Abstract
Introduction
This study aimed to compare the integrity of the hyperreflective layer of the inner choroid in eyes with and without drusen.
Methods
Swept-source optical coherence tomography images of patients with drusen and normal controls were reviewed. Using a line plot of ImageJ, choroidal reflectivity was measured at the subfovea, and the integrity of the hyperreflective layer of the inner choroid was determined.
Results
In total, 63 eyes with drusen and 30 control eyes without drusen were included. The integrity of the hyperreflective layer of the inner choroid was preserved in 81.0% of eyes with drusen and 93.3% of normal controls. The proportion of eyes with the hyperreflective layer did not differ between eyes with and without drusen. Of the 63 subjects with drusen, this hyperreflective layer was observed in all 28 eyes (100%) with pachydrusen but only in 68.6% of the 35 eyes with soft drusen, and its prevalence was significantly different (P = 0.001).
Conclusion
The prevalence of the hyperreflective layer between the choriocapillaris and medium or large choroidal vessels in eyes with soft drusen differed from that in eyes with pachydrusen. These findings support the suggestion that changes within the choroidal stroma may be involved in the pathogenesis of age-related macular degeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The complex composition of the choroid, which includes blood vessels and various stromal components, contributes to the broad spectrum of irregular reflectivity observed in optical coherence tomography images. |
This study aimed to investigate the variation in choroidal reflectivity representing the choroidal stroma in eyes with and without drusen. |
What was learned from the study? |
The hyperreflective layer in the inner choroid was identified between the choriocapillaris and the medium or large choroidal vessels. |
The hyperreflective layer was less frequently observed in eyes with soft drusen than in eyes with pachydrusen. |
Measurement of the inner choroidal hyperreflective layer may provide new insights into the study of the pathophysiology of age-related macular degeneration. |
Introduction
Drusen are characteristic features of age-related macular degeneration (AMD) and their pathogenesis has been widely studied [1,2,3,4]. In addition to the degeneration of Bruch’s membrane (BM) and the retinal pigment epithelium (RPE), recent studies using optical coherence tomography (OCT) have suggested a role for the choroid in the development of different types of drusen, such as soft drusen and pachydrusen [2,3,4,5]. The choroid is a highly vascularized tissue that helps maintain homeostasis of the overlying neuroretina [6]. Choroidal vascular flow is purportedly essential for maintaining the supply of oxygen and nutrients to the retina and RPE. In addition to classical techniques for the kinetic measurement of choroidal vascular flow, such as indocyanine green angiography and Doppler flowmetry, a newly proposed static measurement of the absolute and relative proportion of the choroidal vascular lumen using OCT has revealed choroidal vascular variation in eyes with and without various types of drusen [7, 8]. Baek et al. [8] suggested that the large choroidal vessels in Haller’s layer were associated with pachydrusen. The choroidal vascularity index has been used to characterize pathological changes in the choroid in various chorioretinal diseases [7].
Various progressive changes in the choroid owing to aging have been observed [6]. Age-related overall thinning of the choroid has been presented in several studies with histological samples and with OCT [6, 9, 10]. A linear reduction in choroidal blood flow with aging has been reported in many studies with volunteers [6, 11]. While early studies on age-related choroidal changes have focused on the choriocapillaris and choroidal flow [12,13,14,15], in addition to vessels, the choroid is composed of stroma, such as various cells and neurons. Aging changes in choroidal stroma have also been reported in various studies [16, 17]. A recent study showed that the choroidal stromal volume decreased with age [16]. A decrease in hyaluronic acid in the choroid stroma was reported in a previous study [18], and a trend of decreasing content with aging in choroidal melanocytes was observed in another study [19]. However, whether these age-related changes in the choroidal stromal component are associated with the development of drusen remains unclear.
Therefore, in this study, using swept-source (SS)-OCT, we investigated the variation in choroidal reflectivity representing the choroidal stroma in eyes with and without drusen.
