Abstract
Introduction
Cenegermin is approved for treatment of neurotrophic keratopathy (NK) and has been studied in patients with stage 2 or 3 NK. This study evaluated the efficacy and safety of cenegermin in adults with stage 1 NK.
Methods
This was a phase IV, multicenter, prospective, open-label, uncontrolled trial. Adults with stage 1 NK (Mackie criteria) and decreased corneal sensitivity (≤ 4 cm) received 1 drop of cenegermin 20 mcg/ml in the affected eye(s) 6 times/day for 8 weeks with a 24-week follow-up.
Results
Of 37 patients, corneal epithelial healing was observed in 84.8% (95% confidence interval [CI] 68.1–94.9%; P < 0.001) at week 8; 95.2% (95% CI 76.2–99.9%; P < 0.001) of those patients remained healed at the end of the 24-week follow-up (week 32). At week 8, 91.2% (95% CI 76.3–98.1%; P < 0.001) of patients experienced improved corneal sensitivity; this improvement was observed in 82.1% (95% CI 63.1–93.9%; P < 0.001) of patients at week 32. Mean best-corrected distance visual acuity change from baseline at week 8 was − 0.10 logMAR (standard deviation [SD], 0.15; 95% CI − 0.16 to − 0.05; P < 0.001) and at week 32 was − 0.05 logMAR (SD, 0.16; 95% CI − 0.11 to 0.01; P = 0.122). At weeks 8 and 32, 15.2% (95% CI 5.1–31.9%; P < 0.001) and 10.7% (95% CI 2.3–28.2%; P < 0.001) of patients, respectively, had a 15-letter gain from baseline. At least one adverse event (AE) was reported by 73.0% and 45.7% of patients during the treatment and follow-up periods, respectively. The most common treatment-related, treatment-emergent AEs were eye pain (37.8%), blurred vision (10.8%), and eyelid pain (8.1%); these were mostly mild or moderate and were only reported during the treatment period.
Conclusions
These results support the potential use of cenegermin for treating patients with stage 1 NK, and future confirmatory studies would be beneficial to elaborate on these findings.
Trial Registration
DEFENDO; NCT04485546.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Cenegermin is approved for the treatment of neurotrophic keratopathy (NK) based on studies in patients with stage 2 or 3 NK; few data are available on cenegermin use in patients with stage 1 NK. |
This phase IV open-label, uncontrolled clinical trial was designed to evaluate the efficacy and safety of cenegermin in patients with stage 1 NK. |
What was learned from the study? |
In the study, 84.8% (28/33) of patients experienced corneal epithelial healing, with 95.2% of those patients remaining healed at the 24-week follow-up; improvements in corneal sensitivity, visual acuity, and overall dry eye symptoms were also observed. |
Cenegermin was generally well tolerated in this study. Overall, 73.0% (27/37) and 45.7% (16/35) of patients reported ≥ 1 adverse event (AE) during the treatment and follow-up periods, respectively. The three most common treatment-related, treatment-emergent AEs were eye pain (37.8%; 14/37), blurred vision (10.8%; 4/37), and eyelid pain (8.1%; 3/37); these were mostly mild or moderate and were only reported during the treatment period. |
These results support the potential use of cenegermin for treating patients with stage 1 NK. Since this study was open-label and uncontrolled, future confirmatory studies would be beneficial to elaborate on these findings. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24632028
Introduction
Corneal nerves arise from trigeminal nerve bundles, and in response to ocular surface damage, mediate reflexes such as blinking and tear production [1,2,3]. One of their key roles is the release of neuromediators (e.g., substance P), which provide trophic support for corneal tissues, stimulate wound healing, and maintain anatomic integrity [4, 5]. The release of these neuromediators stimulates epithelial cells and keratocytes to release neurotrophins, such as nerve growth factor (NGF), which supports differentiation and maturation of neurons [5]. This dynamic interplay between neurotrophins, corneal nerves, and the corneal epithelium maintains corneal nerve integrity and corneal homeostasis [1, 4,5,6].
Damage to corneal nerves disrupts homeostasis, leading to the degenerative condition neurotrophic keratopathy (NK, or neurotrophic keratitis) [7, 8]. In NK, corneal trigeminal innervation impairment may lead to reduced corneal sensitivity, corneal perforation, and eventual vision loss [1, 7, 9]. Neurotrophic keratopathy is classified into three stages based on Mackie criteria: epithelial alterations and ocular surface irregularities (stage 1), persistent epithelial defects (stage 2), and corneal ulceration (stage 3) [9,10,11]. Key etiologies associated with NK include conditions that damage the ophthalmic branch of the trigeminal nerve, such as ocular herpes infections or zoster keratitis, chronic inflammation and dry eye disease, preserved topical ophthalmic medications, systemic disease (e.g., diabetes), and ocular surgery (e.g., refractive) [7, 12,13,14,15].
