Abstract
Introduction
To evaluate and compare the clinical outcomes of two toric presbyopia-correcting intraocular lenses (IOLs).
Methods
Non-randomized prospective comparative study including 86 eyes of 51 patients (age 43–83 years) that underwent cataract surgery with implantation of one of the following two IOLs: TECNIS Toric Synergy (Johnson & Johnson Vision) (Synergy group) or AT LISA tri toric 939MP (Carl Zeiss Meditec) (ATLISA group). Visual and refractive outcomes were evaluated during a 6-month follow-up.
Results
At 6 months after surgery, all eyes achieved uncorrected distance visual acuity 20/25 or better in both groups, whereas 96.2% and 100% of eyes achieved uncorrected near visual acuity (UNVA) 20/25 or better in the ATLISA and Synergy groups, respectively. All eyes achieved postoperative mesopic UNVA 20/30 or better in both IOL groups; 96.2% and 100% of eyes had a manifest cylinder ≤ 0.50 D at 6 months in ATLISA and Synergy groups, respectively. Mean magnitude of error was 0.04 ± 0.20 and − 0.04 ± 0.09 D in ATLISA and Synergy groups, respectively (p = 0.05). In the defocus curve, significant differences were found between IOL groups for most of distance-corrected visual acuities, except those corresponding to defocus of 0 D (p = 0.268) and − 1 D (p = 0.361).
Conclusions
The two toric presbyopia-correcting IOLs evaluated provide an efficacious astigmatic correction combined with a successful distance, intermediate and near visual rehabilitation. The visual performance seems to be better for most visual demands with the TECNIS Toric Synergy IOL, especially for distances closer than 40 cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
This study was conducted to compare two different commercially available presbyopia-correcting toric intraocular lenses (IOLs), a trifocal diffractive toric IOL and the toric version of a diffractive-based IOL combining an extended depth of focus (EDOF) and a multifocal pattern. |
This is crucial for determining the real clinical performance of currently available IOLs and consequently to take more optimized clinical decisions. |
What was learned from the study? |
Although both toric presbyopia-correcting IOLs provide an efficacious visual rehabilitation in eyes with corneal astigmatism, a better visual performance is achieved with the hybrid IOL combing the EDOF and diffractive multifocal pattern for most visual demands, but especially for distances closer than 40 cm. |
A functional near visual outcome is maintained with both toric presbyopia-correcting IOLs under mesopic light conditions. |
The efficacy of astigmatic correction is good with both toric presbyopia-correcting IOLs, although there is a trend to worse rotational instability with the trifocal diffractive toric IOL that should be confirmed in future studies with larger samples. |
Introduction
The presence of residual astigmatism after implantation of a multifocal intraocular lens (IOL) due to non-corrected pre-existing corneal astigmatism can lead to a clinically significant degradation of visual performance [1, 2], even when the magnitude of this astigmatism is low [3]. This can lead to the need for a corneal laser retreatment or even a piggyback implantation including a toric IOL to achieve a full refractive correction allowing a satisfactory visual rehabilitation across all distances [4]. Toric multifocal IOLs were developed to solve this situation, providing not only the visual restoration at all distances but also allowing a complete correction of the spherocylindrical error, avoiding or minimizing the presence of residual astigmatism [5].
Several studies have demonstrated the efficacy of toric multifocal IOLs to provide an efficacious correction of pre-existing corneal astigmatism while providing good visual acuity at different distances [6,7,8,9,10,11,12,13,14]. Among them, diffractive trifocal IOLs are the most commonly used; they combine a toric surface with a diffractive one, which is normally equivalent to that used in the non-toric version of the IOL [6,7,8,9,10,11,12,13,14]. Recently, a new toric multifocal IOL was developed by the manufacturer Johnson & Johnson that combines a diffractive-based extended depth of focus profile (EDOF) with a diffractive multifocal pattern, which is commercially known as Tecnis Synergy™ IOL [15]. This hybrid optical approach is efficacious for delivering a continuous high-contrast vision from far through near, even in low lighting conditions [15,16,17,18,19,20,21,22,23,24,25,26]. To date, only De Rojas et al. [20] have evaluated the outcomes of this new toric presbyopia-correcting IOL, but in a subsample of 22 eyes implanted with the toric version of Tecnis Synergy™ from a total sample of 104 eyes implanted with the non-toric version. However, these authors did not provide a specific analysis of the efficacy of the astigmatic correction of the toric version of the IOL. The aim of the current study was to evaluate and compare the clinical outcomes of a trifocal diffractive toric IOL with those obtained with the toric version of this new diffractive-based IOL combining an EDOF and a multifocal pattern.
