learned from the study? |
The Mendelian randomization (MR) analysis revealed specific associations between IMIDs and AMD risk. Crohn’s disease, rheumatoid arthritis, and type 1 diabetes were correlated with an elevated risk of AMD. Multiple sclerosis appeared to have a protective influence against AMD. |
Genetic evidence supports IMID–AMD causality, guiding future research and therapeutic strategies. These findings have implications for future research and the development of strategies for the prevention and treatment of AMD. |
The identification of specific IMIDs associated with AMD risk provides potential targets for therapeutic interventions. |
Introduction
With the progression of time, the demographic trend of an aging global population is accelerating. Forecasts predict a twofold increase in individuals aged 60 years or older by 2050, escalating from 962 million in 2017 to an estimated 2.1 billion [1]. This significant and continuous demographic expansion highlights the growing impact of the senior population segment. The significant increase in the incidence of age-associated diseases, particularly age-related macular degeneration (AMD) [2], is closely associated with this demographic change. AMD is identified as the leading cause of visual impairment and blindness among the senior populace in industrialized nations. Future projections indicate that the worldwide prevalence of AMD is expected to rise to 288 million by 2040 [3]. AMD presents considerable physical and psychological challenges to those affected and its escalating incidence contributes to an increased societal burden.
AMD is an intricate ocular pathology predominantly affecting the macula, a diminutive structure located posteriorly in the retina, crucial for mediating acute detail and chromatic perception [4]. Initial manifestations of AMD are characterized by the accumulation of drusen, yellowish-white deposits comprising proteins and lipids, coupled with anomalous pigmentation. Progression of AMD divides into two distinct phenotypes: the atrophic (dry) and the neovascular (wet) forms [5]. Dry AMD, constituting 80–85% of all advanced AMD cases, is typified by a profusion of drusen. The primary etiology of vision impairment in AMD is attributed to the advanced dry variant, known as geographic atrophy [6], which leads to the functional debilitation and subsequent loss of photoreceptor cells. Despite concerted efforts to mitigate the progression of this AMD variant, it remains recalcitrant to current therapeutic interventions. Conversely, wet AMD is characterized by choroidal neovascularization, the proliferation of aberrant blood vessels from the choroid penetrating the retina, culminating in retinal edema [7]. This phenotype can be treated with pharmaceuticals derived from nucleic acids, monoclonal antibodies, or chimeric proteins, such as anti-vascular endothelial growth factor drugs [7], which are expected to promote reperfusion of choroidal capillaries and improve the patient’s condition. Hence, early detection of this severe, age-related disorder is imperative for substantially improving the quality of life in the aging demographic.
Immune-mediated inflammatory diseases (IMIDs) represent a spectrum of clinically common disorders with heterogeneous presentations, including inflammatory bowel disease (IBD) which encompasses Crohn’s disease (CD) and ulcerative colitis (UC), rheumatoid arthritis (RA), ankylosing spondylitis (AS), MS multiple sclerosis (MS), and asthma, among others [8]. At present, the therapeutic approaches for managing IMIDs are still insufficient [9]. IMIDs and AMD are both characterized as inflammatory conditions, sharing not only common pathological features but also overlapping risk factors such as age, smoking, obesity, family history, and gender [10]. Emerging evidence suggests a potential association within the IMID spectrum and the pathogenesis of AMD [11,12,13,14]. Notably, the connection between RA and AMD has been extensively explored, with multiple observational studies indicating a significant link [11]. This may be because they are regulated, to some extent, by the complement pathway of the immune response and the role of the inflammatory cytokine tumor necrosis factor alpha (TNFα) [15]. Concurrently, some drugs used to treat RA, including hydroxychloroquine (HCQ), have also been employed for the treatment of AMD. McGeer and Sibley initially reported that their patients with RA, following treatment with HCQ, were relatively spared from the development of AMD [16], suggesting a potential protective effect of HCQ against AMD. Yahalomi’s study found that patients undergoing HCQ treatment experienced slower progression of AMD and fewer formations of vitreous drusen [17]. However, the overall effectiveness and mechanism of action of these treatments in this context remain. Despite certain similarities, the precise relationship between these conditions requires further in-depth investigation. However, as a result of the inherent limitations of observational studies, including susceptibility to remaining confounding and reverse causation, it is necessary to establish a novel research strategy for exploring the causal relationship between IMIDs and AMD.