Stage 1 NK treatment is intended to improve the quality of corneal epithelium, avoid epithelial breakdown, and preserve corneal transparency [16]. Stage 2 NK treatment aims to promote healing of the epithelial defect and prevent corneal ulcer development [17]. Stage 3 NK treatment aims to reduce corneal thinning, further corneal damage, and perforation [7]. Medical management recommendations include cenegermin ophthalmic solution, preservative-free tear substitution, and autologous serum eyedrops [15]. Nonsurgical interventions include corneal therapeutic contact lenses and punctal occlusion to prevent eyelid friction and increase the retention of natural tears [1, 7]. Surgical interventions, including tarsorrhaphy, amniotic membrane transplant, corneal transplants, and neurotization, are reserved for severe refractory cases in late NK stages but may compromise the patient's appearance [1, 5, 15, 16, 18]. Moreover, in advanced NK, disease progression becomes increasingly irreversible. Even with medical intervention, suboptimal NK management can potentially lead to vision impairment and, in more severe cases, functional or anatomical loss of an eye [1, 16].
Cenegermin-bkbj 0.002% (20 mcg/ml; recombinant human NGF; Oxervate®) received breakthrough therapy designation, was granted fast track and priority review by the US Food and Drug Administration (FDA), and in 2018 became the first topical biologic approved for NK treatment [9, 19, 20]. Currently, cenegermin is the only US FDA-approved treatment for NK and is approved for all stages of NK. Evidence from two randomized controlled trials (NGF0212 [REPARO] and NGF0214) demonstrated efficacy and safety of cenegermin 20 mcg/ml in patients with stage 2 or 3 NK [21, 22]. At week 8 in REPARO and NGF0214, respectively, 72.0% (36/50) and 65.2% (15/23) of cenegermin-treated patients versus 33.3% (17/51) and 16.7% (4/24) of vehicle-treated patients had complete corneal healing (REPARO: treatment difference, 38.7%; 97.06% confidence interval [CI], 18.7–58.6%; P < 0.001; NGF0214: treatment difference, 48.6%; 95% CI 24.0–73.1%; P < 0.001) [19, 21, 22]. Reports of cenegermin’s impact in patients with stage 1 NK are currently limited to two retrospective studies that evaluated corneal integrity and visual acuity [23, 24].
The aim of this phase IV, multicenter, prospective, open-label trial was to evaluate the efficacy and safety of cenegermin in patients with stage 1 NK.
Methods
Study Design
DEFENDO was a phase IV, multicenter, prospective, single-arm, open-label trial (ClinicalTrials.gov, NCT04485546) conducted in five US study centers. Institutional review board approval was obtained for the study protocol, protocol amendments, informed consent forms, and any other relevant study-related documents at each center (Sterling IRB, 8203-DJSchanzlin, 8203-EJHolland, 8203-GJBerdy, 8203-MMassaroGiordano; and WCG-WIRB, 20202809). The study complied with the Declaration of Helsinki, relevant parts of the Code of Federal Regulations Title 21, and good clinical practice and good laboratory practice guidelines. Written informed consent was obtained from all patients before study initiation.
Patients
Adults aged ≥ 18 years with stage 1 NK were eligible for study inclusion. To be eligible for inclusion in this study, patients were required to have stage 1 NK per investigator assessment, defined by Mackie criteria and characterized by superficial punctate keratopathy (i.e., grade 3 fluorescein corneal staining per the National Eye Institute [NEI]) and evidence of decreased corneal sensitivity (≤ 4 cm using the Cochet–Bonnet esthesiometer) in the central qualifying zone. Key inclusion criteria also included no improvement in NEI zone between screening and study baseline.
Key exclusion criteria included evidence of active ocular infection; use of nondiagnostic medications (e.g., topical drops for clinical testing) that could have induced corneal toxicity between screening and baseline; prior NK-related surgical procedures such as tarsorrhaphy (amniotic membrane transplantation was permitted under specific circumstances); or ocular surgery within 90 days before the baseline visit. Complete inclusion and exclusion criteria are available in the Supplementary Methods.
Study Procedures
After the screening visit, patients underwent a 2-week washout period during which they could use only commercially available preservative-free artificial tears up to four times per day as needed and were required to track usage daily in a patient diary. Patients received 1 or 2 biweekly cartons (plus pipettes and adaptors) sufficient for 2 weeks of cenegermin dosing at visit 2 (baseline), visit 3 (week 2), visit 4 (week 4), and visit 5 (week 6). Patients were advised to store the weekly carton(s) containing the cenegermin vials in the refrigerator between 36 and 46 °F (2–8 °C). Patients then applied 1 drop of cenegermin 20 mcg/ml to the study eye 6 times per day at 2-h intervals for 8 weeks. A vial opened for daily use could be stored in the original biweekly carton in the refrigerator between 36 and 46 °F (2–8 °C) or at room temperature up to 77°F (25 °C) for up to 12 h while dosing. During the treatment period, use of commercially available preservative-free artificial tears was not permitted. Patients were then followed for another 24 weeks. Patients who were completely healed at week 8 were not permitted to use any treatment during the follow-up, except for commercially available preservative-free artificial tears as needed. Patients who were not completely healed and did not meet discontinuation criteria could continue any treatment at the discretion of the physician, with treatment tracked as concomitant medication.
