Abstract
Introduction
There is growing evidence that confirms morphological changes in the posterior structures in patients with keratoconus (KC); however, isolated alterations could have been missed. This study assesses choroidal thickness (CT) in the fovea and beyond in KC eyes.
Methods
This prospective case–control and non-randomized study enrolled 107 eyes, 62 eyes of 62 patients with KC, and 45 age-matched eyes of 45 control subjects with axial length in the range of 22–26 mm. Swept-source optical coherence tomography (SS-OCT) was performed to manually measure the subfoveal choroidal thickness (SCT) using a single-line scan. CT was obtained automatically from the Early Treatment Diabetic Retinopathy Study (ETDRS) grid using the 12-lines radial scan pattern. A two-way repeated-measures analysis of variance (ANOVA) was conducted to evaluate CT variations among macular eccentricity, parafoveal area, and the interaction between both factors. CT was compared in all parafoveal areas between groups and subgroups of KC.
Results
SCT was significantly thicker in KC eyes (357 ± 57 µm) than in healthy eyes (325 ± 63 µm) (p < 0.001). Significant choroidal thickening was observed in the central ring and outer and inner rings of the temporal, superior, and inferior parafoveal macular areas (p < 0.001), except in the outer ring of the nasal macular zone (p > 0.05) of KC compared to healthy eyes. The CT significantly decreased from the center to the outer ring regardless of the presence of KC (p < 0.001). The choroid in the nasal macular zone was significantly thinner than that in the temporal, superior, and inferior parafoveal areas (p < 0.001).
Conclusions
The choroidal structure increased its thickness not only in the subfoveal area, but also in eight parafoveal areas of the ETDRS grid encompassing a wider area of macular examination. These findings demonstrate and corroborate that keratoconus is not a purely corneal disease. Furthermore, it confirms the role that the choroidal structure has in the pathophysiology of keratoconus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Keratoconus (KC) is not a purely corneal disease. There is growing evidence that confirms morphological changes of the posterior structures in the disease process. |
Most studies have focused on the manual measurement of choroidal thickness (CT), however, isolated alterations in the choroidal profile could have been missed. |
The aim of the present study was to assess the CT in patients with KC using the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. |
What was learned from the study? |
CT was increased under the fovea and beyond in KC eyes. |
There was a choroidal thickening in eight out of nine parafoveal sectors of the ETDRS grid in KC providing data on a much wider area of macular examination. |
The CT significantly decreased from the center to the outer ring regardless of the presence of KC. |
Subclinical KC showed a choroidal thickening although the disease is not yet well established. |
Introduction
Keratoconus (KC) is a progressive corneal disorder known to have a high impact on young patients’ quality of life worldwide, making it a true public health issue [1]. It is a bilateral, typically asymmetrical and sporadic disease that results in corneal protrusion and thinning, irregular astigmatism, progressive myopia, and visual acuity reduction [2]. Although it has historically been classified as a non-inflammatory condition, the growing evidence for inflammatory pathway activation, such as the increased levels of proinflammatory cytokines in the tear film of patients with KC and oxidative stress [3], should allow to redefine KC as a chronic inflammatory corneal disease [4, 5]. Moreover, atopia, eczema, allergy, and eye rubbing are associated risk factors that increase the development of KC [6]. In addition, KC can be linked to systemic pathologies such as Down’s syndrome and Turner’s syndrome, and Leber’s congenital amaurosis, etc. [7].
In recent times, more attention has been given to the surrounding structures of the eye, and it has been questioned whether KC is a purely corneal disorder or a corneal manifestation of a systemic condition [4, 5]. Some authors [8, 9] have confirmed anterior segment alterations in the scleral tissue, such as a homogeneity loss in collagen fibers [8], which affects the scleral geometry increasing the corneo-scleral shape of patients with KC [9]. However, interactions between anterior and posterior structures of the eye may be taking place and the internal ophthalmic structures may also be involved in the disease pathogenesis, which remains uncertain and is constantly under-changing [4].
Since optical coherence tomography (OCT) was established as a potential device to analyze in vivo posterior segment structures, the quantitative assessment of the retina and choroid has become a valuable tool in monitoring retinal diseases such as diabetic retinopathy, macular degeneration, etc. [10]. Currently, there is enough evidence that confirms morphological changes in different layers of posterior segment in KC subjects. Özsaygılı et al. [11] observed an alteration in some retinal layers’ thickness along with increasing KC severity. Nonetheless, the choroidal tissue is also subjected to morphological variations [12,13,14,15]. Gutierrez-Bonet et al. [12] used the enhanced deep imaging optical coherence tomography (EDI-OCT) technique to confirm that KC eyes presented thicker subfoveal choroidal thickness (SCT) than patients without KC. These findings have been contrasted a posteriori by many other authors [13,14,15]. It should be noted that in all of these studies, choroidal thickness (CT) was manually quantified with a caliper using a single-line, fovea-centered scan protocol. However, swept-source OCT has integrated built-in mapping software, and CT can be automatically and objectively calculated and shown with nine subfields defined by the Early Treatment Diabetes Retinopathy Study (ETDRS) style grid. This scan protocol avoids the loss of isolated alterations in the CT profile of previous works, their subjectivity, and added information about unexplored parafoveal areas in KC.
