Abstract
Introduction
To evaluate the efficacy and safety of Quantum Molecular Resonance (QMR) treatment in patients with severe dry eye disease (DED), as well as its effects on aqueous-deficient (ADDE), evaporative (EDE), and mixed (MDE) dry eye.
Methods
In this prospective, interventional study, 81 patients were randomly allocated to received four treatment sessions of QMR at 1-week intervals (Rexon-Eye®, Resono Ophthalmic, Trieste, Italy) (QRM group) or tear substitute four times daily, containing 0.15% sodium hyaluronate and 3% trehalose (Thealoz Duo®, Thea Pharma, France) (SH-TH group). Outcome measures included ocular surface disease index (OSDI) questionnaire, tear meniscus height (TMH), tear breakup time (TBUT), non-invasive breakup time (NIBUT), corneal fluorescein staining (CFS), lipid layer thickness (LLT), tear film osmolarity (OSM), and meibomian gland dysfunction (MGD) grade, which were assessed at baseline and 1-month and 3-month follow-up.
Results
The QMR group achieved better improvements than the SH-TH group in OSDI and SANDE questionnaires, NIBUT, LLT, and CFS. The mean differences between the groups were as follows: OSDI (− 12.4 ± 0.25 points, P = 0.01), SANDE (10.6 ± 1.7 points, P = 0.01), NIBUT (2 ± 0.25 s, P = 0.01), LLT (18.7 ± 0.7 nm, P = 0.01), and CFS (1.2 ± 0.1 points, P = 0.02). In subgroups analysis, QMR treatment demonstrated a beneficial role to improve DED symptoms and signs in ADDE, EDE, and MDE.
Conclusion
QMR is an effective and well-tolerated treatment that seems to improve DED symptoms and signs in patients with severe DED. However, further studies are needed to confirm this.
Trial Registration
ClinicalTrials.gov identifier NCT06119386.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Current treatments for dry eye disease (DED) require chronic use with possible side effects in some of them. Therefore, there is an unmet need for novel treatments that target the specific mechanism involved in the pathogenesis of DED. |
This study evaluates the efficacy and safety of Quantum Molecular Resonance (QMR) treatment in patients with severe DED. |
What was learned from the study? |
Four sessions of QMR treatment seems to improve symptoms and signs in aqueous-deficient dry eye (ADDE), evaporative dry eye (EDE), and mixed dry eye (MDE), which suggests that this treatment could be an effective and safe option to address DED. However, further studies are needed. |
Introduction
Dry eye disease (DED) is a multifactorial, chronic disease of the ocular surface that affects up to 30% of adults over the age of 50, it is more frequent in women, and its prevalence increases with age [1, 2]. According to the recent definition provided by The Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II, DED is characterized by loss of homeostasis and tear film instability, where hyperosmolarity and inflammation play a key role in its pathogenesis [1, 3]. In addition, corneal and conjunctival epithelial cell damage, apoptosis, and metaplasia as well as inflammation and imbalance of cytokines on the ocular surface are also key factors of DED [1, 3,4,5], which may lead to a wide variety of ocular symptoms, such as foreign body sensation, burning, and visual disturbances, affecting patients’ quality of life [6,7,8].
Dry eye diagnosis could be established by a combination of objective and subjective tests [9]. Regarding DED symptoms assessment, the ocular surface disease index (OSDI) and the symptom assessment in dry eye (SANDE) questionnaires are the most commonly used in clinical studies [9,10,11,12]. Both questionnaires determine the severity of DED symptoms with a score from 0 to 100. However, the OSDI questionnaire requires the patient to read, understand, and answer 12 questions, whereas the SANDE questionnaire only includes two questions on a visual analog scale and provides clinicians with a quick and reliable assessment of DED symptoms [13, 14]. Regarding DED signs assessment, classic methods, such as tear film breakup time (TBUT), Schirmer test (ST), and corneal fluorescein staining (CFS), have been widely used, but these depend on the skill of the examiner and influence tear film stability [9, 15, 16]. Therefore, objective, non-invasive tests, such as non-invasive tear film breakup time (NIBUT), tear meniscus height (TMH), and lipid layer thickness (LLT), are preferred in the assessment of patients with DED [9, 17]. In addition, new devices that automatically perform objective, non-invasive tests have been developed, which reduce observer bias in some tests, such as meibography, and do not alter tear film stability, resulting in a potential screening tool for DED [18, 19].