Methods
The Institutional Review Board (IRB) of the Korea University Hospital approved the study design (IRB number: 2023AN0096). Informed consent was waived for this study, as it was a retrospective review of anonymized image data. This study adhered to the tenets of the Declaration of Helsinki. After reviewing the medical records of subjects who visited retina clinics, we included the eyes of subjects with drusen (≥ 125 µm in the smallest diameter) [20, 21]. We also excluded eyes with only reticular pseudodrusen, as well as those with geographic atrophy, disciform scarring, choroidal neovascularization (CNV), or pigmented epithelial detachment in AMD-affected eyes. Other exclusion criteria were as follows: (1) those with other chorioretinal diseases including central serous chorioretinopathy, retinal vascular occlusion, diabetic retinopathy, macular hole, and uveitis; (2) those with inherited retinal dystrophy; (3) those with a history of intravitreal injection, laser treatment, vitrectomy, and other ocular surgery except cataract surgery; and (4) patients with high myopia (axial length ≥ 28.0 mm or mean spherical equivalent < −6.50 D). For comparison, we also included normal eyes of subjects who had undergone OCT examination but had no apparent chorioretinal disease in either eye, except for the idiopathic epiretinal membrane in one eye. We also excluded images with poor quality to prevent measurement of imaging parameters. For subgroup analysis, drusen were classified into two subgroups: soft drusen and pachydrusen. We determined soft drusen and pachydrusen using color fundus photography and OCT, according to previously described criteria [2, 4]. Several eyes with soft drusen also had reticular pseudodrusen (subretinal drusen deposits), and these were classified into the soft drusen group.
OCT Measurements
The SS-OCT device (DRI OCT Triton, Topcon Corp., Tokyo, Japan) uses a central wavelength of 1050 nm, and it operates at a speed of 100,000 A-scans per second with a horizontal resolution of 20 μm and an axial resolution of 8 μm. High-resolution B-scan images averaged from eight B-scans obtained using a five-line cross-scan protocol centered on the fovea were used. A horizontal line scan crossing the macular center was selected for the measurement. On the horizontal B-scan images from SS-OCT, we measured choroidal thickness in the subfoveal area using a previously presented method [5]. Two independent observers (Y.K. and J.O.) determined subfoveal choroidal thickness. The average of the measurements of the subfoveal choroidal thickness of the two observers was used for analysis. Using built-in software, a retinal thickness map was automatically generated from a 12-mm × 9-mm macular volume scan centered between the optic disc and fovea. The central macular thickness was defined as the mean retinal thickness on the 1000-μm-diameter inner central circle in the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. The segmentation line and the position of the center of the ETDRS grid were reviewed by a retinal specialist (Y.K.) and adjusted, if necessary.
Measurement of Vitreous, RPE-BM Complex, and Choroidal Reflectivity
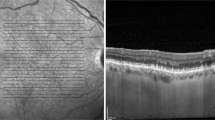
The reflectivity of the vitreous, RPE-BM complex, and choroid was measured at the foveal center. The reflectivity was determined on the horizontal B-scan images of the SS-OCT using the “line plot” tool of ImageJ software (National Institutes of Health, imagej.nih.gov/ij) (Fig. 1). After exporting the B-scan image from SS-OCT, the TIFF image was uploaded to ImageJ. After changing to an 8-bit image, the scale of the images was converted from pixels to micrometers. A linear “line plot” tool was applied to measure the reflectivity, and the profile of the line plot was determined using a “plot profile” function in ImageJ. The width of the line plot was set at 500 μm. To measure the reflectivity of the vitreous, RPE, and choroid, the linear line plot was set perpendicular to the BM. The mean vitreous reflectivity was determined using a linear line plot in a 500-μm × 500-μm area of the vitreous at the foveal center. The peak reflectivity of the RPE complex was determined as the outermost highest reflectance of the RPE-BM complex layer. The reflectivity of the choroid generally decreases with distance from the RPE-BM complex. The inverted reflectivity of the choroid was determined when there was a zone of increasing reflectance. Within areas with inverted choroidal reflectivity, the highest reflectivity was defined as the peak reflectance of the choroid.
Determining choroidal reflectivity. A Choroidal reflectivity was determined on horizontal B-scan images of the swept-source OCT using a line plot of ImageJ with a width of 500 µm. The 500-µm-wide line plot was set perpendicular to Bruch’s membrane (blue arrow). B Using the “plot profile” function of ImageJ, the reflectivity of the choroid was determined. The reflectivity profile plot shows several inflection points, with peak reflectance corresponding to the ellipsoid zone (purple arrow), retinal pigment epithelium (blue arrow), and the choroidal–scleral junction (green arrow). The hyperreflective layer of the inner choroid (red arrow), which had the highest reflectivity within areas with inverted choroidal reflectivity, was observed between the choriocapillaris (CC) and medium-sized and large choroid vessel in Sattler’s and Haller’s layers. CC choriocapillaris, OCT optical coherence tomography
Determination of the Hyperreflective Layer of the Inner Choroid
The hyperreflective layer of the inner choroid was identified when the peak reflectivity of the choroid was located between the choriocapillaris layer and the medium or large choroidal vessels (Figs. 1 and 2). The optical intensity ratio (OIR) of the hyperreflective layer of the inner choroid was defined as the mean OCT reflectivity of the peak reflectivity of the inner choroid, normalized to the mean reflectivity of the vitreous. To obtain the OIR [22] of the hyperreflective layer of the inner choroid, we calculated the peak choroid OIR by dividing the average intensity of the hyperreflective layer of the inner choroid by the mean vitreous intensity. The distance between the RPE-BM complex and the hyperreflective layer was measured. In eyes with extracellular deposits between RPE and BM, the distance between the BM and the hyperreflective layer was measured.