Hence, the main purpose of this study was to measure CT using the ETDRS style-grid to compare it to a healthy population which allows a more accurate and a wider morphological evaluation of the choroid for the scientific community. Indeed, it could be useful in clinical practice to create a normalized database in KC and to speed up the measurement process employed until now.
Methods
Data Collection
This prospective cross-sectional study was conducted in the anterior segment, cornea, and external disease unit of the Foundation of Medical Ophthalmology (FOM), Valencia, Spain, between April 2019 and March 2020. The Ethics Committee of the FOM Hospital approved the protocol and procedures of the study (PI93). This research was adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the participants.
All subjects were recruited from FOM and Optic and Optometry Department from University of Valencia, Valencia, Spain. Only one eye was randomly selected to avoid the possible bias through the choice of both eyes. Inclusion criteria for both populations were age ranged between 18 and 55 years, to avoid the age impact on CT [16], and axial length (AL), ranged between 22 and 26 mm, given the negative relation with CT [17]. All patients underwent a comprehensive ophthalmic examination including corrected distance visual acuity (CDVA), refractive error, slit-lamp biomicroscopy, AL measurement (IOL Master 700; Carl Zeiss Meditec, Jena, Germany), tomographic analysis (Pentacam HR; Oculus Optikgeräte GmbH, Wetzlar, Germany), intraocular pressure (IOP) and fundus examination.
Two groups of patients were considered to participate in the study, healthy subjects (control group, CG) and subclinical or previously diagnosed patients with KC (KC group, KG) by the corneal specialist, Cristina Peris-Martínez. The inclusion criteria in healthy eyes were: mean spherical equivalent (SE) between-5 diopters (D) and + 2 D, CDVA ≥ 0.0 log MAR (20/20) and no clinical signs on slit lamp. KC eyes have to present at least one KC clinical sign on slit lamp (Fleischer ring, Vogt striae, stromal thinning or anterior stromal scar) and an asymmetric bowtie pattern in corneal topography. Patients with any history of ocular trauma, retinal or other eye diseases, previous crosslinking (CXL), eye surgery, and the use of contact lenses and topical medications at least 2 weeks before the examination were excluded from the study. KC eyes with atopia or other external corneal diseases related to dryness and inflammation were excluded because the potential influencing of the inflammatory load in their orbit and their relationship with CT. [18]
The sample size was determined using a statistical power analysis with Software G* Power [19] and previously published data regarding CT in KC [12, 13, 15]. The software calculations suggested that at least 45 participants in each group would produce a statistical power of 80% to detect significant CT differences between CG and KG at a significant level of 0.05. However, KG was increased by 40% considering the possible disturbance of corneal opacity in OCT measurements.
The sample was stratified by considering the Belin-Ambrosio enhanced ectasia display (BAD-D index) and topographic KC classification (TKC) from Pentacam. Each eye was classified as control, BAD-D < 1.30 D, and KC, BAD-D ≥ 1.30 D. KC eyes were graded into four subgroups: (1) subclinical KC: TKC = possible or nothing, BAD-D ≥ 1.3 and < 3.5, and fellow eye with diagnosed KC; (2) Grade 1 or mild KC: TKC = 1 or 1–2 and BAD-D ≥ 3.5 and < 5.5; (3) Grade 2: TKC = 2 and BAD ≥ 5.5 and < 10; (4) Moderate KC: TKC = 2–3, 3 or 3–4 and BAD-D ≥ 10. The Pentacam software provided a cut-off value of 1.6 D and 3.0 of BAD-D to distinguish between normal, suspect and KC eyes, however, in order to have balanced groups this value was modified to 1.3 D and 3.5 D. If BAD-D index was out of established range more than 0.70 D, it was considered as mandatory criteria. This value was selected considering the KC repeatability study of Kreps et al. [20] The following corneal metrics provided by Pentacam were considered: thinnest corneal thickness (TCT), apical corneal thickness (APT), maximum keratometry (Kmax), and total corneal astigmatism (ACt).