Endpoints and Assessments
The primary endpoint was the percentage of patients who experienced corneal epithelial healing at week 8. Corneal epithelial healing was defined by the absence of persistent epithelial staining abnormalities related to the disease. The assessment considered punctate staining and other clinical manifestations in stage 1 NK (e.g., fluorescein pooling or diffusion, epithelial scarring) and their persistence. Two trained readers from the independent central reading center who were board-certified ophthalmologists assessed the baseline staining patterns of each patient and their evolution over time to ensure objective, standardized, and unbiased grading of study images. Secondary endpoints included the percentage of patients with corneal epithelial healing at week 4; the percentage of patients who experienced corneal epithelial healing at week 8 and remained healed throughout the follow-up period; and the following assessments at weeks 4, 8, and 32: mean change from baseline in corneal sensitivity by Cochet–Bonnet, percentage of patients who experienced improvements in corneal sensitivity, mean change from baseline in best-corrected distance visual acuity (BCDVA) and percentage of patients with a 15-letter gain in BCDVA (Early Treatment Diabetic Retinopathy Study [ETDRS]), change from baseline in Schirmer I and tear film breakup time (TFBUT) tests, and change from baseline in quality-of-life (QoL) as assessed with the Impact of Dry Eye on Everyday Life (IDEEL) and EuroQoL 5-dimension 5-level (EQ-5D-5L) questionnaires. The IDEEL and EQ-5D-5L questionnaires were completed by patients before any ophthalmic procedures at any visit. The IDEEL questionnaire was designed to assess health-related QoL by representing the patient perspective on the impact of dry eye on function across three modules: dry eye impact on daily life (five-point scale ranging from all of the time to none of the time), dry eye symptom-bother (four-point scale ranging from not at all to very much), and dry eye treatment satisfaction (five-point scale ranging from none of the time to all of the time). Module subscores and total scores were calculated from 0 to 100. The EQ-5D-5L questionnaire is a standardized measure of health status in five areas—mobility, self-care, usual activities, pain/discomfort, and anxiety/depression—with a rating scale of 1 (no problems) to 5 (extreme problems). The questionnaire also asks the patient to rate their current health (“how good or bad your health is today”) on a scale from 0 (worst health you can imagine) to 100 (best health you can imagine). Adverse events (AEs), serious AEs (SAEs), and treatment-emergent AEs (TEAEs) were monitored throughout the study and categorized according to the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0.
Statistical Analysis
A sample size of 35 patients who completed 8 weeks of treatment was determined to be adequate to achieve a lower limit of 95% CI equal to 40% and 54% when the percentage of patients who experienced epithelial healing was 58% at 4 weeks and 72% at 8 weeks, respectively (based on the percentage of patients who achieved complete corneal healing by week 8 in the NGF0212 trial [72%]) [22]. The full analysis set (FAS) included all enrolled patients who received at least one dose of study drug. The full treated set included all enrolled patients who received treatment with the study drug through week 8. All safety analyses were conducted on the FAS. All efficacy analyses were conducted on an as-observed case basis, with no imputation of missing data performed.
Frequencies and percentages are presented for categorical variables. Continuous variables are summarized with descriptive statistics. Analyses for responder outcomes included percentage of response and exact 95% CIs. Analyses of change from baseline and continuous outcomes included mean and associated asymptotic 95% CIs. Missing data were not imputed in the analyses. Not all patients who enrolled in the study had data to assess at each endpoint; thus, all patient percentages reported were calculated using the number of patients with available data for each endpoint as the denominator.
The percentages of patients who had corneal epithelial healing, a 15-letter gain in visual acuity, and improved corneal sensitivity were analyzed post hoc with a one-sample binomial test. Mean changes from baseline in corneal sensitivity and BCDVA were analyzed post hoc with descriptive statistics and using one-sample t tests at each time point. Other secondary and exploratory endpoint results were considered statistically significant when the 95% CI did not include 0. Of note, some secondary and exploratory endpoints were also analyzed at each time point by using univariate one-sample binomial or t test depending on the nature of each endpoint. These inferential analyses were univariate and used for descriptive purposes.
Results
Patient Demographics, Baseline Characteristics, and Compliance
Thirty-seven patients were enrolled in the FAS (Table 1). The mean (standard deviation [SD]) age was 64.6 (11.9) years, 75.7% (28/37) of patients were female, and most identified as White (86.5%; 32/37) and not Hispanic/Latino (94.6%; 35/37). The causes of NK in these patients were difficult to determine; Supplementary Table S1 includes a summary of patients’ most common ocular medical histories. Of the 37 patients, 36 (97.3%) had an ocular medical history, with the most common system organ class being eye disorders (86.5%; 32/37), followed by surgical and medical procedures (81.1%; 30/37). The most common MedDRA preferred terms were dry eye (56.8%; 21/37), intraocular lens implant (51.4%; 19/37), cataract operation (37.8%; 14/37), and cataract (32.4%; 12/37).