Although DED may be classified in aqueous-deficient dry eye (ADDE), evaporative dry eye (EDE), and mixed dry eye (MDE), the evidence suggests that all forms of DED have an evaporative component since ocular surface hyperosmolarity only can arise in response to evaporation [3]. Meibomian gland dysfunction (MGD) is the most common form of EDE and its first line of treatment usually includes warm compresses, eyelid hygiene, oral antibiotics, and preservative-free tear substitutes containing lipid supplements [20,21,22]. However, these treatments require chronic instillations with possible side effects related to some of them [23, 24]. Therefore, novel treatments that target the specific mechanism involved in the pathogenesis of DED have emerged, such as microblepharoexfoliation (MBE) [25], vectoral thermal pulsation (VTP) [26], intense pulse light (IPL) [27], and low-level light therapy (LLLT) [28]. Recently, Quantum Molecular Resonance (QMR) has been proposed as a novel therapy for DED, demonstrating promising results [29,30,31,32,33]. QMR involves passing an electric current at a low intensity and high frequency (4–64 MHz) through contact electrodes [30, 32]. Previous studies have demonstrated the wound healing properties and anti-inflammatory effects of QMR on the ocular surface [29,30,31,32,33]. However, most of these studies evaluated only patients with mild to moderate DED with a short-term follow-up period [29,30,31,32].
Therefore, this study aims to evaluate the efficacy and safety of QMR treatment in patients with severe DED after 3-month follow-up, as well as to analyze its effects on ADDE, EDE, and MDE.
Methods
Study Design
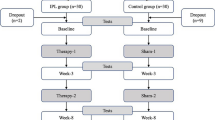
This prospective, randomized interventional study was carried out at the Tedesco Eye Center (Girifalco, CZ, Italy) between November 2022 and February 2023. This study fulfilled all the requirements of the Declaration of Helsinki and was approved by the center’s internal review board (Approval Nr. DB13385-2568932022, ClinicalTrials.gov identifier NCT06119386). Before initiating the study, informed and written consent was obtained from each patient. Patient consent was received for Fig. 2.
Patients
Eligible patients were adults > 18 years old with a self-reported history of DED in both eyes for ≥ 6 months who met the following inclusion criteria in ≥ 1 eye at screening and randomization: OSDI score ≥ 33 points and TBUT < 10 s [9]. In addition, patients were divided into ADDE, EDE, and MDE subgroups according to the following criteria: TMH < 0.20 mm and ST I < 10 mm/5 min for ADDE; meibomian gland expressibility score between 1 and 2 points, meibum quality between 4 and 13 points, and LLT < 40 nm for EDE; and both sets of criteria were considered for MDE [9, 34].
Patients were excluded from participation if they met any of the following criteria: skin pathologies that prevent QRM treatment; all corneal disorders that affect diagnostic test, such as active corneal infection and corneal dystrophies; active ocular allergies; intraocular surgery or laser ocular surgery within the previous 6 months; use of topical antibiotics and anti-inflammatory treatments, including steroids and non-steroidal anti-inflammatory drugs; contact lens wearers; pregnant or lactating women; and patients who did not understand or comprehend the informed consent. A washout period of 1 week was considered before QMR treatment.
Treatments
Eligible patients were randomized in a 1:1 ratio to received either four treatment sessions of QMR treatment at 1-week intervals (Rexon-Eye®, Resono Ophthalmic, Trieste, Italy) (QMR group) (Figs. 1 and 2) or four times daily tear substitute containing 0.15% sodium hyaluronate and 3% trehalose (Thealoz Duo®, Thea Pharma, France) (SH-TH group). QMR treatment was performed using specific contact electrodes built into a mask, which is worn by the patient over closed eyes. In addition, a disposable waterproof facial strip, worn between the mask and the eyelid surface, was used to uniformly spread the electric current over the whole ocular surface, and also ensuring personal hygiene. The device provides an interface showing the applied power with a custom unit scale, marked from 0 to 10, and the duration of the treatment. The duration of each treatment session was 20 min, using an intensity of 5 units, which correspond to an average power of 12 W, with 60 V voltage and 200 mA current between the goggle electrode and the neutral plate electrode.