Mendelian randomization (MR), as a research method, utilizes genetic variations in non-experimental data to infer the causal relationships between exposure factors and outcome variables. These exposure factors may encompass various elements, like biological markers, dietary habits, anthropometric measurements, lifestyle factors, or additional potential risk factors, which may influence the outcomes [18]. MR is considered a reliable approach to overcome the potential limitations seen in observational studies and to assess causal relationships. This is because that genetic variations are randomly allocated at conception and are less likely to be affected by external confounding factors [19].
As of now, we have not come across any reports on MR studies investigating the relationship between IMIDs and AMD. Moreover, there have been no dedicated randomized controlled trials exploring the relationship between the two. Therefore, the present MR analysis was carried out to explore the association between IMIDs and AMD.
Methods
Study Design
In this study, a two-sample MR analysis method was employed, and then instrumental variables (IVs) were obtained for various IMIDs based on public summary data. The method contributes to the effective addressing of confounding bias present in conventional epidemiological studies and to investigating the causal relationship between these diseases and AMD [20]. Three assumptions must be satisfied to consider a genetic variant as a valid IV for MR analysis:
-
1.
The variant is associated with the exposure.
-
2.
The variant is not associated with the outcome via a confounding pathway.
-
3.
The variant does not directly affect the outcome; instead, it may indirectly affect the outcome via the exposure [21].
In addition, multivariate Mendelian randomization (MVMR) analysis was conducted to assess whether IMIDs were independently associated with AMD. On the basis of the results of univariable MR analysis, we specifically examined whether digestive system diseases and skeletal system diseases were independently related to AMD [22]. Figure 1 illustrates the study design.
A The design of univariable Mendelian randomization. The crosses mean that genetic variants are not associated with confounders or cannot be directly involved in outcome but via the exposure pathway. Solid paths are significant; dashed paths should not exist in the Mendelian randomization study. B The design of multivariate Mendelian randomization (MVMR). MVMR considered whether Crohn’s disease and ulcerative colitis are independent within inflammatory bowel disease and whether they have a causal relationship with age-related macular degeneration. It also examined the independence of ankylosing spondylitis and rheumatoid arthritis within the study and their causal relationship with age-related macular degeneration. AMD age-related macular degeneration, RA rheumatoid arthritis, IBD inflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, MS multiple sclerosis, AS ankylosing spondylitis, T1D type 1 diabetes
Data Sources
In this study, the AMD data were sourced from the FinnGen consortium. According to its summary statistics, the dataset comprises 3763 cases (including dry, wet, or a combination of both AMD types) and 205,359 controls, covering a total of 16,380,424 single nucleotide polymorphisms (SNPs) [23]. The official description of this dataset is “age-related loss of vision in the central portion of the retina (macula), secondary to retinal degeneration.” The dataset is large with regard to its sample size and offers a novel perspective compared with previous MR analyses related to AMD. Moreover, data on IMIDs in this study were obtained on the basis of the open Genome-Wide Association Studies (GWAS) database of the Medical Research Council Integrated Epidemiology Unit (IEU) (https://gwas.mrcieu.ac.uk/) [24]. These datasets encompass CD, UC, RA, MS, and AS, all of which are derived from the European populations [25,26,27]. Table 1 displays more detailed information of phenotype and consortium.