Of the 37 enrolled patients, eight (21.6%) discontinued the study. Reasons for discontinuations were AEs (n = 3), a decrease in BCDVA by > 10 ETDRS letters (n = 1), death (n = 1, from myocardial infarction and deemed unrelated to study drug), principal investigator decision (n = 1), protocol deviation (n = 1), or relocation (n = 1). Dispositions are available in Fig. 1. The primary analysis was based on observed cases; three patients were excluded from the FAS because of lack of fluorescein corneal staining assessment at week 8 or at early discontinuation. An additional patient was excluded from the FAS by the research center because of lack of uploaded photos of the stained cornea for grading.
Summary of patient disposition. AE adverse event, BCDVA best-corrected distance visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, logMAR logarithm of the minimum angle of resolution, NEI National Eye Institute, NK neurotrophic keratopathy, PI principal investigator. aDue to myocardial infarction and judged unrelated to cenegermin
Corneal Epithelial Healing
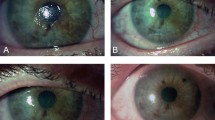
At the end of the treatment period (week 8), 84.8% (28/33; 95% CI 68.1–94.9%; P < 0.001) of patients experienced corneal epithelial healing in the FAS (Fig. 2A). At the end of the 24-week follow-up period (week 32), 95.2% (20/21; 95% CI 76.2–99.9%; P < 0.001) of patients who achieved corneal epithelial healing at week 8 (n = 28) and returned for assessment at week 32 (n = 21) remained healed (full treated set). Figure 2B shows representative images of corneal epithelial healing observed by slit-lamp at weeks 4, 8, and 32.
Summary of corneal epithelial healing. A Percentage of patients who achieved corneal epithelial healing from baseline to week 8 and percentage of patients healed at week 8 who remained healed at week 32. The n/n represents the patients with corneal epithelial healing (numerator) and patients with available data at each time point (denominator). B Slit-lamp photos from a representative patient case at baseline, week 4, week 8, and week 32 demonstrating corneal epithelial healing after treatment with cenegermin. Analyses performed at weeks 4 and 8 were conducted in the full analysis set. Analysis performed at week 32 was conducted in the full treated set. CI confidence interval. aP values derived from one-sample binomial test (H0 = 0.5), post hoc analysis. 95% CI based on Clopper–Pearson exact method
Corneal Sensitivity
Mean corneal sensitivity increased from baseline (2.28 cm; SD, 1.27) to week 8 (4.12 cm; SD, 1.61) and week 32 (4.16 cm; SD, 1.79). Mean change from baseline in corneal sensitivity was 1.87 cm (n = 34; SD, 1.46; 95% CI 1.36–2.38; P < 0.001) at week 8 and 1.79 cm (n = 28; SD, 1.71; 95% CI 1.12–2.45; P < 0.001) at week 32 (Fig. 3A). At weeks 8 and 32, 8.8% (3/34) and 17.9% (5/28) of patients did not demonstrate an increase from baseline, respectively. The mean percentage of patients with improved corneal sensitivity at week 8 was 91.2% (31/34; 95% CI 76.3–98.1%; P < 0.001; Fig. 3B). At week 32, 82.1% (23/28; 95% CI 63.1–93.9%; P < 0.001) of patients had improvements in corneal sensitivity.
Summary of change in corneal sensitivity. A Mean change from baseline in corneal sensitivity, as assessed in the National Eye Institute zone used to qualify in the study eye and measured with a Cochet–Bonnet esthesiometer. Lower bars indicate the baseline value and upper, lightly shaded bars represent change from baseline; n represents the number of patients with available data at each time point. B Percentage of patients with improvement from baseline in corneal sensitivity (red/blue sections), defined as a change > 0 cm in the study eye. Analyses were conducted in the full analysis set; n/n represents the number of patients with an improvement from baseline in corneal sensitivity (numerator) and patients with available data at each time point (denominator). CI confidence interval. aP values derived from one-sample t test (H0 = 0.0), post hoc analysis. 95% CI based on Clopper–Pearson exact method. bP values derived from one-sample binomial test (H0 = 0.5), post hoc analysis. 95% CI based on Clopper–Pearson exact method
Visual Acuity
A decrease in BCDVA logarithm of the minimum angle of resolution (logMAR) indicates improvement in visual acuity. Mean change from baseline to week 8 in BCDVA was − 0.10 logMAR (n = 33; SD, 0.15; 95% CI − 0.16 to − 0.05; P < 0.001; Fig. 4A). At week 32, mean visual acuity was still slightly decreased from baseline by − 0.05 logMAR (n = 28; SD, 0.16; 95% CI − 0.11 to − 0.01; P = 0.122). The mean percentage of patients with a 15-letter gain in BCDVA was 15.2% (5/33; 95% CI 5.1–31.9%; P < 0.001) at week 8 and 10.7% (3/28; 95% CI 2.3–28.2%; P < 0.001; Fig. 4B) at week 32.