Image Acquisition and Data Analysis
Posterior segment exploration was carried out after the initial screening of all participants after a break and wash-out period. The same trained examiner (Neus Burguera-Giménez) performed the choroidal analysis between 3:00 and 7:00 PM to limit the confounding effects of diurnal variations of CT [21]. Choroidal images were obtained using the DRI-1 Swept-Source (Topcon Medical, Tokyo, Japan), which presents the following specifications: a 1050-nm wavelength light source, a scanning speed of 100,000 A scans/s, an angle picture of 43°, and an in-depth and lateral resolution of 5 and 20 μm, respectively. Two pattern scans, both centered on the fovea, were used: one single-line and 12 radial-line B-scans. The OCT image of 12 mm provided by the single-line scan was used to determine the SCT by two masked observers (Neus Burguera-Giménez, María Amparo Díez-Ajenjo). SCT was manually quantified just under the fovea as the perpendicular distance between the outer boundary of the retinal pigment epithelium (RPE) and the inner boundary of the choroid-sclera junction (Fig. 1A). The OCT image of the 12 radial-line B-scans was used for the quantitative analysis of CT values and CT topography in the nine subfields based on the ETDRS grid.
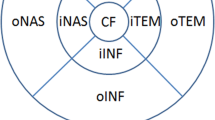
Scan patterns selected to analyze choroidal thickness (CT). A B-scan obtained from one single-line scan, where, subfoveal choroidal thickness (SCT) was manually measured as the axial distance between the retinal pigment epithelium (RPE) (dashed red line) and choroidal-scleral junction (dashed blue line). B Choroidal thickness (CT) map obtained from 12-mm radial B-scan, where CT was automatically provided in the central ring (1 mm in diameter), central CT (cCT), and in different macular sectors based on the Early Treatment Diabetic Retinopathy Study (ETDRS) style grid. The inner ring (3 mm in diameter) which includes the nasal inner macula (iNAS), superior inner macula (iSUP), temporal inner macula (iTEM), and inferior inner macula (iINF). The outer ring involves the nasal outer macula (oNAS), superior outer macula (oSUP), temporal outer macula (oTEM) and inferior outer macula (oINF)
A three-dimensional topographic map was automatically generated, which provided CT in nine subfields defined by the ETDRS style-grid (Fig. 1B). The subfoveal macula (1-mm central ring, cCT), inner ring (3 mm in diameter) which includes the nasal inner macula (iNAS), superior inner macula (iSUP), temporal inner macula (iTEM), and inferior inner macula (iINF). The outer ring includes the nasal outer macula (oNAS), superior outer macula (oSUP), temporal outer macula (oTEM) and inferior outer macula (oINF). The device has an automatic layer segmentation that identifies choroidal boundaries for each 12-mm radial scan. Then, using the built-in segmentation-modifying tool, each line was inspected individually by the two observers to verify both boundaries and if both agreed on the need for manual correction, the original segmentations were modified. Swept-source optical coherence tomography (SS-OCT) provided a choroidal index quality (CIQ) to evaluate the quality of the choroidal images. Hence, only scans with a CIQ score of ≥ 60 were included in the current study.
Statistical Analysis
Statistical analysis was performed using SPSS software version 28.0 (Chicago, IL, USA) for Mac OS. Quantitative variables were described using mean and SD values, and categorical variables were described using absolute and relative frequencies. The Kolmogorov–Smirnov test was used to verify the normal distribution in CG and KG, whereas KC subgroups were tested using the Shapiro–Wilk test. Welch’s t test was applied to correct for unequal sample size in the KC subgroups. A two-way repeated measures analysis of variance (ANOVA) was conducted to assess if there were any CT differences within rings (1-, 3- and 6-mm diameters, three levels), parafoveal sectors (nasal, temporal, superior, and inferior, four levels), and to check the interaction between rings and parafoveal areas. In all cases, post hoc analysis with Bonferroni adjustment was conducted if Mauchly’s sphericity test was not violated (p > 0.05). If not, the assumption of sphericity was not met and was calculated according to Greenhouse and Geisser [22] to correct the repeated-measures ANOVA. The Mann–Whitney U test or independent t test was used to compare parameters between groups. Spearman’s correlation (r) analysis was used to evaluate the possible correlation between anterior and posterior segment parameters. Agreement between cCT and SCT obtained from two pattern-scans were displayed using Bland–Altman plot. The level of significance was set at p < 0.05, for all comparisons (two-tailed).
Results
One hundred and seven eyes of 107 patients, 62 eyes of 62 patients with KC and 45 eyes of 45 age-matched healthy subjects were included (CG 32 ± 9.1 years, KG 30 ± 12 years; p = 0.33). Mean SE of the CG and KG was − 0.57 ± 1.3 D and − 2.19 ± 2.57 D (p = 0.002), and mean AL was 23.42 ± 0.92 mm and 24.1 ± 1.12 mm (p < 0.001). The demographic, refractive, and ocular characteristics of both groups are summarized in Table 1.