Genetic Instrumental Variable Selection
The process of selecting IVs was roughly divided into three steps. First, to satisfy the assumptions of MR analysis, SNPs whose p values were lower than the genome-wide significance level (5 × 10−8) were selected. Additionally, to guarantee a strong relationship between IVs and exposure factors, weak IVs whose F values (according to the formula [R2/(R2 − 1)] × [(N − K − 1)/K]) were less than 10 were eliminated. Second, those selected IVs should pass the independence test. Genetic distance is the length of linkage disequilibrium region. Consequently, 1000 Genomes Project European sample data were used as a reference panel for determining linkage disequilibrium among SNPs. In other words, linkage disequilibrium level (r2) of SNPs was set as 0.001, while genetic distance was 10,000 kb. Furthermore, SNPs whose r2 level was > 0.001 and those with the greatest significance were eliminated to reduce the impact of linkage disequilibrium and to maintain the independence of those chosen IVs [28]. Third, MR-PRESSO package was used to exclude outliers. Finally, we evaluated and eliminated SNPs associated with other phenotypes, like potential confounders and mediators (e.g., body mass index), according to the PhenoScanner database V2 [29] (Supplementary Table 8 displays data on specific SNPs). After these three steps, IVs closely related to the exposure, weakly related to the outcome, but not related to confounders were obtained. More details of the SNP selection process are shown in Table 2.
In two-sample MR analysis, we used the inverse variance-weighted (IVW) method to assess the causality between IMIDs and AMD. IVW is a traditional analysis approach used to combine Wald ratio estimates from diverse related IVs [30]. In the absence of horizontal pleiotropy, the IVW method is identified as the unbiased and most effective approach to estimate causality in MR studies. Additionally, we employed the weighted median method for MR analysis as well. This method is identified as the reliable approach for generating effective causal estimates, even though as much as 50% of genetic instruments (i.e., IVs) are ineffective or are affected by pleiotropic effects [31]. This method takes the median of diverse IV estimates to determine the causal effect estimate, and weights it with the inverse of corresponding variance. Moreover, we applied the Simple and Weight mode methods as additional reference standards. In case of inconsistent results among these methods, the priority was given to IVW as the primary outcome [32].
Furthermore, to investigate the direct impact of different IMIDs on AMD, we conducted a MVMR analysis. The MVMR method allows one to simultaneously consider multiple risk factors, such as different immune mediators, and their potential interactions [33]. First of all, researchers collected genetic data related to IMIDs and selected SNPs associated with immune mediators or immune-related gene expression levels as IVs. Subsequently, they utilized the MVMR method to combine all IVs for estimating the causality between IMIDs and AMD while taking into account the interactions among multiple exposure factors. Through conditional analysis, researchers were able to assess the interrelationships among these factors, thereby gaining a more comprehensive understanding of their impact on AMD. The MVMR method facilitates the further exploration of multifactor disease relationships and offers a more comprehensive insight into the complex association between IMIDs and AMD.
Sensitivity Analysis
To ensure the reliability of MR analysis, we employed multistep validation. Firstly, Cochran’s Q statistic was used to assess the heterogeneity among IVs. Subsequently, MR-PRESSO was applied to detect and remove SNPs showing great outlier values before conducting a reiteration of MR analysis [34]. Next, a test for pleiotropy was performed, and the detection of pleiotropy indicated that the reliability of causality between exposure and outcome was substantially compromised [35]. Finally, the leave-one-out analysis was carried out by eliminating one SNP each time and determining the pooled effect of the rest of the SNPs to observe if there were significant changes in results upon removing specific SNPs. Any significantly altered results after removing a particular SNP suggested that this SNP substantially affected the outcome, and this was undesirable. Conversely, the ideal scenario was that the outcome remained insignificantly altered after SNP removal. Table 3 shows the sensitivity analysis results.
Result Reporting and Software
Our MR analysis results were represented by estimates of odds ratio (OR) or β values and corresponding 95% confidence interval (CI), according to binary or continuous variables. P < 0.05 (two-sided) indicated statistical significance. Two estimate types were expressed as point estimates. Data were processed and analyzed by R version 4.3.0 [36], along with Storm Statistical Platform (www.medsta.cn/software).
Ethical Approval
Primary data were not utilized in this work, which avoided the need for ethical approval.
Results
F statistics were determined for diverse SNPs according to exposure and outcome data; as a result, all SNPs satisfied the standard of F > 10, indicating no weak instrument bias. On the basis of MR-Egger regression, its intercept suggested no horizontal pleiotropy among SNPs associated with exposure factor (Supplementary Fig. 1).