Clinical Endpoints
Clinical endpoints were assessed at baseline (1 day) and two follow-up visits: month 1 (4 ± 0.5 weeks) and month 3 (12 ± 1 weeks). All clinical endpoints were performed in the sequence proposed by Ballesteros et al. [19] to best preserve the integrity of the tear film to avoid affecting test results. In addition, they were obtained in standard environmental conditions in the same room by a trained optometrist.
Dry Eye Symptoms
The OSDI and SANDE questionnaires were used to evaluate DED symptoms severity, ranging from 0 (no ocular surface disease) to 100 (severe ocular surface disease) points [10, 12]. Both questionnaires were completed in consultation at all follow-up visits.
Tear Film Stability and Volume
Tear film stability was automatically evaluated with NIBUT (expressed in seconds, s) by projecting the Keratograph 5M® (Oculus Optikgeräte GmbH, Wetzlar, Germany) Placido rings onto the corneal surface, recording the time between the last blink and the initial distortion of the ring pattern. Fluorescein TBUT (expressed in seconds, s) was also evaluated. Patients were instructed to blink and then stare without blinking, recording the time between the blink and the initial appearance of a dark spot. Three consecutive measurements of NIBUT and TBUT were averaged for statistical analysis. In addition, the Lipiview II® ocular surface interferometer (Johnson & Johnson, NJ, USA) and the TearLab® osmolarity system (TearLab corporation, CA, USA) were used to assess LLT (expressed in nanometers, nm) and tear film osmolarity (OSM, expressed in milliosmole, mOsm), respectively. Regarding tear volume, TMH was evaluated using the tear film scanning function of the Keratograph 5M® device, which allows for capturing images of the lower tear film meniscus determining its height.
Ocular Surface Staining
CFS was subjectively and invasively evaluated with the modified Oxford scale, ranging from grade 0 (no epithelial staining) to grade 5 (severe epithelial staining) [35]. Prior to assessing CFS, a single drop of unit dose saline was instilled onto a fluorescein impregnated strip. The lower right lid was then pulled down and the strip was tapped onto the lower tarsal conjunctiva. The same procedure was performed on the left eye. A cobalt-blue filter with yellow Kodak Wratten 12 barrier filter was used for better detection of CFS.
Meibomian Gland Analysis
Meibography was performed on the upper and lower eyelids to evaluate MGD. The loss area of meibomian glands was automatically assessed with the Keratograph 5M® device, which incorporates the JENVIS Grading Scale software, classifying MGD in four grades: grade 0 (no gland loss), grade 1 (loss in an area smaller than 1/3), grade 2 (loss in an area between 1/3 and 2/3), and grade 3 (loss in an area greater than 2/3).
Safety Assessment
Safety assessment included adverse events (AEs), best-corrected visual acuity (BCVA), intraocular pressure (IOP), slit-lamp biomicroscopy, and dilated funduscopy.
Statistical Analysis
Statistical analyses were performed with SPSS statistics software, version 28.0 (IBM Corporation, NY, USA). A total sample size of 62 patients was estimated using the GRANMO calculator, version 7.12 (Municipal Institute of Medical Research, Barcelona, Spain). Estimation was based on a statistically significant paired difference at 95% confidence and with 80% power of 6.45 ± 0.77 s in NIBUT based on previous studies [31,32,33].