Summary of change in visual acuity. A Mean change from baseline to week 32 in BCDVA test. The n represents the number of patients with available data at each time point. B Percentage of patients who achieved a 15-letter gain in BCDVA test (red/blue sections) from baseline to week 32. Fifteen-letter gain in BCDVA was defined as change from baseline of logMAR ≤ − 0.3 in study eye. The n/n represents the number of patients with 15-letter gain in BCDVA test from baseline (numerator) and patients with available data at each time point (denominator). Analyses were conducted in the full analysis set. BCDVA best-corrected distance visual acuity, CI confidence interval, logMAR logarithm of the minimum angle of resolution. aP values derived from one-sample t test (H0 = 0.0), post hoc analysis. 95% CI based on Clopper–Pearson exact method. bP values derived from one-sample binomial test (H0 = 0.0), post hoc analysis. 95% CI based on Clopper–Pearson exact method
Schirmer I Test
The mean change from baseline in the Schirmer I test was 4.13 mm (n = 32; SD, 7.72; 95% CI 1.34–6.91; P < 0.05 based on evaluation of 95% CI) at week 4 and 2.22 mm (n = 32; SD, 8.02; 95% CI − 0.67 to 5.11; P ≥ 0.05 based on evaluation of 95% CI) at week 8 (Supplementary Figure S1).
TFBUT Test
The mean change from baseline in TFBUT (average of two measurements) was 0.05 s (n = 33; SD, 2.25; 95% CI − 0.75 to 0.85; P ≥ 0.05 based on evaluation of 95% CI) at week 8 and − 0.26 s (n = 28; SD, 1.97; 95% CI − 1.03 to 0.50; P ≥ 0.05 based on evaluation of 95% CI) at week 32 (Supplementary Figure S2).
IDEEL
Health-related QoL outcomes reported by patients using the IDEEL questionnaire were grouped by six domains within three modules: dry eye impact on daily life, dry eye symptom-bother, and dry eye treatment satisfaction (Supplementary Figure S3; Supplementary Table S2). Improvements in IDEEL subscores for impact on work and emotional impact were observed at week 8 (mean change from baseline, + 21.25 [n = 16; SD, 23.13; 95% CI 8.92–33.58] and + 10.49 [n = 34; SD, 17.87; 95% CI 4.26–16.73], respectively, P < 0.05 based on evaluation of 95% CI) and at week 32 (+ 26.15 [n = 13; SD, 24.42; 95% CI 11.40–40.91] and + 14.37 [n = 28; SD, 17.88; 95% CI 7.44–21.30], respectively, P < 0.05 based on evaluation of 95% CI). Improvements in treatment-effectiveness score were also observed at week 32 (mean change from baseline, + 15.50 [n = 25; SD, 29.76; 95% CI 3.21–27.79], P < 0.05 based on evaluation of 95% CI). Symptom-bother scores progressively improved over time, with notable improvement at the end of the treatment and follow-up periods (mean change from baseline, week 8: − 6.51 [n = 34; SD, 17.10; 95% CI − 12.48 to − 0.54]; week 32: − 12.54 [n = 28; SD, 13.25; 95% CI − 17.68 to − 7.41], P < 0.05 based on evaluation of 95% CI); the improvement at week 32 was clinically meaningful [25].
EQ-5D-5L
EQ-5D-5L, which evaluates overall QoL, had minimal improvement at the end of the follow-up period (mean change from baseline, + 2.89 [n = 28; SD, 18.00; 95% CI − 4.09 to 9.87]; P ≥ 0.05 based on evaluation of 95% CI; Supplementary Figure S4; Supplementary Table S3). However, the EQ-5D-5L overall score was relatively low at baseline, signifying minimal baseline dissatisfaction.
Safety
Overall, 73.0% (27/37) of patients reported ≥ 1 AE during the treatment period, and 45.7% (16/35) reported ≥ 1 AE during the follow-up period (Table 2). Most AEs were mild (27.0% [10/37] on treatment; 8.6% [3/35] during follow-up) or moderate (32.4% [12/37] on treatment; 31.4% [11/35] during follow-up).
Treatment-related TEAEs were reported by 54.1% (20/37) of patients during the treatment period and by 5.7% (2/35) during the follow-up period. The three most common treatment-related TEAEs, eye pain (37.8%; 14/37), blurred vision (10.8%; 4/37), and eyelid pain (8.1%; 3/37), were mostly mild or moderate and only reported during the treatment period; in median, eye pain occurred 5 days after the beginning of the treatment, blurred vision occurred after 1.5 days, and eyelid pain occurred after 3.5 days. Of note, during the follow-up period, 5.7% (2/35) of patients reported blurred vision deemed unrelated to cenegermin.
Three (8.1%) patients experienced AEs that led to withdrawal from the study. Two (5.4%) withdrew during the treatment period owing to AEs deemed possibly related to cenegermin treatment (one patient with severe corneal edema and moderate skin blister [both unresolved]; one patient with moderate corneal edema and moderately reduced visual acuity [both resolved]). The patient who experienced moderately reduced visual acuity that resolved was the only patient (2.7%) who experienced a reduction in visual acuity (defined as a decrease in BCDVA by > 10 ETDRS letters) during the study. The third patient withdrew during the follow-up period because of severe bacterial conjunctivitis deemed unrelated to cenegermin treatment. None of these events were classified as serious.