The SCT and CT values in each macular subfield of the ETDRS grid by main group are shown in Table 2. The coefficient of variation between the two observers in the manual SCT measurements was less than 4%. Manual correction on the 12 mm radial B-scan cases was needed in 70% of cases for the inner margin of the choroidal junction. The mean SCT was significantly higher in the KG than in the CG (357 ± 63 μm vs. 325 ± 57 μm, p = 0.01). The mean CT was significantly greater in the KG than in the CG in the central ring (331 ± 56 μm vs. 300 ± 53 μm, p = 0.006) and in seven parafoveal sectors of the ETDRS grid (all macular areas, p < 0.05), except in the outer nasal ring (KG, 223 ± 55 μm vs. CG, 237 ± 51 μm, p = 0.197). The topographic maps of both types of patients are shown in Fig. 2. The differences between both groups per each parafoveal sector are illustrated in Table 3. In the subfoveal area (SCT, cCT), there was a similar difference between groups (32 and 31 μm, respectively), whereas the nasal macular zone increased to a lesser extent in the inner (24 μm, 8.66%) and outer ring (14 μm, 6.30%) than in the temporal, superior, and inferior macular areas.
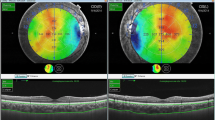
Early Treatment Diabetic Retinopathy Study (ETDRS) topographic map in a healthy subject (A) and in a grade 2 keratoconus (KC) patient (B) with its corresponding B-scan (bottom). The dashed white line, represented in the lower B-scan, shows the choroidal difference (white arrow) between both subjects. Significant choroidal thickening was observed in KC eyes in eight out of nine macular sectors of the ETDRS grid, except in the outer ring of the nasal area
There was an insignificant increase in CT from subclinical KC to grade 1 in most macular areas of the ETDRS grid, whereas from grade 1 to grade 3, it remained stable (p > 0.05, one-way ANOVA using Welch correction for unequal samples), Table 4.
Two-way repeated-measures ANOVA revealed that there was a large and significant effect of macular eccentricity and parafoveal sector on CT (p < 0.001 in both groups and factors). There was a considerable and significant interaction between eccentricity and parafoveal sector (p < 0.001 in both groups), Fig. 3. Hence, the correction was applied using one-way repeated-measures ANOVA for both factors. The results showed that the CT of the iNAS and oNAS were significantly thinner compared to the rest of the parafoveal sectors (p < 0.001 in all multiple pair-wise comparisons in both groups). Meanwhile, CT significantly decreased from subfoveal area with increasing macular eccentricity, regardless of the parafoveal area and group observed (p < 0.001, Fig. 4).
Interaction graphs for control (A) and keratoconus (B) groups obtained from the two-way repeated measures analysis of variance (ANOVA). Dots and error bars indicate the choroidal thickness (CT) means and standard deviations (SDs) of each ring: central, inner, and outer in the different parafoveal sectors of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. The upper box indicates the p value and effect size (ηp2) of the macular ring and parafoveal sector (green: healthy, pink: keratoconus). The lower violet box shows the interaction between both factors and their effect size
Topographic variations of choroidal thickness (CT) with macular eccentricity in control (blue line) and keratoconus (red line) groups in the nasal (A), temporal (B), superior (C), and inferior (D) parafoveal sectors. Dots and error bars indicate the choroidal thickness (CT) means and standard deviations (SDs). *A bold asterisk indicates the significant differences obtained from the one-way analysis of variance (ANOVA) repeated measurement when post hoc analysis with Bonferroni adjustment was conducted for pairwise macular eccentricity comparisons (***p < 0.001). Central choroidal thickness (cCT), nasal inner macula (iNAS), superior inner macula (iSUP), temporal inner macula (iTEM), inferior inner macula (iINF), nasal outer macula (oNAS), superior outer macula (oSUP), temporal outer macula (oTEM), and inferior outer macula (oINF)
The agreement between the two scan patterns used to perform SCT and cCT was low. The Bland–Altman plot in Fig. 5 depicts 95% limits of agreement (LOA) of − 27.3 to + 82 μm. The plot also illustrates a possible trend whereby SCT was thicker (mean difference = 27.4 μm) than cCT, which was automatically reported by the device instead of SCT that was manually quantified using a caliper.