Continuous variables were displayed as the mean ± standard deviation (SD), while ordinal categorical variables were expressed as frequencies (n) and percentages (%). Before the analyses, one eye was randomly selected. The randomization scheme was generated using an online randomizer program (https://www.randomization.com). After testing for normality with Kolmogorov–Smirnov test, we performed the paired Student’s t test (parametric) or Wilcoxon’s signed-rank test (nonparametric) to compare intra-group clinical outcomes. Within each group, the increment (Δ) was calculated. It was defined as the change from the last visit (LV) to baseline (B): Δ = LV − B. Inter-group clinical outcomes were analyzed with the unpaired Student’s t test (parametric) or Mann–Whitney’s U test (nonparametric). Between each group, the differences were calculated as ΔQMR group − ΔSH-TH group. Categorical variables were compared using the χ2 test. A P value of less than 0.05 is considered to be statistically significant.
Results
Baseline characteristics of the study populations are shown in Table 1. Eighty-one eyes of 81 patients, 23 (28.3%) men and 58 (71.6%) women with a mean age of 60.7 ± 7.9 years, were enrolled in the study. No significant differences in demographic characteristics or parameters related to DED were detected between both groups at baseline. In addition, all patients completed the study.
Efficacy Endpoints
The efficacy of QMR treatment on DED symptoms and ocular surface parameters during follow-up visits in both groups is shown in Table 2.
Dry Eye Symptoms
After 3 months of follow-up, QMR treatment achieved significant ΔOSDI and ΔSANDE questionnaire reductions of − 23.3 ± 2.2 (P = 0.02) and − 22.7 ± 7.1 points (P = 0.01), respectively. However, only SH-TH treatment led to significant improvement in ΔSANDE questionnaire with a reduction of − 12.1 ± 2.7 points (P = 0.04).
Comparing both groups, the results were in favor QMR treatment with a difference in OSDI and SANDE questionnaire scores of − 12.4 ± 0.25 (P = 0.01) and − 10.6 ± 1.7 points (P = 0.01), respectively.
Tear Film, Ocular Surface Staining, and Meibomian Gland Analysis
After 3 months of follow-up, QMR treatment achieved significant improvements in ΔTBUT and ΔNIBUT of 1.7 ± 0.1 (P = 0.01) and 4.8 ± 0.6 s (P < 0.001), respectively. In addition, ΔTMH, ΔLLT, ΔCFS, and ΔOSM also showed significant improvements of 0.05 ± 0.04 mm (P < 0.001), 10.8 ± 2.4 nm (P = 0.002), − 1.6 ± 0.2 points (P = 0.01), and − 17.4 ± 2.5 mOsm/L (P < 0.001), respectively. Regarding SH-TH treatment, ΔTBUT and ΔNIBUT also achieved significant improvements of 3 ± 0.1 (P = 0.01) and 2.8 ± 0.1 s (P = 0.03) after 3 months of follow-up, respectively. Similar results were reported in ΔCFS and ΔOSS with values of − 0.4 ± 0.05 points and − 10.2 ± 2.3 mOsm/L, respectively. However, ΔLLT showed a significant worsening of − 7.9 ± 3.8 nm (P = 0.002).
Comparing both groups, the differences in NIBUT, LLT, and CFS were in favor of QMR treatment with values of 2 ± 0.25 s (P = 0.01), 18.7 ± 0.7 nm (P = 0.01), and − 1.2 ± 0.1 points (P = 0.02), respectively. The remaining outcomes were not in favor of any treatment group. In addition, no significant improvement in MGD grade was observed within and between groups.
Subgroups Analysis
The effects of QMR treatment on symptoms and ocular surface parameters of patients with ADDE, EDE, and MDE are shown in Tables 3, 4, and 5, respectively. Regarding the ADDE subgroup, significant improvement in ΔOSDI questionnaire, ΔNIBUT, ΔTMH, ΔLLT, and ΔOSM were reported, with values of − 31.3 ± 4.1 points (P < 0.001), 6.4 ± 0.9 s (P < 0.001), 0.09 ± 0.04 mm (P < 0.001), 10.6 ± 2.3 nm (P = 0.02), and − 19.4 ± 2.5 mOsm/L (P < 0.001) after 3 months of follow-up, respectively. Similar results were achieved in the EDE subgroup with significant ΔOSDI, ΔNIBUT, ΔLLT, and ΔOSM improvements of − 17.4 ± 0.75 points (P < 0.001), 4.2 ± 0.4 s (P = 0.01), 14 ± 4.6 nm (P < 0.001), and − 17.6 ± 3.2 mOsm/L (P < 0.001), respectively. However, the MDE subgroup only showed significant improvements in ΔOSDI, ΔNIBUT, and ΔOSM with values of − 35.4 ± 6.4 points (P < 0.001), 6.1 ± 1.2 s (P < 0.001), and − 17.5 ± 1.4 mOsm/L (P < 0.001), respectively.