Methods
Patients
This study was a non-randomized comparative study enrolling a total of 86 eyes of 51 patients undergoing uncomplicated phacoemulsification cataract surgery with implantation of one of the following two presbyopia-correcting toric IOLs: TECNIS Toric Synergy™ (Johnson & Johnson Vision, Jacksonville, FL, USA) or AT LISA tri toric 939MP (Carl Zeiss Meditec, Jena, Germany). Inclusion criteria were subjects with the diagnosis of dysfunctional lens syndrome seeking spectacle independence and predicted postoperative astigmatism of > 0.75 diopters (D). Exclusion criteria included history of ocular trauma, topographic astigmatism of < 1 D, active ocular pathology, large scotopic pupil size (> 6 mm), previous ocular surgery, optic nerve atrophy, aniridia or iris atrophy, use of systemic or topical medication with the potential of affecting the visual function, and systemic diseases with the potential of increasing intraoperative risks or affecting the visual outcomes (e.g., uncontrolled diabetes). Before surgery, all patients were adequately informed about the study and signed a consent form in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Svjetlost Hospital (Zagreb, Croatia) ethics committee (IIIS-ZG21-TS).
Clinical Protocol
The preoperative examination included the following battery of tests and procedures: measurement of monocular uncorrected (UDVA) and corrected (CDVA) distance visual acuity, measurement of monocular uncorrected visual acuity (UNVA) at 40 cm, manifest refraction, optical biometry and keratometry (IOLMaster 700, Carl Zeiss Meditec, Jena, Germany), Goldman tonometry, slit-lamp biomicroscopy, corneal topography (Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany) and fundus evaluation. The ETDRS chart was used for measuring all visual acuities throughout the study.
All IOL powers were calculated using the Barrett Toric calculator with measured posterior astigmatism. For both IOL groups, the lens power nearest to emmetropia was chosen, with the SIA set to 0.5 D at 90° for all surgeries. For astigmatic correction, a lens power was selected that was nearest to emmetropia and that avoided causing an axis flip when accounting for the SIA of the incision at 90°.
Postoperatively, patients were evaluated at 1 day, 1 week, 1 month, 3 months and 6 months after surgery. At 1 day, an analysis of the integrity of the status of the anterior segment by slit-lamp biomicroscopy and tonometry was performed. At 1 week and 1 month postoperatively, the following examinations were performed: measurement of monocular UDVA, CDVA and UNVA, manifest refraction, slit-lamp biomicroscopy and applanation tonometry. In the two last postoperative visits (3 and 6 months after surgery), the same protocol of measurements was followed, with the inclusion of additional tests: measurement of monocular CDVA, measurement of monocular UNVA under mesopic conditions (3 cd/m2), IOL rotation and monocular measurement of the defocus curve only at 6 months after surgery. IOL rotation was checked during surgery using ZEISS CALLISTO eye computer-assisted cataract surgery system (Carl Zeiss Meditec, Jena, Germany) with the Reference image function from IOL master 700. A pyramidal ocular wavefront device (Osiris-T, CSO, Florence, Italy) was used for postoperative IOL axis position checks (Fig. 1).
Surgery
All surgeries were performed by the same experienced surgeon (NG) using a standard technique of sutureless microincision phacoemulsification. The first step was the instillation of anaesthesia and mydriatic drops and then the surgery was initiated with the creation of the corneal incision (incision size: 2.75 mm) at 90º meridian. After this, a manual creation of the capsulorhexis was performed and the phacoemulsification process initiated. After eliminating the content within the capsular bag, the IOL was inserted into it through the main incision using the injector developed by the manufacturer for this purpose. A combination of topical antibiotic and steroid was prescribed to be applied postoperatively four times daily for 3 weeks.