Representative cases of the hyperreflective layer of the inner choroid. In most of the eyes with normal fundus (A) or pachydrusen (B), the peak choroidal reflectance (red arrowheads) was located between the choriocapillaris and a large choroid vessel layer. However, the peak choroidal reflectance was not observed in some eyes with soft drusen (C). The blue and green asterisks indicate the retinal pigment epithelium and choroidal–scleral junction, respectively
Statistical Analysis
SPSS Statistics (version 20.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. Continuous variables were compared using the independent t-test, and categorical variables were compared using the chi-squared test or Fisher’s exact test. Linear correlations between the parameters were analyzed using Pearson’s correlation test. A P value < 0.05 was considered statistically significant.
Results
General Characteristics
We included 93 eyes from 93 subjects. The mean age of the 93 subjects was 66.5 ± 15.0. The study included 46 women and 47 men. The mean subfoveal choroidal thickness was 224.6 ± 121.7 (66.5–619.0) μm. Of the 93 eyes, the integrity of the hyperreflective layer of the inner choroid was preserved in 80 (86.0%). In 80 eyes with the hyperreflective layer of the inner choroid, the mean peak reflectance of the hyperreflective layer was 145.3 ± 15.1. The peak reflectance of the hyperreflective layer did not correlate with age (r = 0.135, P = 0.231). The OIR of the hyperreflective layer did not correlate with age (r = 0.165, P = 0.145). The mean distance between the hyperreflective layer and the RPE-BM complex was 45.6 ± 10.1 μm.
Comparison of the Hyperreflective Layer of the Inner Choroid Between Eyes With and Without Drusen
Of the 93 eyes, 63 eyes of 63 subjects had drusen. Thirty eyes of 30 subjects in the control group had no fundus lesions or history of chorioretinal diseases. The mean age was different between eyes with and without drusen (P < 0.001) (Table 1). The central macular and subfoveal choroidal thickness did not differ between the groups. The proportion of eyes with the hyperreflective layer of the inner choroid was different between those with (81.2%) and without drusen (93.3%) (Table 1). However, it was not statistically significant (P = 0.213). No differences between groups were observed in the peak reflectance of the hyperreflective layer in the inner choroid or the OIR of the peak reflectance, or in the distance between the hyperreflective layer and the RPE-BM complex.
Subgroup Analysis for Comparison of the Hyperreflective Layer of the Inner Choroid Between Eyes with Pachydrusen and Soft Drusen
Of the 63 subjects with drusen, 35 had soft drusen, and 28 had pachydrusen. The mean age was lower in the eyes with pachydrusen (P < 0.001) (Table 2). The central macular thickness did not differ between the groups (P = 0.958), but the subfoveal choroidal thickness was thicker in the pachydrusen group (P < 0.001). The proportion of eyes with the hyperreflective layer of the inner choroid differed between eyes with soft drusen and pachydrusen (P = 0.001). The hyperreflective layer was observed in all eyes (100%) with pachydrusen but only in 68.6% of eyes (n = 24) with soft drusen (Fig. 3). Drusen were more frequently observed within 500 μm of the foveal center in eyes with drusen than those with pachydrusen (P < 0.001).
When comparing 28 eyes with pachydrusen and 24 eyes with soft drusen that had a hyperreflective layer of the inner choroid, the OIR of the peak reflectance of the hyperreflective layer did not differ between the groups. The distance between the hyperreflective layer and the RPE-BM complex also did not differ.