Safety Endpoints
No significant changes of BCVA, IOP, slit-lamp biomicroscopy, and dilated funduscopy were observed after QMR treatment (data not shown). In addition, no AEs were documented during treatment sessions with QMR, and throughout the follow-up period.
Discussion
Tear film hyperosmolarity is considered the trigger for the ocular surface inflammatory mechanism resulting in DED symptoms and signs [3, 36]. Therefore, new treatments that target the specific mechanism involved in the pathogenesis of DED and improve the tear film stability and restore the homeostasis of the ocular surface are under research [37, 38]. The aim of this study is to evaluate the efficacy and safety of QMR treatment in patients with severe DED, as well as its effects on ADDE, EDE, and MDE.
Quantum Molecular Resonance Efficacy
In this study, QMR treatment achieved significant improvements in OSDI and SANDE questionnaires, NIBUT, LLT, and CFS compared to SH-TH treatment after 3 months of follow-up. In addition, QMR treatment was beneficial for ADDE, EDE, and MDE, with similar result between the subgroups.
Several studies have evaluated the effects of QMR treatment on DED symptoms and signs [29,30,31,32,33]. Pedrotti et al. [29] reported that 12 sessions of QMR treatment significantly improved OSDI questionnaire, TBUT, CFS, and ST after 1 year of follow-up in patients with ADDE. Although ST was performed during the screening stage in our study, it was not used as a clinical endpoint because of its invasiveness and association with CFS, which could affect our results [39, 40]. For this reason, tear volume was evaluated by TMH, showing a significant improvement after 3 months of follow-up. Ferrari et al. [30] reported similar results in OSDI questionnaire, NIBUT, and CFS after four treatment sessions of QMR. In addition, this study also reported significant improvements in meibum quality and the number of expressible meibomian glands after 1 month of follow-up. Although meibomian gland secretion was not assessed in our study, a significant LLT improvement was reported in patients with EDE, which is consistent with the results reported by Ferrari et al. [30] In addition, Trivli et al. [32] reported significant improvements in DED symptoms and signs after 1 month of follow-up in patients with MDE, which is also in harmony with the results reported in our study.
Although the mechanism underlying QMR treatment remains unclear, it is hypothesized that electrical stimulation of the ethmoidal nerve may modulate the activity of lacrimal and meibomian gland, thereby improving tear film stability [20, 41]. In addition, similarly to Corneal Cross Linking [4], QMR treatment also seems to produce anti-inflammatory effects by reducing tissue infiltration of leukocytes and modulating metalloproteinase (MMP) expression [42]. It is well known that MMPs may be an indicator for tear film OSM, playing a key role in the initiation and maintenance of ocular surface damage [3, 43]. In particular, MMP-9 contributes to corneal epithelial barrier instability, with increased corneal epithelial desquamation and corneal surface irregularity [44, 45]. Recently, Trivli et al. [32] demonstrated a significant reduction of MMP-9 expression on the ocular surface in patients with DED after treatment with QMR, which was associated with a significant reduction in CFS. Although our study did not analyze MMP levels, tear film OSM was evaluated, showing a significant reduction after 3 months of follow-up. Overall, these hypotheses support the results reported in this study. However, further studies are needed to confirm this.
Quantum Molecular Resonance Safety
In this study, the absence of reported AEs after QMR treatment aligns with the findings from other studies conducted by Ferrari et al. [30], Trivli et al. [32], Kavroulaki et al. [31], and Foo et al. [33]. This consistent pattern of safety across multiple studies underscores the robustness of QMR as a safe therapeutic option for DED. In addition, the safety profile of QMR treatment becomes even more significant when compared to alternative novel therapies, such as IPL, whose main AEs may include blistering, cheek swelling, and loss of eyelashes [46].