Statistical Analysis
The statistical data analysis was performed using SPSS software, version 22.0, for Windows (SPSS, Chicago, IL, USA). Normality of all data distributions was initially evaluated using the Kolmogorov-Smirnov test. Paired Student t or Wilcoxon tests were used to analyse the differences between pre- and postoperative visits depending on whether or not the data samples were normally distributed. For the comparison between IOL groups, unpaired Student t or Wilcoxon tests were used to analyse the differences between IOL groups depending on whether or not the data samples were normally distributed. A p value < 0.05 was considered statistically significant. To avoid the bias associated with the inclusion of both eyes when analysing monocular data due to the inherent correlation between fellow eyes, the analysis of monocular data was only performed for right eyes.
The Alpins vector analysis method was used for the analysis of the astigmatic changes occurring after surgery [27, 28]. The following vectors were determined and evaluated: targeted induced astigmatism (TIA) as the vector of intended change in cylinder for each treatment, surgically induced astigmatism (SIA) as the vector of the real change achieved and difference vector (DV) as the additional astigmatic change that would enable the initial surgery to achieve its intended target. Additionally, the magnitude of error (ME) (difference between the magnitude of SIA and TIA) and the angle of error (AE) (angle described by the vectors of SIA and TIA) were calculated.
Results
A total of 86 eyes of 51 patients with mean age of 59.2 years (SD 9.4, median 53.7, range 43–83 years) were enrolled. The sample comprised 29 men (56.9%) and 22 women (43.1%). A difference close to the limit of statistical significance among IOL groups was found in age (ATLISA vs Synergy groups: 56.3 ± 9.1 vs 50.8 ± 9.1, p = 0.049). In contrast, no significant differences between groups were found in sex distribution (p = 0.842). Table 1 summarizes the preoperative clinical data obtained in the IOL groups of the sample evaluated. As shown, no significant differences were found between groups in axial length (AXL), keratometry, anterior chamber depth (ACD) or IOL power (p ≥ 0.329).
Visual Acuity Outcomes
In both IOL groups, a significant improvement was observed at 1 week after surgery in monocular UDVA and UNVA as well as in monocular CDVA (p < 0.001). When comparing the visual outcomes between IOL groups during the postoperative follow-up (Table 2), no significant differences were found in any visual acuity measure (p ≥ 0.075).
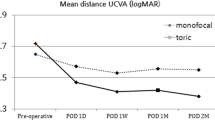
Figure 2 shows the distribution of 3- and 6-month postoperative monocular distance and near visual acuity data in the sample evaluated. At 6 months after surgery, a total of 100% (26/26) of eyes achieved UDVA of 20/25 or better in the ATLISA group, with also 100% (19/19) of eyes achieving this level of UDVA in the Synergy group (Fig. 1). Likewise, a total of 80.8% (21/26) and 100% (19/19) of eyes achieved UDVA of 20/20 or better. Concerning UNVA, a total of 96.2% (25/26) of eyes achieved a value of 20/25 or better in the ATLISA group, whereas this percentage was 100% (19/19) in the Synergy group (Fig. 2). Concerning postoperative mesopic UNVA, all eyes achieved a value of 20/30 or better at 3 and 6 months after surgery in both IOL groups.
Refractive Outcomes
No significant differences between IOL groups were found in the manifest residual sphere and cylinder during the whole follow-up (p ≥ 0.113). Residual sphere was within ± 0.50 D in all cases during the postoperative follow-up, except in one eye of the Synergy group at 6 months. Concerning cylinder, a total of 96.2% (25/26) of eyes in the ATLISA group had a manifest cylinder of ≤ 0.50 D at the end of the follow-up (Fig. 3). In the Synergy group, all eyes had a manifest cylinder of ≤ 0.50 D at 6 months (Fig. 3).
Table 3 summarises the outcomes of the vector analysis of the astigmatic changes achieved with the toric IOL implantation in the two IOL groups of the sample evaluated. As shown, only a difference in the limit of statistical significance (p = 0.05) between IOL groups was found in magnitude of error (ME), with a more positive value in the ATLISA group. No differences between IOL groups were found in difference vector (DV) and angle of error (AE) (p ≥ 0.613).