Univariate Mendelian Randomization
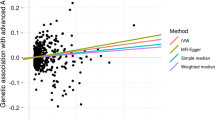
As for MR analysis using AS, CD, IBD, MS, RA, T1D, and UC as exposures and AMD as the outcome, after filtering, there were altogether 5, 110, 131, 3, 64, 37, and 86 SNPs enrolled. According to two-sample MR analysis results, except for UC, all the other IMIDs showed a significant causal relationship with AMD. Specifically, the following results were obtained by the IVW method: the p values and ORs (95% CI) of the IVW method were 0.036 and 3.58E10 (2.24–5.72E10) for AS, 0.007 and 1.05 (1.01–1.1) for CD, 0.033 and 1.05 (1–1.1) for IBD, 0.008 and 2.78E−18 (2.23E−31 to 3.48E−05) for MS, 0.0001 and 1.09 (1.04–1.15) for RA, 0.001 and 1.05 (1.02–1.09) for T1D, and 0.5 and 1.01 (0.96–1.07) for UC, respectively (Fig. 2).
In summary, patients with AS, CD, IBD, RA, and T1D were more likely to develop AMD as a secondary condition. Interestingly, our statistical results even suggested that the presence of MS was a protective factor against AMD. Moreover, the MR-Egger regression intercept results revealed no evidence of pleiotropy, with a p value greater than 0.05. Supplementary Figs. 1–3 present all the results of MR analysis, assessments of heterogeneity, and the “leave-one-out” analysis.
Multivariable Mendelian Randomization
In the previous univariable MR analysis, we identified shared IVs among several IMIDs in the digestive system disease group; similarly, the skeletal system disease group exhibited the same situation. Considering that it might potentially affect the reliability of MR analysis results, separate MVMR analyses were conducted for both the digestive system and the skeletal system to validate the associations of digestive system diseases and skeletal system diseases with AMD.
The results of mutual MVMR analysis indicated that the causal relationship between CD and AMD was consistent with the univariable MR analysis results, suggesting that CD might be a contributing factor for the onset of AMD (p = 0.03, OR 1.068, 95% CI 1.005–1.136). The estimated effect of RA on AMD was also comparable to the univariable IVW estimate (p = 0.01, OR 1.010, 95% CI 1.018–1.182). In contrast, the multivariable MR estimates for other autoimmune diseases and their association with AMD were not statistically significant. To sum up, the results from the MVMR study emphasized a certain causal association between CD and AMD, and highlighted the potential link between RA and AMD (Fig. 3).
Discussion
The present work focused on comprehensively investigating the possible causality between IMIDs and AMD. To achieve this goal, we selected multiple autoimmune diseases as exposures and AMD as the outcome. After rigorous IV selection, univariable and multivariable MR analyses were performed. Following thorough sensitivity analysis, the following research findings were obtained:
-
1.
The genetically predicted CD, RA and T1D might be the causative factors for AMD.
-
2.
There was no causal relationship between UC, AS, and AMD.
-
3.
The presence of MS might potentially have a protective effect against AMD.
IMIDs constitute a diverse and widespread spectrum of diseases driven by immune, inflammatory, and genetic pathways, which have increased the risk of developing AMD via various biological mechanisms [11,12,13, 37]. One of the primary pathological characteristics of these diseases is the alteration of cellular homeostasis in the body, with a central and plausible mechanism involving systemic inflammation. IMIDs can result in the production of oxidized lipoproteins and free radicals, causing stress responses in retinal tissues [38]. This process can activate local microglial cells and induce the accumulation of subretinal products, thereby triggering localized inflammatory responses. Consequently, macrophages, T lymphocytes, and mast cells infiltrate the macular region to initiate the formation of drusen [39,40,41]. On the other hand, low-grade inflammation may persist for decades during the course of IMIDs and intensify with age, leading to pathological changes that disrupt ocular homeostasis; particularly, it involves the activation of microglial cells and the complement system, ultimately promoting the development of AMD [42].