Eight patients (21.6%) experienced SAEs: two (5.4%) during the treatment period and seven (20.0%) during the follow-up period, of which only one was ocular in nature (worsening anterior uveitis during the follow-up period that resolved by the end of the study) and considered possibly related to cenegermin treatment.
Discussion
Cenegermin-bkbj (rhNGF) 20 mcg/ml ophthalmic solution is approved by the FDA for the treatment of NK [19]. The pivotal trials leading to its approval included patients with stage 2 or 3 NK [21, 22]. Although these trials demonstrated efficacy and safety of cenegermin, patients with stage 1 NK per Mackie classification were not included in the trials. The goal of initiating NK treatment at stage 1 is to prevent progression to later stages when significant visual loss may occur [1]. Therefore, we evaluated efficacy and safety of cenegermin in patients with stage 1 NK. Oxervate® is commercially available and approved for all stages of NK; hence, for ethical reasons, we conducted a post-marketing open-label trial.
Most patients achieved corneal epithelial healing after 8 weeks of cenegermin treatment, similar to previous trials conducted in patients with stage 2 and 3 NK [21, 22]. We observed improvements in both corneal sensitivity and visual acuity during treatment, with prolonged effects observed through the follow-up period. Corneal sensitivity is a key indicator of corneal health [2, 26], and increased corneal sensitivity suggests improvement in corneal nerve function [10, 27]. In addition, deterioration of vision and loss of corneal sensitivity become more difficult to address in later stages of NK [1]. Our findings suggest that cenegermin treatment could be considered in earlier stages of NK. Although there are data to suggest that cenegermin treatment may increase corneal innervation [10, 27,28,29], including in patients with stage 1 NK [24, 30], most studies were uncontrolled and included small numbers of patients. The incorporation of in vivo confocal microscopy into future prospective, randomized controlled trials could help elucidate the impact of cenegermin on corneal reinnervation in patients early in the disease continuum.
A 15-letter improvement on the eye chart (equivalent to a 0.3 logMAR decrease) is considered clinically significant in patients’ perception of vision [31]. Therefore, our findings suggest that visual acuity was improved after 8 weeks of treatment; however, these improvements decreased slightly between week 8 and the end of the follow-up period. This decrease could be explained in part by the haze that often clouds vision temporarily during corneal epithelial healing [21].
The IDEEL questionnaire measured dry eye–related QoL outcomes, which were improved at week 32, notably in emotional impact and impact on work as early as 8 weeks. With the EQ-5D-5L questionnaire, minimal improvements were observed at the end of the follow-up period. However, baseline EQ-5D-5L scores were low, suggesting that disease symptoms were not yet affecting patients’ overall health. Good overall initial health could partially explain why little improvement was observed with the EQ-5D-5L scores. Although it is common for patients with NK, particularly those with advanced stage disease, not to display symptoms owing to the nature of the condition [5], patients with stage 1 NK may report symptoms similar to dry eye disease, including foreign body or burning sensation, blurred vision, fluctuating vision, and photophobia [32]. Because the IDEEL questionnaire focuses on dry eye symptoms (including those reported in patients with stage 1 NK), whereas the EQ-5D-5L questionnaire more broadly assesses overall health status (e.g., mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), our results suggest that dry eye symptoms may have a notable impact on patients with stage 1 NK, even if it has yet to affect the patients’ perceptions of their overall health.
Most AEs and TEAEs were mild to moderate. The most common TEAE was eye pain, which encompassed the terms eye soreness, eye pain, left upper-lid soreness, left lower-lid soreness, ocular ache, ocular soreness, soreness, and eye tenderness and may reflect the known effect of NGF associated with nociceptor sensitization [22, 33]. Most TEAEs were transient during the treatment phase, with only one patient experiencing an ocular treatment-related TEAE during the follow-up period. Of note, eye pain, blurred vision, and eyelid pain with a possible or probable relation to cenegermin were only reported during the treatment period and not during the follow-up period, suggesting that these are short-term ocular TEAEs that typically resolve after the completion of cenegermin treatment. Few serious TEAEs emerged, and only two patients withdrew from the study due to drug-related AEs. Regarding the SAE of worsening anterior uveitis (reported during the follow-up period and deemed possibly related to cenegermin), the patient’s persistent herpes zoster ophthalmicus may have been a contributing factor, as the patient reported poor compliance with prescribed topical steroids. The anterior uveitis subsequently resolved and the patient was able to complete the study.
Altogether, our results are in line with 2 retrospective studies evaluating the efficacy of cenegermin in patients with stage 1 NK [23, 24]. At a time when limited data on the effect of cenegermin in patients with stage 1 NK were available, these retrospective studies have been informative regarding the effect of cenegermin in patients with stage 1 NK. However, with retrospective studies being generally more prone to bias and not permitting determination of causation, it was crucial to design a prospective trial to confirm the observed benefits of using cenegermin early on in the development of NK. Because the current FDA-approved indication for cenegermin is for patients with all stages of NK [19], there were ethical considerations precluding the incorporation of a randomized control group into this study given the progressive nature of NK. If future randomized controlled trials on the safety and efficacy of cenegermin in patients with stage 1 NK can be designed in a way that takes this ethical consideration into account, including trials designed to assess the safety and efficacy of multiple courses of cenegermin in patients with incomplete healing at the end of the standard 8-week treatment period, such studies would be beneficial to further strengthen the current data.