Defocus Curve Outcomes
Figure 4 displays the mean monocular defocus curve in each IOL group in the sample evaluated. Significant differences were found between IOL groups for most of the distance-corrected visual acuities measured, except for that corresponding to the defocus level of 0 D (p = 0.268). Specifically, visual acuities were significantly better in the Synergy group for most of defocus levels, except for − 2.50 D and 0.50 D, for which the opposite trend was found.
IOL Rotation
Figure 5 displays mean IOL rotation during the follow-up in the two IOL groups of the current sample. As shown, significant differences were found between IOL groups at 1-month follow-up (p = 0.049), with a very small trend to a more negative IOL rotation in the Synergy group. However, this trend was not present during the rest of follow-up, with no significant differences existing between groups. The percentage of eyes with an IOL rotation within ± 3º was 50.0% (13/26) and 100.0% (19/19) in ATLISA and Synergy groups, respectively. Likewise, 76.9% (20/26) and 100.0% (19/19) of eyes showed an IOL rotation within ± 5º in ATLISA and Synergy groups, respectively.
Adverse Events
The development of posterior capsular opacification (PCO) that required YAG capsulotomy was observed in 6 eyes: 5 eyes (19.2%) in the ATLISA group and 1 eye (5.3%) in the Synergy group. Furthermore, excimer laser corneal refractive enhancements were needed at the end of the follow-up in four eyes evaluated in the current series: three eyes (11.5%) in the ATLISA group and one eye (5.3%) in the Synergy group. In all cases, astigmatic correction was needed.
Discussion
The correction of pre-existing corneal astigmatism in eyes implanted with presbyopia-correcting toric IOLs is necessary to obtain a satisfactory visual outcome, especially at intermediate and near distances [1,2,3]. It has been demonstrated that the effect of residual astigmatism on satisfaction was more evident at the 0.75 to 1.00 D level for eyes implanted with either monofocal or multifocal IOLs [29], with especially negative impact of residual astigmatism on intermediate vision with some trifocal IOLs [1]. In the current study, two different types of toric presbyopia-correcting IOLs have been evaluated and compared, a trifocal diffractive and an hybrid model combining a diffractive-based EDOF and a multifocal diffractive pattern, investigating not only the level of visual rehabilitation achieved with them, but also the efficacy of the refractive correction by vector analysis. It was confirmed that the two groups of eyes (each one implanted with one of the two IOLs evaluated) were comparable in terms of anatomical, visual and refractive parameters, with no significant differences existing between them preoperatively in any clinical parameters assessed.
The analysis of the visual outcomes measured with the two IOLs evaluated revealed that there were no significant differences in UDVA, UNVA or CDVA between IOL groups at 1, 3 and 6 months after surgery. Specifically, mean UDVA values of 0.02 ± 0.03 and 0.00 ± 0.01 logMAR were found at 6 months postoperatively in ATLISA and Synergy groups, respectively. Concerning UNVA, mean values of 0.01 ± 0.04 and 0.00 ± 0.00 logMAR were found in these two groups, respectively. The visual outcomes obtained in the ATLISA group were consistent with those reported by Paredes et al. [30] using an optimized preoperative and intraoperative protocol and with those reported by Kretz et al. [31]. In other studies [32,33,34], somewhat poorer UNVAs have been reported with the AT LISA trifocal toric IOL, which can be attributed to several factors such as the procedure used for measuring the visual acuity (optotype used, illumination conditions, etc.), target refraction or age range of included patients. Concerning the Synergy group, the results were similar to those reported with the non-toric version of the IOL by some previous authors [15,16,17, 19]. However, other authors have reported somewhat worse visual outcomes compared to those obtained in the current study when evaluating the clinical performance of the non-toric version of the Tecnis Synergy™ IOL [18, 23]. As previously mentioned, one of the critical factors for these discrepancies in the visual outcomes among clinical studies, which may be caused by the different methodologies used for characterizing such outcomes. Therefore, comparative studies between different modalities of IOLs using the same methodology as in the study reported here are necessary to extract more consistent conclusions about the clinical performance of presbyopia-correcting IOLs.