No previous reports have illustrated a clear causal relationship between MS and AMD. Instead, there are only isolated case reports indicating the induction of macular edema following symptomatic treatment of MS [43], implying a potential protective effect of MS against AMD. From a pathophysiological perspective, patients with MS exhibit elevated levels of sphingosine-1-phosphate (S1P) in their bodies [44]. In vitro, S1P has been recognized to enhance the barrier function via sphingosine-1-phosphate receptor 1 (S1PR1), which may contribute to the inhibition of exudation and choroidal neovascularization, the two key characteristics of exudative AMD [45]. Such indirect effects may mitigate the occurrence of exudative AMD. This underscores the complex roles of S1P and S1PR in the development and maintenance of ocular vasculature. Although our MR analysis results suggested a potential protective effect of MS against AMD, further investigation is warranted in the future.
In research on IBDs, the increased risk of AMD is associated with CD rather than with UC. CD can occur anywhere in the digestive tract, while UC is limited to the colon. A cross-sectional study also detected differences in the impact of these two IBDs on AMD [12]. To be specific, retinal drusen significantly increased in patients with CD, whereas it was less pronounced in patients with UC. The formation of drusen is related to fatty acid oxidation, cell apoptosis, and complement activation [46, 47]. In IBDs, the microbial cell wall lipids exposed to normal intestinal epithelium increase the secretion of C3 [48]. However, in the context of IBD, the defects in intestinal mucosa allow for continuous bacterial exposure, thus activating the alternative complement pathway [49]. C-reactive protein can bind to intestinal bacteria, triggering the classical pathway complement activation, all of which provides the necessary conditions for drusen formation [50]. Nonetheless, the complement involvement differs between CD and UC, with the enhanced complement activation during CD potentially accounting for a higher number of drusen. In CD, greater levels of C3 and C4 can be produced by the intestinal mucosa [51], while the formation of crypt abscesses further upregulates C3 [48, 52, 53]. Additionally, as a result of the more extensive lesion scope in CD than UC, more complement is generated. Increased production of C3 in patients with CD leads to the formation of more vitreous drusen, thereby exacerbating the progression of AMD. This may explain the differential impact of the two IBDs on AMD observed in the MVMR study. Generally, more retinal drusen can be observed in IBDs, accompanied by the extended disease course, a higher complication rate, and an increased risk of related IgA glomerulonephritis.
According to our MR analysis among IMIDs affecting the skeletal system, RA was identified as a risk factor for AMD, while AS was not. Both RA and AS are chronic inflammatory diseases primarily affecting the joints, and they share numerous common risk factors and overlapping immune responses with AMD. We hypothesized that this might be due to the prolonged activation of the shared immune pathways, such as the complement cascade, thus potentially increasing the risk of developing AMD in these patients [47]. Previous research has yielded conflicting results regarding the association between RA and AMD. McGeer suggested that the risk of AMD might decrease among patients with RA [16], which was attributed to the long-term anti-inflammatory treatments. Nevertheless, certain limitations should be noted in the study, including a small sample size, lack of assessment of AMD prevalence, and oversimplification of the pharmacodynamics of RA treatments. Moreover, Keenan’s cross-sectional study found that the risk of subsequent AMD significantly increased in patients with RA [11]. Their study had the strength of a large dataset and extended follow-up period, adding credibility to its findings. Furthermore, a recent study based on the MarketScan database indicated that the diagnosis of RA led to a significantly earlier diagnosis of AMD [54]. Our MR study also corroborated this finding at the genetic level.
While diabetes has long been considered a significant risk factor for AMD [55], previous reports on the association between diabetes and AMD have afforded conflicting results, and studies specifically addressing the relationship between T1D and AMD are lacking. A previous study suggested a protective effect of diabetic retinopathy on the risk of AMD, since inner blood-retinal barrier damage in diabetes was associated with the enhanced outer blood-retinal barrier activity, thus potentially providing a protective mechanism for AMD [56]. However, a meta-analysis found an increased risk of AMD associated with diabetes [57], although it only adjusted for age and sex, leaving potential confounders unaccounted for. Moreover, recent studies have shown that, compared with non-diabetic eyes, the choriocapillaris surrounding the dark halo associated with type 1 macular neovascularization in diabetic eyes exhibits greater hypoperfusion both before starting therapy and after the loading phase. These findings suggest that diabetic retinopathy may be a potential risk factor for the development and progression of late-stage AMD [58]. Compared with previous studies, our research has a significant advantage by utilizing the MR study method, which minimizes confounders, and confirming the causality between T1D and AMD.