Strengths and Limitations
To the best of our knowledge this is the first randomized, interventional study that analyzes the efficacy and safety of QMR treatment in patients with severe DED, as well as its effects on different types of dry eye. However, there are some limitations that need to be addressed. First, masking was not possible because both groups received quite different treatments. Second, since the QMR group did not receive treatment with SH-TH tear substitutes, it may be difficult to specifically determine the beneficial effects in the QMR group over the SH-TH group. Similarly, multiple previous treatments and a 1-week washout might have influenced the outcomes. In addition, it would have been interesting to have a third group receiving both treatments, which would allow a more solid comparison between the QMR and SH-TH treatments, as well as to evaluate whether the combination of both treatments has a synergistic effect. Changes in the expressibility and quality of meibum secretions after treatments could be evaluated as well in future studies. Therefore, there is a need for larger, well-designed, strictly blinded randomized controlled trials evaluating the long-term efficacy and safety of QMR treatment in patients with DED, as well as its effects on different types of dry eye, identifying the minimum number of effective sessions. In addition, it would also be interesting to compare the effects of QMR treatment with other novel therapies, such as MBE, VTP, LLLT, and IPL, as well as their combinations. This would be of special interest in patients with Sjögren’s syndrome and MGD, which are the main cause of ADDE and EDE, respectively.
Conclusions
This study seems to demonstrated that four treatment sessions of QMR treatments improves DED symptoms and signs, with no adverse effects reported. In addition, this treatment also appears to be beneficial for ADDE, EDE, and MDE. These findings suggest that QMR treatment could be an effective and safe option to address DED and its different subtypes. However, further research and well-designed clinical trials are needed to confirm these results and to better understand the underlying mechanisms of QMR treatment in the context of DED.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83. https://doi.org/10.1016/J.JTOS.2017.05.008.
Su P, Chen T, Xie J, et al. Corneal nerve tortuosity grading via ordered weighted averaging-based feature extraction. Med Phys. 2020;47(10):4983–96. https://doi.org/10.1002/mp.14431.
Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. https://doi.org/10.1016/J.JTOS.2017.05.011.
Acar Eser N, Dikmetas O, Kocabeyoglu S, et al. Evaluation of keratoconus disease with tear cytokine and chemokine levels before and after corneal cross-linking treatment. Ocul Immunol Inflamm. 2023;6:1–7. https://doi.org/10.1080/09273948.2023.2165950.
Azzolini C, Pagani IS, Pirrone C, et al. Expression of VEGF-A, Otx homeobox and p53 family genes in proliferative vitreoretinopathy. Mediators Inflamm. 2013;2013:857380. https://doi.org/10.1155/2013/857380.
Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–7. https://doi.org/10.1007/S40135-013-0009-1.
Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21(4):310–6. https://doi.org/10.1097/ICU.0B013E32833A8C15.
Barabino S, Labetoulle M, Rolando M, Messmer EM. Understanding symptoms and quality of life in patients with dry eye syndrome. Ocul Surf. 2016;14(3):365–76. https://doi.org/10.1016/J.JTOS.2016.04.005.
Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–74. https://doi.org/10.1016/j.jtos.2017.05.001.
Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the Ocular Surface Disease Index (OSDI). Invest Ophthalmol Vis Sci. 2011;52(12):8630–5. https://doi.org/10.1167/IOVS.11-8027.
Özcura F, Aydin S, Helvaci MR. Ocular surface disease index for the diagnosis of dry eye syndrome. Ocul Immunol Inflamm. 2007;15(5):389–93. https://doi.org/10.1080/09273940701486803.
Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–7. https://doi.org/10.1016/S1542-0124(12)70053-8.
Amparo F, Schaumberg DA, Dana R. Comparison of two questionnaires for dry eye symptom assessment: the ocular surface disease index and the symptom assessment in dry eye. Ophthalmology. 2015;122(7):1498–503. https://doi.org/10.1016/J.OPHTHA.2015.02.037.