Besides the measurement of visual acuities under photopic conditions, a measurement of UNVA under mesopic conditions was performed, obtaining mean values of 0.16 ± 0.03 and 0.15 ± 0.04 logMAR at 6 months after surgery in ATLISA and Synergy groups, respectively. This difference did not reach statistical significance, demonstrating that both IOLs had a comparable performance under mesopic conditions when working at a distance of 40 cm. These results were comparable to those obtained for the non-toric version of the Tecnis Synergy™ IOL in previous studies [15, 16, 18, 20]. Our research group [15] reported a mean monocular mesopic UNVA measured at 40 cm of 0.14 ± 0.03 logMAR in a series of 103 patients with ages ranging from 35 to 75 years old and implanted with the Tecnis Synergy™ IOL.
The measurement of the defocus curve with the two models of IOL evaluated allowed determining the real visual performance achieved by patients implanted with them across all ranges of distances from near to far. In our series, better distance-corrected visual acuities were found in the Synergy group compared to ATLISA group for most of defocus levels, with the largest differences in the visual acuities corresponding to the defocus levels of − 1.50, − 3.00 and − 3.50 D. This suggests a better visual performance of the Tecnis Toric Synergy™ IOL compared to the AT LISA tri, especially for the closest distances. This is consistent with the results of previous studies evaluating the non-toric version of the hybrid IOL combining the EDOF and multifocal diffractive patterns in which a continuous range of vision was detected, with a visual acuity of 0.10 logMAR or better for the defocus levels between 0.50 and − 2.50 D [16, 17, 19, 22,23,24]. In contrast, in our series, distance and near peaks in the defocus curve could be more clearly distinguished in eyes implanted with the trifocal IOL evaluated, with a reduction of visual acuity for the defocus level of − 1.50 D, but always maintaining the mean values > 0.20 logMAR. This is consistent with the defocus curve profile reported in previous studies evaluating the visual performance with the AT LISA tri toric IOL [11, 32].
The predictability of the refractive correction achieved with the two IOLs evaluated in the current study was high, with a residual sphere within ± 0.50 D in all cases, except in one eye of the Synergy group at 6 months, and a total of 100% and 96.2% of eyes in the AT LISA and Synergy groups with a postoperative manifest cylinder of ≤ 0.50 D at the end of the follow-up. This is consistent with the results of previous series evaluating the trifocal toric IOL [30] and the non-toric version of the hybrid IOL investigated [15,16,17,18,19,20,21,22,23,24,25,26]. Our research group found that 99% of eyes had a spherical equivalent within ± 0.50 D in a sample of 206 eyes implanted with the non-toric version of the Tecnis Synergy™ IOL and De Rojas et al. [20] found that 88% of eyes had a SE within ± 0.50 D in a group of 52 patients implanted with the spherical or the toric version of the Tecnis Synergy™ IOL. All these results confirm that the A-constant for the AT LISA tri and Tecnis Toric Synergy™ IOLs has been optimized and appropriately defined.
The efficacy of the astigmatic correction with the two IOLs evaluated in the current study was high, with no significant differences between TIA and SIA and DV values very close to zero. Likewise, mean ME was very close to zero (AT LISA 0.04 ± 0.20 vs. Synergy − 0.04 ± 0.09, p = 0.05) with both IOLs, although differences were in the limit of statistical significance. This means that there was a slight trend to overcorrection with the trifocal IOL and a trend to undercorrection with the hybrid IOL. These differences may be attributed to differences in the algorithm used to estimate the cylinder power to implant, including how the contribution of the posterior corneal surface is considered in the calculation platform used for each IOL. In any case, these trends can vary among studies because of the specific characteristics of the sample analysed (magnitude and type of astigmatisms) or the method used for obtaining the subjective refraction. For example, Piovella et al. [11] obtained, by means of vector analysis, a mean ME of − 0.16 ± 0.48 D when analysing the efficacy of the astigmatic correction achieved with the AT LISA diffractive trifocal toric IOL. In contrast, Mojzis et al. [32] found the opposite trend with the same trifocal toric IOL, reporting a mean ME of 0.06 ± 0.30 D. In any case, in our series, the astigmatic correction achieved was satisfactory with both IOLs, which was also due to a good rotational behaviour of the IOL. Indeed, mean AE was 0.48 ± 3.21º and 0.38 ± 1.67º in the ATLISA and Synergy groups, respectively, which was associated with mean rotation levels of around ≤ 2º. These results are consistent with IOL rotation values reported by previous authors for some multifocal toric IOLs [10, 35, 36].