Comparison of Variables in Eyes with Soft Drusen According to the Presence or Absence of the Hyperreflective Layer of the Inner Choroid
Of the 35 eyes with soft drusen, the mean subfoveal choroidal thickness was 167.5 ± 70.2 and 139.5 ± 70.2 μm in eyes with and without the hyperreflective layer, respectively (P = 0.281) (Table 2). Among them, 18 showed drusen within 500 μm of the foveal center (Table 3). The proportion of eyes with or without the hyperreflective layer of the inner choroid did not differ between eyes with and without drusen within 500 μm of the foveal center (P = 0.803).
Discussion
The variation in reflectivity in the choroid depends mainly on the distribution of the vascular lumen [17, 23]. Choroidal vessels have been classified into three layers according to vessel dimensions: the choriocapillaris, Sattler’s layer, and Haller’s layer. Although Sattler's layer and Haller's layer are not easily distinguishable in OCT images of all eyes, the morphology of medium or large choroidal vessels in Sattler’s or Haller’s layer can be determined as a hyporeflective lumen surrounded by the hyperreflective vascular wall or stromal tissues in OCT images [23]. Aside from medium or large vessels in Sattler’s and Haller’s layers, vessels in the choriocapillaris layer cannot be separated as each vessel in OCT B-scan images. In addition, en face OCT angiography images have shown blood flow in the choriocapillaris [24], but it is difficult to discriminate each vessel in the choriocapillaris slab. Instead, the choriocapillaris layer can be easily identified as a linear hyporeflective area between the RPE layer and the hyperreflective layer beneath the choriocapillaris layer [24]. In this study, we obtained the reflectivity of the hyperreflective layer comprising the outer margin of the choriocapillaris layer. The hyperreflective layer in the inner choroid was present in almost all eyes with a normal fundus in this study. The origin of the hyperreflective layer in the inner choroid remains unclear. Its location corresponds to the area of the choroidal stroma and the medium-sized vessel walls of the inner choroid (Fig. 1). Recent studies using en face OCT have suggested that melanin pigmentation contributes to hyperreflectivity in this area [25,26,27]. These results suggest that the hyperreflective layer in the inner choroid may be a characteristic finding representing the pigmentation of the inner choroid.
In this study, we focused on choroidal reflectivity and hypothesized that the hyperreflective layer in the inner choroid in OCT B-scans may represent variation in the stromal component of the choroid [25, 28]. We compared the proportion of eyes with the hyperreflective layer in the inner choroid between those with soft drusen and pachydrusen. The prevalence of the hyperreflective choroid layer differed between the eyes with soft drusen and pachydrusen. It is unclear how the difference in the prevalence of the hyperreflective layer contributes to the difference in the phenotype of drusen, such as soft drusen or pachydrusen. One possibility is that the variation in choroidal vascular and stromal components associated with soft drusen or pachydrusen may be related to the difference in the prevalence of the hyperreflective layer in the inner choroid. Another possibility is that the change within choroidal stromal components, such as melanin pigment, in eyes with soft drusen could be related to the difference in the prevalence of the hyperreflective layer in the inner choroid. In a previous study [27], changes in choroidal reflectivity on OCT B-scan images were reported in eyes with a sunset glow fundus due to Vogt–Koyanagi–Harada disease. In another study [26], the hyperreflective choroidal foci on OCT decreased in the inner choroid of eyes with sunset glow fundus. These findings may support the notion that depigmentation in the choroid could be associated with the development of soft drusen, which is a hallmark of classic AMD. However, this requires further research. Melanin in the choroid has been suggested to protect the choriocapillaris, choroidal vessels, and the neuroretina [29, 30]. Melanin depletion in the inner choroid may be related to the development of soft drusen. In a previous study with histological samples, Weiter et al. [19] showed that the total choroidal melanin density did not change with age. In our study, the peak reflectance of the hyperreflective layer did not correlate with age. However, the hyperreflective layer was more frequently missing in the eyes with soft drusen. Further studies with more cases are needed to evaluate the quantitative relationship between the peak reflectance of the hyperreflective layer and the development of soft drusen.