Rodriguez-Garcia A, Ruiz-Lozano RE, Bustamante-Arias A, Pantaleon-Garcia J, Hernandez-Quintela E, Navas A. Reply to Letter to the Editor: Correlation and level of agreement between the ocular surface disease index and the symptom assessment in dry eye questionnaires: a survey-based study. Curr Eye Res. 2023;48(11):1086–8. https://doi.org/10.1080/02713683.2023.2255397.
Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19(5):644–9. https://doi.org/10.1097/00003226-200009000-00009.
Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–30. https://doi.org/10.1167/IOVS.10-5390.
Ozulken K, Aksoy Aydemir G, Tekin K, Mumcuoğlu T. Correlation of non-invasive tear break-up time with tear osmolarity and other invasive tear function tests. Semin Ophthalmol. 2020;35(1):78–85. https://doi.org/10.1080/08820538.2020.1730916.
Sánchez-González MC, Capote-Puente R, García-Romera MC, et al. Dry eye disease and tear film assessment through a novel non-invasive ocular surface analyzer: the OSA protocol. Front Med (Lausanne). 2022. https://doi.org/10.3389/FMED.2022.938484.
Ballesteros-Sánchez A, Gargallo-Martínez B, Gutiérrez-Ortega R, Sánchez-González JM. Intra-observer repeatability assessment of the S390L Firefly WDR slit lamp in patients with dry eye disease: objective, automated and non-invasive measures. Eye Contact Lens. 2023;49(7):283–91.
Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–78. https://doi.org/10.1167/IOVS.10-6997C.
Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–64. https://doi.org/10.1167/IOVS.10-6997G.
Bonzano C, Borroni D, Lancia A, Bonzano E. Doxycycline: from ocular rosacea to COVID-19 anosmia. New insight into the coronavirus outbreak. Front Med (Lausanne). 2020. https://doi.org/10.3389/FMED.2020.00200.
Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. https://doi.org/10.1016/J.JTOS.2017.05.006.
O’Neil EC, Henderson M, Massaro-Giordano M, Bunya VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30(3):166–78. https://doi.org/10.1097/ICU.0000000000000569.
Ballesteros-Sánchez A, Gargallo-Martínez B, Gutiérrez-Ortega R, Sánchez-González JM. Eyelid exfoliation treatment efficacy and safety in dry eye disease, blepharitis, and contact lens discomfort patients: a systematic review. Asia-Pac J Ophthalmol. 2023;12(3):315–25. https://doi.org/10.1097/APO.0000000000000607.
Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016;10:1385–96. https://doi.org/10.2147/OPTH.S109663.
Karaca EE, Evren Kemer Ö, Özek D. Intense regulated pulse light for the meibomian gland dysfunction. Eur J Ophthalmol. 2020;30(2):289–92. https://doi.org/10.1177/1120672118817687.
Park Y, Kim H, Kim S, Cho KJ. Effect of low-level light therapy in patients with dry eye: a prospective, randomized, observer-masked trial. Sci Rep. 2022;12(1):3575. https://doi.org/10.1038/s41598-022-07427-6.
Pedrotti E, Bosello F, Fasolo A, et al. Transcutaneous periorbital electrical stimulation in the treatment of dry eye. Br J Ophthalmol. 2017;101(6):814–9. https://doi.org/10.1136/BJOPHTHALMOL-2016-308678.
Ferrari G, Colucci A, Barbariga M, Ruggeri A, Rama P. High frequency electrotherapy for the treatment of meibomian gland dysfunction. Cornea. 2019;38(11):1424–9. https://doi.org/10.1097/ICO.0000000000002063.
Kavroulaki D, Konstantinidou E, Tsiogka A, Rallis K, Mavrikakis E. Quantum molecular resonance electrical stimulation as a beneficial and safe treatment for multifactorial dry eye disease. Cureus. 2023. https://doi.org/10.7759/CUREUS.39695.
Trivli A, Karmiris E, Dalianis G, Ruggeri A, Terzidou C. Evaluating the efficacy of Quantum Molecular Resonance (QMR) electrotherapy in mixed-type dry eye patients. J Optom. 2023;16(2):128–34. https://doi.org/10.1016/J.OPTOM.2022.06.003.