This study has several limitations that should be acknowledged. First, the number of cases per group is limited, with future studies with larger samples sizes being necessary to confirm our outcomes. Accordingly, it was not possible to perform sub-analysis as a function of axial length, differentiating the short and long eyes. Second, a hydrophilic IOL with a plate haptic design has been compared with an IOL of hydrophobic acrylic material and C-haptics with a different diameter and therefore differences between IOLs could not be only attributable to differences in the optical design of the IOL. However, the discrepancies in terms of material, lens diameter and design of the haptic could mainly have a role in the differential behaviour in terms of rotational stability and PCO incidence, and such differences have not been found in the current study. This suggests that the contribution of these potential differences is minimal and specially with low clinical relevance. Mihaltz et al. [37] conducted a prospective, randomised study to interocularly compare the visual performance after implantation of two different toric IOLs with different haptic designs: AT TORBI 709 M IOL from Carl Zeiss Meditec (plate haptic) and Tecnis toric IOL (C-haptic) from Johnson & Johnson. They found that both toric IOLs successfully reduced ocular astigmatism, with the Tecnis IOL showing better positional stability. In our series, differences did not reach statistical significance although fewer eyes implanted with the AT LISA IOL showed an IOL rotation within ± 5º (76.9%) compared to those implanted with the Synergy IOL (100.0%). Future studies with larger samples sizes must confirm the statistical significance of this trend. Finally, other aspects that could have influenced the outcomes have not been evaluated in the current study, such as capsular back size, time of rotation after surgery or postoperative course of the patients (hours after surgery resting in bed/going home, movements). This should be investigated further in future studies.
Conclusion
In conclusion, the two toric presbyopia-correcting IOLs evaluated provide an efficacious correction of pre-existing corneal astigmatism and visual rehabilitation across a great variety of distances. However, the visual performance achieved with the hybrid IOL combining the EDOF and diffractive multifocal pattern seems to be better for most visual demands, especially for distances closer than 40 cm. Furthermore, the near visual outcome is maintained with both toric presbyopia-correcting IOLs within functional levels under mesopic light conditions. It should be considered that this study compares very different IOLs in terms of material and geometry, not only in terms of optical design, and it is difficult to extract consistent conclusions about the exact cause for some of the differences detected. Future comparative studies with other types of multifocal toric IOLs as well as long-term studies should be conducted.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cervantes-Coste G, Tapia A, Corredor-Ortega C, Osorio M, Valdez R, Massaro M, Velasco-Barona C, Gonzalez-Salinas R. The influence of angle alpha, angle kappa, and optical aberrations on visual outcomes after the implantation of a high-addition trifocal IOL. J Clin Med. 2022;11:896.
Berdahl JP, Hardten DR, Kramer BA, Potvin R. Effect of astigmatism on visual acuity after multifocal versus monofocal intraocular lens implantation. J Cataract Refract Surg. 2018;44:1192–7.
Zheleznyak L, Kim MJ, MacRae S, Yoon G. Impact of corneal aberrations on through-focus image quality of presbyopia-correcting intraocular lenses using an adaptive optics bench system. J Cataract Refract Surg. 2012;38:1724–33.
Gundersen KG, Makari S, Ostenstad S, Potvin R. Retreatments after multifocal intraocular lens implantation: an analysis. Clin Ophthalmol. 2016;10:365–71.
Zvorničanin J, Zvorničanin E. Premium intraocular lenses: the past, present and future. J Curr Ophthalmol. 2018;30:287–96.
Yoo YS, Paik DW, Lim DH, Chung TY. One-year long-term clinical outcomes following diffractive trifocal toric intraocular lens implantation: retrospective observational case series study. Ann Transl Med. 2022;10:1159.