Bland–Altman plot of the agreement in choroidal thickness measurements between the two scan patterns used: single-line (subfoveal choroidal thickness, SCT) and 12 single radial scans (central choroidal thickness, cCT). The blue line represents the mean choroidal thickness (CT) measurement difference between the two methods and the red dotted lines represent the 95% limits of agreement. SD standard deviation
There was a moderate correlation among all CT values and age, AL, and SE (r < − 0.3, p < 0.05; r > 0.5, p < 0.001; and r > 0.3, p < 0.001, respectively) and there was no correlation with the cylinder (p > 0.05). Partial correlation adjusted for age, gender, SE, and AL showed that there was no association of any CT area with TCT, APT, Kmax, ACt, BAD-D, and TKC (all p > 0.05).
Discussion
The aim of the current study was to assess CT using the ETDRS grid to evaluate whether there were differences in KC eyes compared to healthy subjects under the fovea and beyond. Choroidal thickening in KC has been previously described by numerous authors [12,13,14,15, 23,24,25], who quantified CT in different eccentricities using a single-line fovea-centered scan protocol with diverse OCT devices (Table 5). However, isolated alterations in the macular CT profile could have been missed and the subjectivity of the manual measurement could have affected the CT values, in contrast to our study.
The findings of the present study have shown that there was a significant choroidal thickening of approximately 29–34 μm in patients with KC not only under the subfoveal area, SCT, but also in eight out of nine parafoveal areas of the ETDRS grid, which depended on each observed parafoveal sector and ring (Table 3). Although an increase of 14 μm in the outer nasal area with respect to the healthy eyes was observed, this difference was not statistically significant. This could be because the gradual thickness decrease from the subfoveal zone to the external nasal macular zone was significantly greater than that of the other three zones (superior, inferior, and temporal). Consequently, CT does not increase in the same proportion as in these other regions, as Breher et al. [26] reported in healthy subjects and Singh et al. [27] who also described that the variation in choroidal vascularity is greater in the nasal macular area than in the rest of macular areas.
Our results are consistent with those of previous studies regarding CT in patients with KC where SCT increased at different naso-temporal macular eccentricities [12,13,14,15]. However, the SCT of our KC eyes was lower than that reported by Gutierrez-Bonet et al. [12] (369 μm) and Pinheiro-Costa et al. [13] (371.50 μm) who included established KC with TKC 1–2 and with a mean BAD-D of 9.25 ± 5.23 D, respectively. These values are consistent with KC grade 1–2 of the present study (375 μm), hence, this difference is due to the inclusion of subclinical KC in the main KC group, whereby SCT was 356 μm, and consequently, mean SCT might be reduced. Conversely, Yilmaz et al. [23] confirmed that the choroid of KC pediatric subjects does not suffer thickness changes because of the age of patients (12 ± 2 years), since KC disease has not yet manifested, and the alteration of CT would occur in puberty when the initial manifestations of the disease appear.
In general, the choroid profile was significantly greater in eyes with KC irrespective of manual or automatic measurements. In contrast, although an increment of the CT profile from subclinical KC to grade 1 was observed, the insignificant differences between each KC subgroup and control healthy subjects have also been described by other authors, who did not find a correlation between TKC and any point of SCT [12] and progressive and non-progressive KC, which presented similar SCT [24]. It should be noted that when factors associated with the CT profile were controlled, our findings demonstrated that TKC and BAD-D index were not linked with any CT of the ETDRS grid, as well as TCT, APT, Kmax, and ACt. Nonetheless, it would be interesting to increase the sample size of each subgroup to verify the trend observed in the current investigation. Notwithstanding that these findings have no statical significance, there is an important clinical relevance behind it because it could optimize the care protocol for patients with keratoconus. This could help to generate standard values of CT based on the ETDRS topographic map according to the severity of KC, which would facilitate the evaluation of this type of patients in the clinical practice, and allow specialists to consider whether they are within the pre-established standards. On the other hand, subclinical KC presented a clinically relevant choroidal thickening even though the disease is not well established which provide additional information about another possible optimal variable for the characterization of the disease.
Although the exact pathophysiological mechanism of gross CT changes in KC remains unknown, based on the extensive evidence in the literature [11,12,13,14,15], it can be associated with the underlying characteristic inflammatory mechanism of patients with KC [3,4,5]. The elevated expression of proinflammatory proteins, such as IL-1-, IL-6, and TNF-α, among others, in corneal tissue [28] and tear film [29], produce extracellular matrix degradation and, consequently, stromal thinning activated by matrix metalloproteinases. As some histological studies confirm, the choroid has been implicated in the pathogenesis of inflammatory diseases linked to uveitis, such as Vogt–Koyanagi–Harada (VKH) syndrome [30], Behçet’s disease [31], or psoriasis [32], etc. Elevated numbers of inflammatory cell have been observed at the choroidal stromal level, which produces greater vascular dilatation and stromal infiltration. This contributes to choroidal thickening during the inflammatory period and subsides after cortisone treatment [33]. Recently, Gutierrez-Bonet et al. [25] described that the vascular area of KC eyes undergoes a larger increment than the stromal area of healthy control subjects. They attributed this change to the augmentation in stromal infiltration and vascular dilatation, with the latter being the main cause of choroidal thickening. Moreover, Moleiro et al. [18] suggested that atopia could play a possible role in the choroidal profile of KC eyes due to the thickened choroid in patients with atopic KC compared to non-atopic KC.