Foo VHX, Liu YC, Tho B, Tong L. Quantum molecular resonance electrotherapy (Rexon-Eye) for recalcitrant dry eye in an Asian population. Front Med (Lausanne). 2023. https://doi.org/10.3389/FMED.2023.1209886.
Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–49. https://doi.org/10.1167/IOVS.10-6997F.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–50. https://doi.org/10.1097/00003226-200310000-00008.
Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013;11(4):246–58. https://doi.org/10.1016/J.JTOS.2013.07.003.
Barabino S, Benitez-Del-Castillo JM, Fuchsluger T, et al. Dry eye disease treatment: the role of tear substitutes, their future, and an updated classification. Eur Rev Med Pharmacol Sci. 2020;24(17):8642–52. https://doi.org/10.26355/EURREV_202009_22801.
Zeng J, Lin C, Zhang S, et al. Isolation and identification of a novel anti-dry eye peptide from tilapia skin peptides based on in silico, in vitro, and in vivo approaches. Int J Mol Sci. 2023;24(16):12772. https://doi.org/10.3390/ijms241612772.
Li N, Deng XG, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5(4):478–81. https://doi.org/10.3980/J.ISSN.2222-3959.2012.04.14.
Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106(4):803–10. https://doi.org/10.1016/S0161-6420(99)90170-7.
Brinton M, Kossler AL, Patel ZM, et al. Enhanced tearing by electrical stimulation of the anterior ethmoid nerve. Invest Ophthalmol Vis Sci. 2017;58(4):2341–8. https://doi.org/10.1167/IOVS.16-21362.
Fraccalvieri M, Salomone M, Di Santo C, Ruka E, Morozzo U, Bruschi S. Quantum molecular resonance technology in hard-to-heal extremity wounds: histological and clinical results. Int Wound J. 2017;14(6):1313–22. https://doi.org/10.1111/IWJ.12805.
Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366–403. https://doi.org/10.1016/J.JTOS.2017.03.006.
Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–8. https://doi.org/10.1016/J.OPHTHA.2016.07.028.
Lanza NL, Valenzuela F, Perez VL, Galor A. The matrix metalloproteinase 9 point-of-care test in dry eye. Ocul Surf. 2016;14(2):189–95. https://doi.org/10.1016/J.JTOS.2015.10.004.
Toyos R, Toyos M, Willcox J, Mulliniks H, Hoover J. Evaluation of the safety and efficacy of intense pulsed light treatment with meibomian gland expression of the upper eyelids for dry eye disease. Photobiomodul Photomed Laser Surg. 2019;37(9):527–31. https://doi.org/10.1089/photob.2018.4599.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Drafting the paper: Antonio Ballesteros-Sánchez and José-María Sánchez-González; Data collection: Giovanni Roberto Tedesco, Carlos Rocha-De-Lossada, Fedele Russo and Antonio Spinelli; Statistical analysis: Irene Ingrande; Data collection, concept and design, supervision and drafting the paper: Davide Borroni.
Corresponding author
Ethics declarations
Conflict of Interest
Antonio Ballesteros-Sánchez, José-María Sánchez-González, Giovanni Roberto Tedesco, Carlos Rocha-De-Lossada, Fedele Russo, Antonio Spinelli, Irene Ingrande, and Davide Borroni have nothing to disclose.
Ethical Approval
This study fulfilled all the requirements of the Declaration of Helsinki and was approved by the internal review board for Tedesco Eye Center (Approval Nr. DB13385-2568932022). Before initiating the study, informed and written consent was obtained from each patient. Patient consent was received for Fig. 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ballesteros-Sánchez, A., Sánchez-González, JM., Tedesco, G.R. et al. Efficacy and Safety of Quantum Molecular Resonance Electrotherapy in Patients with Aqueous-Deficient, Evaporative and Mixed-Type Dry Eye: A Randomized Interventional Study. Ophthalmol Ther 13, 495–507 (2024). https://doi.org/10.1007/s40123-023-00868-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00868-w