Ackerman M, Lawless M, Levitz L, Bhatt U, Reich JA, Sutton G, Roberts TV, Tenen A, Kaur A, Hodge C. Visual and refractive efficacy of Panoptix toric intraocular lens in a clinical setting. Clin Ophthalmol. 2022;16:4227–37.
Tekce A, Gulmez M. Comparison of visual and refractive outcomes of diffractive bifocal toric and trifocal toric intraocular lenses 12 months after implantation in patients with moderate to high myopia. Int Ophthalmol. 2021;41:3029–40.
Kohnen T, Lwowski C, Hinzelmann L, Ahmad W, Petermann K, Hemkeppler E, Pawlowicz K, Böhm M. Presbyopia correction in astigmatic eyes using a toric trifocal intraocular lens with quadrifocal technology. J Refract Surg. 2020;36:638–44.
Ribeiro FJ, Ferreira TB. Comparison of visual and refractive outcomes of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2020;46:694–9.
Piovella M, Colonval S, Kapp A, Reiter J, Van Cauwenberge F, Alfonso J. Patient outcomes following implantation with a trifocal toric IOL: twelve-month prospective multicentre study. Eye (Lond). 2019;33:144–53.
Rementería-Capelo LA, Contreras I, García-Pérez JL, Blázquez V, Ruiz-Alcocer J. Visual quality and patient satisfaction with a trifocal intraocular lens and its new toric version. J Cataract Refract Surg. 2019;45:1584–90.
Poyales F, Garzon N. Comparison of 3-month visual outcomes of a spherical and a toric trifocal intraocular lens. J Cataract Refract Surg. 2019;45:135–45.
Ferreira TB, Ribeiro FJ. Prospective comparison of clinical performance and subjective outcomes between two diffractive trifocal intraocular lenses in bilateral cataract surgery. J Refract Surg. 2019;35:418–25.
Gabrić N, Gabrić I, Gabrić K, Biščević A, Piñero DP, Bohač M. Clinical outcomes with a new continuous range of vision presbyopia-correcting intraocular lens. J Refract Surg. 2021;37:256–62.
Ribeiro FJ, Ferreira TB, Silva D, Matos AC, Gaspar S. Visual outcomes and patient satisfaction after implantation of a presbyopia-correcting intraocular lens that combines extended depth-of-focus and multifocal profiles. J Cataract Refract Surg. 2021;47:1448–53.
Baur ID, Auffarth GU, Łabuz G, Stengele A, Hallak MK, Khoramnia R. Clinical evaluation of reading performance in refractive lens exchange with a diffractive continuous-range-of-vision intraocular lens. Am J Ophthalmol. 2023;250:25–37.
Chang DH, Hu JG, Lehmann RP, Thompson VM, Tsai LH, Thomas EK. Clinical performance of a hybrid presbyopia-correcting intraocular lens in patients undergoing cataract surgery in a multicenter trial. J Cataract Refract Surg. 2023;49:840–7.
Khoramnia R, Baur ID, Łabuz G, Köppe MK, Hallak MK, Auffarth GU. Functional outcomes after bilateral refractive lens exchange with a continuous-range-of-vision intraocular lens. J Cataract Refract Surg. 2023;49:1011–7.
De Rojas JO, Sandoval HP, Potvin R, Solomon KD. Visual outcomes, quality of vision, patient satisfaction and spectacle independence after bilateral implantation of the Synergy™ intraocular lens. Clin Ophthalmol. 2023;17:2277–85.
Shin DE, Lee H, Kim TI, Koh K. Comparison of visual results and optical quality of two presbyopia-correcting intraocular lenses: TECNIS symfony versus TECNIS Synergy™. Eur J Ophthalmol. 2022;32:3461–9.
Ozturkmen C, Kesim C, Karadeniz PG, Sahin A. Visual acuity, defocus curve and patient satisfaction of a new hybrid EDOF-multifocal diffractive intraocular lens. Eur J Ophthalmol. 2022;32:2988–93.
Shin DE, Lee H, Koh K. Comparative analysis of a presbyopia-correcting intraocular lens that combines extended depth-of-focus and bifocal profiles with a standard monofocal intraocular lens. BMC Ophthalmol. 2022;22:302.
Benyoussef AA, Reboux N, Cochener B. Comparison of bilateral reading performance among two presbyopia-correcting intraocular lenses. J Refract Surg. 2022;38:428–34.