Another proposed theory can be explained by alterations in the distribution of collagen fibers [34]. The corneal stroma is mainly composed of collagen type I, which suffers a significant reduction and shows a disorganized distribution in ectatic corneas. Since collagen type I is the main component of the media and adventitia of blood vessels and taking into account that the choroid is a highly vascularized structure mainly composed of blood vessels, the homogenization loss of these fibers could affect the blood vessel size and diameter, which could be translated with choroidal thickening in KC eyes.
The topographical variations in the CT profile using the ETDRS grid have been reported by numerous investigations [35, 36] and have enhanced the knowledge about CT profiles in healthy eyes without pathologies. However, this is the first study to analyze topographical variations in the KC disease. Our results showed that macular ring and parafoveal sector were two factors that had significant effects on the CT profile in both populations. The choroid shows its minimum thickness in the nasal macular area, being thickest in the central, temporal, and superior areas, followed by the inferior area, regardless of the presence of KC. These findings are supported by previous investigations in eyes without pathologies [35, 36]. However, based on the two theories that explain the mechanism of retinal thickness changes in KC, such as, the compensatory response of the posterior segment according to the Stiles–Crawford effect (SCE) of the first kind and another compensatory mechanism known as photostasis [37], and considering the increased astigmatism as KC progresses [1,2,3], we could expect that the CT profile in KC would be thickened to a greater extent in the superior and inferior hemifields of the ETDRS grid. According to this, our findings in KC showed that in the inner and outer rings of the superior parafoveal zone there was slight choroidal thickening (increment of 11.37 and 11.81%, respectively) compared to the horizontal areas. Meanwhile, the inferior macular area was the second increased zone in both rings (increments of 9.90 and 9.20%, respectively).
In the present study, CT was performed using two different measurement methods: the value measured manually with a caliper at the subfoveal level through the one-line scan pattern, and the value reported automatically by the device itself using a radial scan pattern of 12 lines. Our results showed that manually quantified CT was significantly higher than that automatically provided in the central ring of the ETDRS grid. This is because of the differences in the nature of the measurements. The value obtained through the caliper measures a specific point, while the value of the central ring measures the entire 1-mm ring that results from the average of the 12 B-scans, in which multiple points have been considered. This difference between both exploration methods was observed in healthy subjects and in patients with KC, which confirms that the difference is constant regardless of the presence or absence of the pathology. Therefore, when comparing the results with those described by other authors, it is important to consider the method used to obtain CT values, since similar measurement methods should be compared. These findings are consistent with those previously described by Moussa et al. [36]
Our study has several limitations. The measurement of SCT using a caliper could be a limitation; however, considering that the CT profile was measured objectively by the topographic map, this limitation was discarded. Both groups presented significant SE and AL differences, and CT profile was correlated with both factors; however, significant choroidal variations were observed in eyes with high myopia, SE > -6 D and AL > 26 mm [17], not included in the present study. It has to be noted that there is a negative relationship between AL and CT. Eyes with high myopia present a choroidal thinning. Therefore, if the choroidal thinning would affect CT in KG, the differences between both groups would be lower than that observed in the current study. In addition, these differences possibly would be non-statistically significant, unlike the findings of our study. Another aspect to consider is that most patients were White people, which may also limit the generalization of these results to other populations, considering the close relationship between KC and lineage [38]. The bounded sample size could be another limitation, as well as the unbalanced size of KC subgroups, but it should be taken into consideration that KC is a rare disease with less prevalence in white patients.
Conclusions
The results of the present study confirm that the choroidal structure increased its thickness not only in the subfoveal area but also in eight parafoveal areas of the ETDRS grid. These findings evidence that there is a choroidal thickening on a much wider region of macular examination which avoid the loss of isolated alteration of the choroidal profile of the previous studies. There is not a correlation between the increase of CT and KC degree. However, the current study demonstrated that subclinical KC showed a choroidal thickening although the disease is not yet well established. Indeed, this increase was lower than in cases with KC clinically well established. Moreover, as the literature confirm, our results corroborate that KC is not a purely corneal disease, and contribute to the role of the choroidal structure in the pathophysiology of KC. Further studies are required to generalize the present results in KC that may be used to create new insights regarding the CT profile and to establish a normalized database in patients with KC depending on KC severity using the topographic map based on the ETDRS grid.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author Neus Burguera Giménez (neus.burguera@uv.es) on reasonable request.