This study had some limitations, including the small number of patients, the sex distribution (75.7% female), the absence of a control group, and the unmasked design of the study. The sex distribution in this study may be a reflection of the NK disease state as supported by several recent studies that demonstrate a higher percentage of females with NK than males [24, 34, 35]. Also, the relatively short period of follow-up (24 weeks) might limit long-term interpretation of results from one course of treatment. However, the ongoing NGF0122 DEFENDO long-term extension trial (NCT05552261) has been designed to evaluate clinical outcomes at 24 and 30 months after treatment with cenegermin in patients with stage 1 NK who completed the DEFENDO trial.
Conclusions
The primary efficacy endpoint, corneal epithelial healing at the end of treatment, was met by 84.8% of patients. Key secondary endpoints, including improvement in corneal sensitivity and visual acuity at the end of treatment, were met by 91.2% and 15.2% of patients, respectively. Eye pain was the most commonly reported AE during the treatment period (37.8% of patients). Overall, cenegermin treatment resulted in improvements in several primary and secondary efficacy endpoints and was generally well tolerated in this study. These efficacy and safety results support the potential use of cenegermin for treating patients with stage 1 NK. Since this study was open-label and uncontrolled, future confirmatory studies would be beneficial to elaborate on these findings.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–31.
Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137–45.
Peterson DC, Hamel RN. Corneal reflex. Treasure Island: StatPearls Publishing; 2022.
Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–42.
Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232(4):717–24.
Di G, Qi X, Zhao X, Zhang S, Danielson P, Zhou Q. Corneal epithelium-derived neurotrophic factors promote nerve regeneration. Investig Ophthalmol Vis Sci. 2017;58(11):4695–702.
Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–7.
Feinberg K, Tajdaran K, Mirmoeini K, Daeschler SC, Henriquez MA, Stevens KE, et al. The role of sensory innervation in homeostatic and injury-induced corneal epithelial renewal. Int J Mol Sci. 2023;24(16):12615.
Sheha H, Tighe S, Hashem O, Hayashida Y. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973–80.
Mastropasqua L, Lanzini M, Dua HS, D’Uffizi A, Di Nicola M, Calienno R, et al. In vivo evaluation of corneal nerves and epithelial healing after treatment with recombinant nerve growth factor for neurotrophic keratopathy. Am J Ophthalmol. 2020;217:278–86.
Mackie I. Neuroparalytic keratitis. In: Fraunfelder F, Roy F, Meyer S, editors. Current ocular therapy. 4th ed. Philadelphia: WB Saunders; 1995.
Roth M, Dierse S, Alder J, Holtmann C, Geerling G. Incidence, prevalence, and outcome of moderate to severe neurotrophic keratopathy in a German tertiary referral center from 2013 to 2017. Graefes Arch Clin Exp Ophthalmol. 2022;260(6):1961–73.
Saad S, Abdelmassih Y, Saad R, Guindolet D, Khoury SE, Doan S, et al. Neurotrophic keratitis: frequency, etiologies, clinical management and outcomes. Ocul Surf. 2020;18(2):231–6.
Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003;17(8):989–95.
Dana R, Farid M, Gupta PK, Hamrah P, Karpecki P, McCabe CM, et al. Expert consensus on the identification, diagnosis, and treatment of neurotrophic keratopathy. BMC Ophthalmol. 2021;21(1):327.
Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9.
NaPier E, Camacho M, McDevitt TF, Sweeney AR. Neurotrophic keratopathy: current challenges and future prospects. Ann Med. 2022;54(1):666–73.
Letko E. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch Ophthalmol. 2001;119(5):659.
Oxervate [prescribing information]. Boston: Dompé; 2019.
US Food and Drug Administration. FDA approves first drug for neurotrophic keratitis, a rare eye disease [news release]. 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-neurotrophic-keratitis-rare-eye-disease. Accessed 5 Oct 2023 (Published August 22, 2018)
Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14–26.
Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–43.
Epitropoulos AT, Weiss JL. Topical human recombinant nerve growth factor for stage 1 neurotrophic keratitis: retrospective case series of cenegermin treatment. Am J Ophthalmol Case Rep. 2022;27: 101649.
Saricay LY, Bayraktutar BN, Lilley J, Mah FS, Massaro-Giordano M, Hamrah P. Efficacy of recombinant human nerve growth factor in stage 1 neurotrophic keratopathy. Ophthalmology. 2022;129(12):1448–50.
Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. 2011;9:111.
Brennan NA, Bruce AS. Esthesiometry as an indicator of corneal health. Optom Vis Sci. 1991;68(9):699–702.