Certain limitations should be noted in the present work. First, genome-wide association study data only recruited individuals of European ancestry, which may limit the generalizability of our results to other populations. More studies are warranted for validating the applicability of our results in other ethnic groups. Secondly, resource constraints prevented us from accessing the updated individual-level statistical data. Additionally, the FinnGen Gwas database does not provide detailed staging information for patients with AMD, hindering precise guidance for each specific subtype. Finally, there were only three IVs for MS and AMD, which may inevitably introduce bias.
Conclusion
Our MR analysis reveals that CD, RA, and T1D are related to a higher risk of AMD, while MS appears to have a protective effect against AMD. Moreover, the identification of specific IMIDs linked to an increased risk of AMD presents potential targets for innovative therapeutic interventions.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-116. https://doi.org/10.1016/S2214-109X(13)70145-1.
Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. https://doi.org/10.1038/s41572-021-00265-2.
Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila). 2017;6(6):493–7. https://doi.org/10.22608/APO.2017251.
Kakigi CLM, Singh K, Wang SY, Enanoria WT, Lin SC. Self-reported calcium supplementation and age-related macular degeneration. JAMA Ophthalmol. 2015;133(7):746–54. https://doi.org/10.1001/jamaophthalmol.2015.0514.
Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127(5):616–36. https://doi.org/10.1016/j.ophtha.2019.11.004.
Abdin AD, Devenijn M, Fulga R, Langenbucher A, Seitz B, Kaymak H. Prevalence of geographic atrophy in advanced age-related macular degeneration (AMD) in daily practice. J Clin Med. 2023;12(14):4862. https://doi.org/10.3390/jcm12144862.
Viggiano P, Grassi MO, Pignataro M, et al. Topographical analysis of the choriocapillaris reperfusion after loading anti-VEGF therapy in neovascular AMD. Transl Vis Sci Technol. 2022;11(9):18. https://doi.org/10.1167/tvst.11.9.18.
McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21(10):680–6. https://doi.org/10.1038/s41577-021-00603-1.
Kerrigan SA, McInnes IB. Reflections on “older” drugs: learning new lessons in rheumatology. Nat Rev Rheumatol. 2020;16(3):179–83. https://doi.org/10.1038/s41584-020-0375-7.
Lee KS, Lin S, Copland DA, Dick AD, Liu J. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J Neuroinflammation. 2021;18(1):32. https://doi.org/10.1186/s12974-021-02088-0.
Keenan TDL, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, osteoarthritis and rheumatoid arthritis: record linkage study. Retina. 2015;35(12):2613–8. https://doi.org/10.1097/IAE.0000000000000651.
Nicklason E, Ham Y, Ng D, et al. Retinal drusen counts are increased in inflammatory bowel disease, and with longer disease duration, more complications and associated IgA glomerulonephritis. Sci Rep. 2022;12:11744. https://doi.org/10.1038/s41598-022-15232-4.
Kim JT, Yoon YH, Lee DH, Joe SG, Kim JG. Dexamethasone intravitreal implant in the silicone oil-filled eye for the treatment for recurrent macular oedema associated with ankylosing spondylitis: a case report. Acta Ophthalmol. 2013;91(4):e331-332. https://doi.org/10.1111/aos.12080.
Wirkkala J, Kubin AM, Ohtonen P, Yliselä J, Siik T, Hautala N. Visual outcomes of observation, macular laser and anti-VEGF in diabetic macular edema in type 1 diabetes: a real-world study. BMC Ophthalmol. 2022;22(1):258. https://doi.org/10.1186/s12886-022-02482-z.
Weigelt CM, Zippel N, Fuchs H, et al. Characterization and validation of in vitro and in vivo models to investigate TNF-α-induced inflammation in retinal diseases. Transl Vis Sci Technol. 2022;11(5):18. https://doi.org/10.1167/tvst.11.5.18.
McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol Aging. 2005;26(8):1199–203. https://doi.org/10.1016/j.neurobiolaging.2005.02.003.