References
Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RPL. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169–72. https://doi.org/10.1016/j.ajo.2016.12.015.
Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. https://doi.org/10.1016/S0039-6257(97)00119-7.
Navel V, Malecaze J, Pereira B, et al. Oxidative and antioxidative stress markers in keratoconus: a systematic review and meta-analysis. Acta Ophthalmol. 2021;99(6):e777–94. https://doi.org/10.1111/AOS.14714.
Ferrari G, Rama P. The keratoconus enigma: a review with emphasis on pathogenesis. Ocul Surf. 2020;18(3):363–73. https://doi.org/10.1016/J.JTOS.2020.03.006.
Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A. Keratoconus: an inflammatory disorder? Eye. 2015;29:843–59. https://doi.org/10.1038/eye.2015.63.
Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263–70. https://doi.org/10.1097/ICO.0000000000002150.
Mas Tur V, MacGregor C, Jayaswal R, O’Brart D, Maycock N. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770–83. https://doi.org/10.1016/j.survophthal.2017.06.009.
Kopaeva VG, Zatulina NI, Legkikh LS. Clinical and morphological aspects of the state of the sclera in various types of genetically determined corneal changes. Vestn Oftalmol. 1992;108(2):17–9.
Piñero DP, Martínez-Abad A, Soto-Negro R, et al. Differences in corneo-scleral topographic profile between healthy and keratoconus corneas. Cont Lens Anterior Eye. 2019;42(1):75–84. https://doi.org/10.1016/j.clae.2018.05.005.
Michalewska, Zofia B, Michalewski, Janusz, Nawrocki J, Erzy. Swept-source OCT, wide-field simultaneous choroid, retina, and vitreous visualization. Retina Today. 2013;(September):50–56.
Özsaygılı C, Yıldırım Y. The relationship between keratoconus stage and the thickness of the retinal layers. Turk J Ophthalmol. 2021;51(2):75–82. https://doi.org/10.4274/tjo.galenos.2020.84748.
Gutierrez-Bonet R, Ruiz-Medrano J, Peña-Garcia P, et al. Macular choroidal thickening in keratoconus patients: swept-source optical coherence tomography study. Transl Vis Sci Technol. 2018;7(3):15. https://doi.org/10.1167/tvst.7.3.15.
Pinheiro-Costa J, Viana Pinto J, Perestrelo S, et al. Increased choroidal thickness in keratoconus patients: perspectives in the disease pathophysiology. J Ophthalmol. 2019;2019:1–7. https://doi.org/10.1155/2019/2453931.
Bilgin B, Karadag AS. Choroidal thickness in keratoconus. Int Ophthalmol. 2020;40(1):135–40. https://doi.org/10.1007/s10792-019-01156-y.
Akkaya S. Macular and peripapillary choroidal thickness in patients with keratoconus. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):664–73. https://doi.org/10.3928/23258160-20180831-03.
Ooto S, Hangai M, Yoshimura N. Effects of sex and age on the normal retinal and choroidal structures on optical coherence tomography. Curr Eye Res. 2015;40(2):213–25. https://doi.org/10.3109/02713683.2014.952828.
Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(2):314-319.e1. https://doi.org/10.1016/J.AJO.2012.07.015.
Moleiro AF, Aires AF, Alves H, et al. The role of atopy in the choroidal profile of keratoconus patients. Clin Ophthalmol. 2021;15:1799–807. https://doi.org/10.2147/OPTH.S301330.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Kreps EO, Jimenez-Garcia M, Issarti I, Claerhout I, Koppen C, Rozema JJ. Repeatability of the Pentacam HR in various grades of keratoconus. Am J Ophthalmol. 2020;219:154–62. https://doi.org/10.1016/j.ajo.2020.06.013.
Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Investig Ophthalmol Vis Sci. 2011;52(8):5121–9. https://doi.org/10.1167/IOVS.11-7364.
Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24(2):95–112. https://doi.org/10.1007/BF02289823.
Yilmaz I, Saracoglu Yilmaz B, Guleryuz NB, Perente I, Ozkaya A, Taskapili M. Assessment of the macula and choroid in pediatric keratoconus patients. Saudi J Ophthalmol. 2018;32(2):126–9. https://doi.org/10.1016/j.sjopt.2017.10.010.
Pinheiro-Costa J, Correia PJ, Pinto JV, et al. Increased choroidal thickness is not a disease progression marker in keratoconus. Sci Rep. 2020;10(1):19938. https://doi.org/10.1038/s41598-020-77122-x.