Moshirfar M, Stapley SR, Corbin WM, Bundogji N, Conley M, Darquea IM, Ronquillo YC, Hoopes PC. Comparative visual outcome analysis of a diffractive multifocal intraocular lens and a new diffractive multifocal lens with extended depth of focus. J Clin Med. 2022;11:7374.
Ferreira TB, Ribeiro FJ, Silva D, Matos AC, Gaspar S, Almeida S. Comparison of refractive and visual outcomes of 3 presbyopia-correcting intraocular lenses. J Cataract Refract Surg. 2022;48:280–7.
Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23:65–75.
Alpins N. Astigmatism analysis by the Alpins method. J Cataract Refract Surg. 2001;27:31–49.
Schallhorn SC, Hettinger KA, Pelouskova M, Teenan D, Venter JA, Hannan SJ, Schallhorn JM. Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J Cataract Refract Surg. 2021;47:991–8.
Paredes B, Mora J, Martín MD, Larrosa A, Piñero DP. Short-term clinical results with a trifocal diffractive toric intraocular lens using an optimized preoperative and intraoperative protocol. Eur J Ophthalmol. 2023. https://doi.org/10.1177/11206721231171427. (Online ahead of print).
Kretz FTA, Breyer D, Klabe K, Hagen P, Kaymak H, Koss MJ, Gerl M, Mueller M, Gerl RH, Auffarth GU. Clinical outcomes after implantation of a trifocal toric intraocular lens. J Refract Surg. 2015;31:504–10.
Mojzis P, Majerova K, Plaza-Puche AB, Hrckova L, Alio JL. Visual outcomes of a new toric trifocal diffractive intraocular lens. J Cataract Refract Surg. 2015;41:2695–706.
Bellucci R, Bauer NJC, Daya SM, Visser N, Santin G, Cargnoni M, Nuijts RMMA, Lisa Toric Study Group. Visual acuity and refraction with a diffractive multifocal toric intraocular lens. J Cataract Refract Surg. 2013;39:1507–18.
Visser N, Nuijts RM, de Vries NE, Bauer NJ. Visual outcomes and patient satisfaction after cataract surgery with toric multifocal intraocular lens implantation. J Cataract Refract Surg. 2011;37:2034–42.
Orts P, Piñero DP, Aguilar S, Tañá P. Efficacy of astigmatic correction after femtosecond laser-guided cataract surgery using intraoperative aberrometry in eyes with low-to-moderate levels of corneal astigmatism. Int Ophthalmol. 2020;40:1181–9.
Zeilinger J, Hienert J, Ruiss M, Pilwachs C, Findl O. Rotational stability of a new toric intraocular lens with an advanced optical profile. J Cataract Refract Surg. 2023;49:584–8.
Miháltz K, Lasta M, Burgmüller M, Vécsei-Marlovits PV, Weingessel B. Comparison of two toric IOLs with different haptic design: optical quality after 1 year. J Ophthalmol. 2018;2018:4064369.
Funding
This study was an investigator Initiated Study supported by an unrestricted research grant from Johnson and Johnson Vision, supporting the authors in terms of providing the intraocular lenses freely and covering expenses associated to the performance of the study. The authors have paid the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Ivan Gabrić designed the study. Ivan Gabrić, Krešimir Gabrić, and Nikica Gabrić collected the data. David P. Piñero helped check the data and wrote the first draft of the manuscript. Ivan Gabrić, Krešimir Gabrić, Nikica Gabrić, and David P. Piñero contributed to the interpretation of the results and critical revision of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors Ivan Gabrić, Krešimir Gabrić, Nikica Gabrić and David P. Piñero report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical Approval
The study was approved by the Svjetlost Hospital (Zagreb, Croatia) ethics committee (IIIS-ZG21-TS) and was conducted in compliance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gabrić, K., Gabrić, N., Piñero, D.P. et al. Comparative Analysis of the Clinical Outcomes of Two Toric Presbyopia-Correcting Intraocular Lenses. Ophthalmol Ther 13, 775–790 (2024). https://doi.org/10.1007/s40123-023-00878-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00878-8