In an earlier study, drusen in AMD were classified as hard drusen, soft drusen, and compound drusen [31], and their pathogenesis has been widely studied [1, 4]. Furthermore, in aging eyes, the association of the aging of the RPE and BM with the development of drusen has been thoroughly described [4, 31]. After the introduction of OCT in AMD studies, choroidal changes were suggested to contribute to the pathogenesis of drusen, which were classified as soft drusen, pachydrusen, and reticular pseudodrusen [2, 3]. Early studies have suggested that choroidal vascular changes with aging contribute to the development of AMD. Choroidal thinning and vascular depletion are suspected to provide an environment conducive to the development of soft drusen or reticular pseudodrusen in eyes with AMD. Zhou et al. [16] showed that the stromal or vascular volume of the choroid decreased with age; however, they also reported that the choroidal vascularity index or choroidal stroma-to-volume ratio did not [16]. The role of the relative depletion of vascular or stromal components in the development of AMD has been questioned. In other studies using OCT angiography [12, 13], a generalized flow deficit in the choriocapillaris of the macular area was correlated with the development of AMD. Furthermore, a focal defect in the choriocapillaris overlying pachyvessels was also suggested to be related to pachydrusen [32]. While previous studies on the relationship between flow deficit in the choriocapillaris/choroid and the phenotype of drusen have been presented [14, 15], it is still not clear why soft drusen are uncommon in Asians with darker pigmentation [3]. The finding of the current study that depigmentation in the inner choroid is more frequent in eyes with soft drusen may provide a new perspective on the role of stroma in the development of AMD.
This study faced some limitations associated with its retrospective nature. Patients with drusen were older than those without. As several previous studies have shown that subfoveal choroidal thickness decreases with age [33, 34], this difference might have an effect on the choroidal reflectivity. However, the small number of cases in this study made it difficult to perform further subgroup analyses after considering the effect of age on subfoveal choroidal thickness. Because there was no difference in the subfoveal choroidal thickness between eyes with and without drusen, we believe that the bias had a limited effect on the results. However, further studies with a large number of cases are needed to investigate the relationship between different variables. Another limitation is that we quantified the reflectivity of the hyperreflective layer of the inner choroid only at the foveal center. We used the SS-OCT device, which has less signal reduction than the spectral domain OCT device, but due to OCT technology principles, the reflectivity of the inner choroid can be affected by the retina overlying it. Retinal thickness can be affected by age [35, 36], which may affect choroidal reflectance. To reduce the influence of the retina, we excluded eyes with diseases within the retina. There was no difference in central macular thickness between the groups. The properties of the RPE-BM complex could also affect our measurements. In this study, eyes with soft drusen more frequently had extracellular deposits within 500 μm of the fovea than eyes with pachydrusen, and the extracellular deposits may have affected the measurement of the choroidal reflectance. However, the peak reflectance of the hyperreflective layer and its OIR did not differ between eyes with soft drusen and pachydrusen. In addition, the proportion of eyes with the hyperreflective layer of the inner choroid did not differ between eyes with and without soft drusen within 500 μm of the foveal center. Further research at various locations of the choroid may provide more information for eyes with and without drusen. Another limitation is that we could only include Asian subjects with pigmented eyes. For the generalization of our results to other races, further studies with different ethnic populations are required.
Conclusion
The prevalence of the hyperreflective layer between the choriocapillaris and the medium or large choroidal vessels in eyes with soft drusen was different from that in eyes with pachydrusen. These findings support the notion that changes in the choroidal stroma, such as changes in reflectance, may have different effects on the development of soft drusen and pachydrusen.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am J Ophthalmol. 1986;101(3):342–53.
Spaide RF. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina. 2018;38(4):708–16.
Cheung CMG, Gan A, Yanagi Y, Wong TY, Spaide R. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol Retina. 2018;2(12):1196–205.
Zhang X, Sivaprasad S. Drusen and pachydrusen: the definition, pathogenesis, and clinical significance. Eye (Lond). 2021;35(1):121–33.
Kim YH, Lee B, Kang E, Oh J. Clustering of eyes with age-related macular degeneration or pachychoroid spectrum diseases based on choroidal thickness profile. Sci Rep. 2021;11(1):4999.
Reiner A, Fitzgerald MEC, Del Mar N, Li C. Neural control of choroidal blood flow. Prog Retin Eye Res. 2018;64:96–130.
Agrawal R, Ding J, Sen P, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 2020;77: 100829.
Baek J, Lee JH, Chung BJ, Lee K, Lee WK. Choroidal morphology under pachydrusen. Clin Exp Ophthalmol. 2019;47(4):498–504.
Sohn EH, Khanna A, Tucker BA, Abràmoff MD, Stone EM, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55(3):1352–60.
Kim YH, Oh J. Choroidal thickness profile in chorioretinal diseases: beyond the macula. Front Med (Lausanne). 2021;8: 797428.
Ito YN, Mori K, Young-Duvall J, Yoneya S. Aging changes of the choroidal dye filling pattern in indocyanine green angiography of normal subjects. Retina. 2001;21(3):237–42.