Yahalomi T, Pikkel Y, Arnon R, Porat D, Pikkel J. The HIT study—the hydroxychloroquine effect in the treatment of patients with age-related macular degeneration: a randomized controlled trial. Medicina (Kaunas). 2023;59(3):551. https://doi.org/10.3390/medicina59030551.
Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2(1):6. https://doi.org/10.1038/s43586-021-00092-5.
Budu-Aggrey A, Paternoster L. Research techniques made simple: using genetic variants for randomization. J Invest Dermatol. 2019;139(7):1416-1421.e1. https://doi.org/10.1016/j.jid.2019.03.1138.
Zhang L, Zhang C, Zhang J, Liu A, Wang P, Xu J. A bidirectional mendelian randomization study of sarcopenia-related traits and knee osteoarthritis. Clin Interv Aging. 2023;18:1577–86. https://doi.org/10.2147/CIA.S424633.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. https://doi.org/10.1007/s10654-017-0255-x.
Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11(2):a038984. https://doi.org/10.1101/cshperspect.a038984.
FinnGen: Unique genetic insights from combining isolated population and national health register data | medRxiv. Accessed Oct 1, 2023. https://www.medrxiv.org/content/https://doi.org/10.1101/2022.03.03.22271360v1.
Conroy M, Sellors J, Effingham M, et al. The advantages of UK Biobank’s open-access strategy for health research. J Intern Med. 2019;286(4):389–97. https://doi.org/10.1111/joim.12955.
Liu JZ, Van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. https://doi.org/10.1038/ng.3359.
Forgetta V, Manousaki D, Istomine R, et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. 2020;69(4):784–95. https://doi.org/10.2337/db19-0831.
Ha E, Bae SC, Kim K. Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann Rheum Dis. 2021;80(5):558–65. https://doi.org/10.1136/annrheumdis-2020-219065.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. https://doi.org/10.1002/gepi.21998.
Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3. https://doi.org/10.1093/bioinformatics/btz469.
Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform. 2016;14(4):173–80. https://doi.org/10.5808/GI.2016.14.4.173.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98. https://doi.org/10.1093/ije/dyx102.
Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186. https://doi.org/10.12688/wellcomeopenres.15555.3.
Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–27. https://doi.org/10.1093/ije/dyy262.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7.
Hemani G, Bowden J, Davey SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–208. https://doi.org/10.1093/hmg/ddy163.
Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. ELife. 2018;7:e34408. https://doi.org/10.7554/eLife.34408.
Allen DW, Liew G, Cho YH, et al. Thirty-year time trends in diabetic retinopathy and macular edema in youth with type 1 diabetes. Diabetes Care. 2022;45(10):2247–54. https://doi.org/10.2337/dc21-1652.
Morohoshi K, Goodwin AM, Ohbayashi M, Ono SJ. Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J Autoimmun. 2009;33(3–4):247–54. https://doi.org/10.1016/j.jaut.2009.09.003.
Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011;95(1):14–25. https://doi.org/10.1016/j.pneurobio.2011.05.011.
Wang X, Wang T, Lam E, Alvarez D, Sun Y. Ocular vascular diseases: from retinal immune privilege to inflammation. Int J Mol Sci. 2023;24(15):12090. https://doi.org/10.3390/ijms241512090.
Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7(1):58–68. https://doi.org/10.1111/j.1474-9726.2007.00351.x.
Nita M, Grzybowski A, Ascaso FJ, Huerva V. Age-related macular degeneration in the aspect of chronic low-grade inflammation (pathophysiological parainflammation). Mediators Inflamm. 2014;2014: 930671. https://doi.org/10.1155/2014/930671.
Li Q, Jing LJ, Li Y, Jia Y. Macular edema after siponimod treatment for multiple sclerosis: a case report and literature review. BMC Neurol. 2023;23:286. https://doi.org/10.1186/s12883-023-03333-0.
McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398(10306):1184–94. https://doi.org/10.1016/S0140-6736(21)00244-0.
Terao R, Honjo M, Totsuka K, Miwa Y, Kurihara T, Aihara M. The role of sphingosine 1-phosphate receptors on retinal pigment epithelial cells barrier function and angiogenic effects. Prostaglandins Other Lipid Mediat. 2019;145: 106365. https://doi.org/10.1016/j.prostaglandins.2019.106365.
Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–71. https://doi.org/10.1146/annurev-genom-090413-025610.
Shaw PX, Zhang L, Zhang M, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109(34):13757–62. https://doi.org/10.1073/pnas.1121309109.
Sünderhauf A, Skibbe K, Preisker S, et al. Regulation of epithelial cell expressed C3 in the intestine—relevance for the pathophysiology of inflammatory bowel disease? Mol Immunol. 2017;90:227–38. https://doi.org/10.1016/j.molimm.2017.08.003.
Johansson MEV, Gustafsson JK, Holmén-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–91. https://doi.org/10.1136/gutjnl-2012-303207.
Chirco KR, Potempa LA. C-reactive protein as a mediator of complement activation and inflammatory signaling in age-related macular degeneration. Front Immunol. 2018;9:539. https://doi.org/10.3389/fimmu.2018.00539.
Ahrenstedt O, Knutson L, Nilsson B, Nilsson-Ekdahl K, Odlind B, Hällgren R. Enhanced local production of complement components in the small intestines of patients with Crohn’s disease. N Engl J Med. 1990;322(19):1345–9. https://doi.org/10.1056/NEJM199005103221903.
Elvington M, Schepp-Berglind J, Tomlinson S. Regulation of the alternative pathway of complement modulates injury and immunity in a chronic model of dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2015;179(3):500–8. https://doi.org/10.1111/cei.12464.
Laufer J, Oren R, Goldberg I, et al. Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn’s disease. Clin Exp Immunol. 2000;120(1):30–7. https://doi.org/10.1046/j.1365-2249.2000.01168.x.
Schnabolk G, Rohrer B, Simpson KN. Increased nonexudative age-related macular degeneration diagnosis among medicare beneficiaries with rheumatoid arthritis. Invest Ophthalmol Vis Sci. 2019;60(10):3520–6. https://doi.org/10.1167/iovs.18-26444.
Feldman-Billard S, Dupas B. Eye disorders other than diabetic retinopathy in patients with diabetes. Diabetes Metab. 2021;47(6):101279. https://doi.org/10.1016/j.diabet.2021.101279.
Cummings M, Cunha-Vaz J. Treatment of neovascular age-related macular degeneration in patients with diabetes. Clin Ophthalmol. 2008;2(2):369–75. https://doi.org/10.2147/opth.s2560.
Chen X, Rong SS, Xu Q, et al. Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS ONE. 2014;9(9):e108196. https://doi.org/10.1371/journal.pone.0108196.
Viggiano P, Miere A, Borrelli E, et al. The Impact of diabetic retinopathy on the choriocapillaris in neovascular AMD. Invest Ophthalmol Vis Sci. 2023;64(14):32. https://doi.org/10.1167/iovs.64.14.32.
Acknowledgments
We would like to express our gratitude to the participants and researchers of the FinnGen study, as well as for the GWAS summary statistics on IMIDs from the IEU database.
Funding
This work was partially supported by the following projects: the National Natural Science Foundation of China (Grant No. 82205197); the National Natural Science Foundation of China (Grant No. 82174442). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Study design: Longyao Zhang, Fuhui Sha; conduct of the study: Hongmei Li; data organization: Fengming Liang; analysis and interpretation: Longyao Zhang, Fengming Liang; manuscript preparation and review: Fuhui Sha, Hongmei Li; read and approved the final version of the manuscript: Fuhui Sha, Hongmei Li, Longyao Zhang, Fengming Liang.
Corresponding author
Ethics declarations
Conflict of Interest
Fuhui Sha, Hongmei Li, Longyao Zhang, and Fengming Liang all declare that they have no conflicts of interest to disclose.
Ethical Approval
Primary data were not utilized in this work, which avoided the need for ethical approval.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sha, F., Li, H., Zhang, L. et al. Evidence for Genetic Causal Relationships Between Multiple Immune-Mediated Inflammatory Diseases and Age-Related Macular Degeneration: A Univariable and Multivariable Mendelian Randomization Study. Ophthalmol Ther 13, 955–967 (2024). https://doi.org/10.1007/s40123-024-00895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00895-1