Gutierrez-Bonet R, Ruiz-Medrano J, Biarnés M, et al. Analysis of choroidal vascularity index in keratoconus patients using swept-source optical coherence tomography-based binarization techniques. J Ophthalmol. 2020;2020:1682463. https://doi.org/10.1155/2020/1682463.
Breher K, Terry L, Bower T, Wahl S. Choroidal biomarkers: a repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl Vis Sci Technol. 2020;9(11):8–8. https://doi.org/10.1167/TVST.9.11.8.
Singh SR, Invernizzi A, Rasheed MA, et al. Wide-field choroidal vascularity in healthy eyes. Am J Ophthalmol. 2018;193:100–5. https://doi.org/10.1016/J.AJO.2018.06.016.
Du G, Liu C, Li X, et al. Induction of matrix metalloproteinase-1 by tumor necrosis factor-α is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Exp Biol Med. 2016;241(18):2033. https://doi.org/10.1177/1535370216650940.
Shetty R, Ghosh A, Lim RR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56(2):738–50. https://doi.org/10.1167/iovs.14-14831.
Fong AHC, Li KKW, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt–Koyanagi–Harada disease. Retina. 2011;31(3):502–9. https://doi.org/10.1097/IAE.0B013E3182083BEB.
Ishikawa S, Taguchi M, Muraoka T, Sakurai Y, Kanda T, Takeuchi M. Changes in subfoveal choroidal thickness associated with uveitis activity in patients with Behçet’s disease. Br J Ophthalmol. 2014;98(11):1508–13. https://doi.org/10.1136/BJOPHTHALMOL-2014-305333.
Türkcü FM, Şahin A, Yüksel H, et al. Evaluation of choroidal thickness in psoriasis using optical coherence tomography. Int Ophthalmol. 2016;36(6):851–4. https://doi.org/10.1007/S10792-016-0212-9.
Kawano H, Sonoda S, Yamashita T, Maruko I, Iida T, Sakamoto T. Relative changes in luminal and stromal areas of choroid determined by binarization of EDI-OCT images in eyes with Vogt–Koyanagi–Harada disease after treatment. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):421–6. https://doi.org/10.1007/S00417-016-3283-4.
Meek KM, Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015;49:1–16. https://doi.org/10.1016/J.PRETEYERES.2015.07.001.
Touhami S, Philippakis E, Mrejen S, et al. Topographic variations of choroidal thickness in healthy eyes on swept-source optical coherence tomography. Investig Opthalmol Vis Sci. 2020;61(3):38. https://doi.org/10.1167/iovs.61.3.38.
Moussa M, Sabry D, Soliman W. Macular choroidal thickness in normal Egyptians measured by swept source optical coherence tomography. BMC Ophthalmol. 2016. https://doi.org/10.1186/s12886-016-0314-1.
Hashemi H, Heirani M, Ambrósio R, Hafezi F, Naroo SA, Khorrami-Nejad M. The link between keratoconus and posterior segment parameters: an updated, comprehensive review. Ocul Surf. 2022;23:116–22. https://doi.org/10.1016/j.jtos.2021.12.004.
Pearson AR, Soneji B, Sarvananthan N, Sanford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye. 2000;14(4):625–8. https://doi.org/10.1038/eye.2000.154.
Acknowledgements
The authors would like to thank the participants of the study and Global Surgical Service S.L. and University of Valencia for their support.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
This study was designed by Neus Burguera-Giménez, Mª. Amparo Díez-Ajenjo and Cristina Peris-Martínez; The methodology, software, data curation, and resources were performed by Neus Burguera-Giménez, Noemí Burguera and Celeste Briceno-Lopez. Neus Burguera-Giménez, Mª. Amparo Díez-Ajenjo and Cristina Peris-Martínez carried out the formal analysis and investigation. Writing—original draft preparation Neus Burguera-Giménez; writing-review and editing: all authors; visualization: all authors; supervision: Mª. Amparo Díez-Ajenjo and Cristina Peris-Martínez; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors Neus Burguera-Giménez, Mª. Amparo Díez-Ajenjo, Noemi Burguera, Celeste Briceno-Lopez, and Cristina Peris-Martínez do not have any conflicts of interest to disclose.
Ethical Approval
This prospective study was approved by the Institutional Ethics Committee of Fundación-Oftalmología Médica (FOM) (PI93) and conducted in accordance with the tenets of the Declaration of Helsinki. All participants provided written informed consent before participating in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burguera-Giménez, N., Díez-Ajenjo, M.A., Burguera, N. et al. Subfoveal and Parafoveal Choroidal Thickening in Patients with Keratoconus Using the ETDRS Grid on Swept-Source OCT. Ophthalmol Ther 13, 509–527 (2024). https://doi.org/10.1007/s40123-023-00858-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00858-y