Braun PX, Mehta N, Gendelman I, et al. Global Analysis of Macular Choriocapillaris Perfusion in Dry Age-Related Macular Degeneration using Swept-Source Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2019;60(15):4985–90.
Tiosano L, Corradetti G, Sadda SR. Progression of choriocapillaris flow deficits in clinically stable intermediate age-related macular degeneration. Eye (Lond). 2021;35(11):2991–8.
Byon I, Ji Y, Alagorie AR, Tiosano L, Sadda SR. Topographic assessment of choriocapillaris flow deficits in the Intermediate age-related macular degeneration eyes with hyporeflective cores inside drusen. Retina. 2021;41(2):393–401.
Wu Z, Zhou X, Chu Z, et al. Impact of reticular pseudodrusen on choriocapillaris flow deficits and choroidal structure on optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2022;63(12):1.
Zhou H, Dai Y, Shi Y, et al. Age-related changes in choroidal thickness and the volume of vessels and stroma using swept-source OCT and fully automated algorithms. Ophthalmol Retina. 2020;4(2):204–15.
Sonoda S, Sakamoto T, Yamashita T, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159(6):1123–31.
Tate DJ Jr, Oliver PD, Miceli MV, Stern R, Shuster S, Newsome DA. Age-dependent change in the hyaluronic acid content of the human chorioretinal complex. Arch Ophthalmol. 1993;111(7):963–7.
Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27(2):145–52.
Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132(5):668–81.
Ferris FL, Davis MD, Clemons TE et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005 ;123(11):1570–4.
Mehta N, Lavinsky F, Gattoussi S, et al. Increased inner retinal layer reflectivity in eyes with acute CRVO correlates with worse visual outcomes at 12 months. Invest Ophthalmol Vis Sci. 2018;59(8):3503–10.
Branchini LA, Adhi M, Regatieri CV, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120(9):1901–8.
Chu Z, Zhang Q, Gregori G, Rosenfeld PJ, Wang RK. Guidelines for imaging the choriocapillaris using OCT angiography. Am J Ophthalmol. 2021;222:92–101.
Kim YH, Oh J. Hyperreflective foci in the choroid of normal eyes. Graefes Arch Clin Exp Ophthalmol. 2022;260(3):759–69.
Kim YH, Togloom A, Oh J. Correlation between hyperreflective foci in the choroid and choroidal discoloration in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2022;63(9):27.
Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):502–9.
Venkatesh R, Reddy NG, Chhablani J. Impact of melanin on choroidal measurements. Med Hypotheses. 2021;146: 110408.
Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol. 2008;84(3):639–44.
Peters S, Lamah T, Kokkinou D, Bartz-Schmidt KU, Schraermeyer U. Melanin protects choroidal blood vessels against light toxicity. Z Naturforsch C J Biosci. 2006;61(5–6):427–33.
Spraul CW, Grossniklaus HE. Characteristics of drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115(2):267–73.
Baek J, Kook L, Lee WK. Choriocapillaris flow impairments in association with pachyvessel in early stages of pachychoroid. Sci Rep. 2019;9(1):5565.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–5.
Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–9.
Eriksson U, Alm A. Macular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol. 2009;93(11):1448–52.
Nieves-Moreno M, Martínez-de-la-Casa JM, Morales-Fernández L, Sánchez-Jean R, Sáenz-Francés F, García-Feijoó J. Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS ONE. 2018;13(3):e0194169.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and its publication, including the journal’s Rapid Service Fee, were supported by the Korea Medical Device Development Fund grant (Seoul, Korea) funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health and Welfare, Ministry of Food and Drug Safety) (Project Number: RS-2020-KD000026, KMDF_PR_20200901_0026). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Jaeryung Oh contributed to the study conception and design, data collection, analysis and/or interpretation, original draft preparation, and manuscript review. Young Ho Kim contributed to the study design, data collection, analysis and/or interpretation, drafting and editing of the article, and manuscript review. Cheolmin Yun contributed to the study conception, data interpretation, and manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Young Ho Kim, Cheolmin Yun, and Jaeryung Oh declare that they have no financial or nonfinancial competing interests in any of the products described in this study.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Korea University Hospital (IRB number: 2023AN0096), and the requirement for individual consent for this retrospective analysis was waived.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, Y.H., Yun, C. & Oh, J. Integrity of the Hyperreflective Layer in the Inner Choroid in Eyes with Drusen. Ophthalmol Ther 13, 529–540 (2024). https://doi.org/10.1007/s40123-023-00865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00865-z