Pedrotti E, Bonacci E, Chierego C, De Gregorio A, Cozzini T, Brighenti T, et al. Eight months follow-up of corneal nerves and sensitivity after treatment with cenegermin for neurotrophic keratopathy. Orphanet J Rare Dis. 2022;17(1):63.
Pieragostino D, Lanzini M, Cicalini I, Cufaro MC, Damiani V, Mastropasqua L, et al. Tear proteomics reveals the molecular basis of the efficacy of human recombinant nerve growth factor treatment for neurotrophic keratopathy. Sci Rep. 2022;12(1):1229.
Hao M, Cheng Y, Wu J, Cheng Y, Wang J. Clinical observation of recombinant human nerve growth factor in the treatment of neurotrophic keratitis. Int J Ophthalmol. 2023;16(1):60–6.
Balbuena-Pareja A, Bogen CS, Cox SM, Hamrah P. Effect of recombinant human nerve growth factor treatment on corneal nerve regeneration in patients with neurotrophic keratopathy. Front Neurosci. 2023;17:1210179.
Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50(8):3629–35.
Ruiz-Lozano RE, Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A. The molecular basis of neurotrophic keratopathy: diagnostic and therapeutic implications. A review Ocul Surf. 2021;19:224–40.
Jankowski MP, Koerber HR. Neurotrophic factors and nociceptor sensitization. In: Kruger L, Light AR, editors. Translational pain research: from mouse to man. Boca Raton: CRC Press/Taylor & Francis; 2010.
Bian Y, Ma KK, Hall NE, Elze T, Lorch A, Miller JW, et al. Neurotrophic keratopathy in the United States: an Intelligent Research in Sight Registry analysis. Ophthalmology. 2022;129(11):1255–62.
Roumeau S, Dutheil F, Sapin V, Baker JS, Watson SL, Pereira B, et al. Efficacy of treatments for neurotrophic keratopathy: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2623–37.
Acknowledgements
The authors thank the patients, patients’ caregivers and family members, investigators, and site staff who participated in this study.
Medical Writing, Editorial, and Other Assistance
Writing and editorial assistance was provided under the direction of the authors by Deborah Lew, PhD, and Claire Levine, MS, ELS, of MedThink SciCom and funded by Dompé farmaceutici S.p.A.
Funding
Funding for this study and the journal’s Rapid Service Fee was provided by Dompé farmaceutici S.p.A.
Author information
Authors and Affiliations
Contributions
Study conception and design: GP, FM; acquisition of data: PH, MM-G, DS, EH, GB; statistical analysis and interpretation of data: GG, FM; critical review and final approval of manuscript: PH, MM-G, DS, EH, GB, GG, GP, FM.
Corresponding author
Ethics declarations
Conflict of Interest
Pedram Hamrah has received financial support from CooperVision, Dompé farmaceutici S.p.A., Novartis, Ocular Therapeutix, and OKYO Pharma and has served as a contractor/consultant for Dompé farmaceutici S.p.A., Kala Pharmaceuticals, Novaliq, Novartis, OKYO Pharma, and Oyster Point Pharma. Mina Massaro-Giordano has served as a consultant for Claris Bio, Dompé farmaceutici S.p.A., Kala Pharmaceuticals, Lynthera, Oyster Point, and Tarsus. David Schanzlin has no conflicts of interest to disclose. Edward Holland has served as a consultant for Alcon. Gregg Berdy has served as a researcher for Aerie (now Alcon), Alcon, Allakos, Allergan/AbbVie, Bausch + Lomb, ClarisBio, Dompé, Kala, Novaliq (now Bausch + Lomb), Tarsus, Tear Care, Tear Film Innovations, and Tear Solutions; has consulted for Aerie (now Alcon), Alcon, Allergan/AbbVie, Bausch + Lomb, Dompé, Novartis, Sun, Tarsus, Tear Care, and Tear Solutions; and has lectured for Aerie (now Alcon), Alcon, Allergan/AbbVie, Bausch + Lomb, Dompé, Novartis, Sun, Tarsus, and Tear Care. Giovanni Goisis was an employee of Dompé farmaceutici S.p.A. at the time of this study but is no longer affiliated with the company. Georgea Pasedis and Flavio Mantelli are employees of Dompé farmaceutici S.p.A.
Ethical Approval
Institutional review board approval was obtained for the study protocol, protocol amendments, informed consent forms, and any other relevant study-related documents at each center (Sterling IRB, 8203-DJSchanzlin, 8203-EJHolland, 8203-GJBerdy, 8203-MMassaroGiordano; and WCG-WIRB, 20202809). The study complied with the Declaration of Helsinki, relevant parts of the Code of Federal Regulations Title 21, and good clinical practice and good laboratory practice guidelines. Written informed consent was obtained from all patients before study initiation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hamrah, P., Massaro-Giordano, M., Schanzlin, D. et al. Phase IV Multicenter, Prospective, Open-Label Clinical Trial of Cenegermin (rhNGF) for Stage 1 Neurotrophic Keratopathy (DEFENDO). Ophthalmol Ther 13, 553–570 (2024). https://doi.org/10.1007/s40123-023-00866-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00866-y