Abstract
Introduction
This study explored the structural and microvascular changes in the optic nerve head (ONH) of patients with intracranial hypertension (IH) by using swept-source optical coherence tomography (SS-OCT)/OCT angiography (OCTA) and evaluated their association with clinical features.
Methods
The optic disc morphology, peripapillary retinal nerve fiber layer (pRNFL), ganglion cell–inner plexiform layer (GCIPL), and microvascular densities of the nerve fiber layer plexus (NFLP), superficial vascular plexus (SVP), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) were measured by the SS-OCT/OCTA tool. Frisen score, visual acuity, and intracranial pressure were assessed and recorded in patients with IH.
Results
Sixty-one patients with IH and 65 controls were included in this study. Patients with IH showed thicker pRNFL and GCIPL thickness with larger ONH rim area when compared to controls (P < 0.001). Microvascular densities were increased in NFLP while densities were reduced in SVP, ICP, and DCP when compared to controls (P < 0.001). Structural thickness and microvascular densities were significantly correlated with Frisen scores (P < 0.05) and intracranial pressure (P < 0.05) in patients with IH.
Conclusion
Structural and microvasculature variations of the ONH were found in patients with IH compared to controls. Importantly, we showed that structural and microvascular changes in the ONH were correlated with their Frisen score and intracranial pressure in patients with IH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Optic never head (ONH) is the main ocular site for intracranial hypertension; however, very little is known about microvessels in the ONH and their clinical manifestations. |
Could the microvessels in the ONH reflect the clinical manifestations such as intracranial pressure and severity of papilledema? |
What was learned from the study? |
When compared to controls, patients with intracranial hypertension (IH) had structural and microvascular vascular abnormalities; these changes in IH correlated with their intracranial pressure and the severity of papilledema. |
Imaging the optic nerve may be a potential tool for monitoring the structural and microvascular changes in patients with IH. |
Introduction
Intracranial hypertension (IH) is a clinical disorder characterized by signs and symptoms of increased intracranial pressure. Clinical features including papilledema and visual loss and/or impairment strongly indicate that increased intracranial pressure affects the optic nerve head (ONH), an observation that has been supported by numerous studies [1, 2]. The long-term consequence of papilledema can lead to optic nerve atrophy [3, 4], ultimately resulting in poor quality of life [5].
A sensitive and reliable method for monitoring papilledema development as a result of increased intracranial pressure is lacking, even though the severity of papilledema and vision loss constitutes a foundation for therapeutic interventions. It has been emphasized that more sensitive methods are needed for monitoring the ONH changes in IH and their response to treatment, as well as their long-term visual outcome [6].
Optical coherence tomography (OCT) is suggested as a reliable technique for in vivo visualization of the macula and ONH. Longer wavelengths and faster imaging speed enable swept-source OCT (SS-OCT), which can simultaneously image the ONH and microvasculature with OCT angiography (OCTA), to give retinal imaging with an increasing depth of detail.
There is little information available about ONH changes in IH utilizing SS-OCT. Using the spectral domain OCT (SD-OCT), previous reports [7,8,9] evaluated the morphological changes around the ONH in patients with IH compared to controls. With the longer wavelength, SS-OCT/OCTA would provide a more accurate tissue profile and information about the microvasculature around the ONH in patients with IH. The current study aimed to assess the structural and microvascular changes in the ONH of patients with IH and evaluate their association with clinical features using the SS-OCT/OCTA.
Methods
Study Population
Patients with IH were enrolled from April 2021 to March 2023 in the Neurology Department of West China Hospital. IH diagnoses were made following international diagnostic standards [10] which revealed an elevated intracranial pressure greater than 250 mmH2O confirmed by lumbar puncture. The exclusion criteria were (1) the presence of other cerebral vascular diseases (e.g., arteriovenous malformation, aneurysm, and major artery stenosis or occlusion); (2) the presence of intracranial infections, including meningitis caused by bacteria, fungus, and virus; (3) presence of other neurological diseases (e.g., brain tumor, hydrocephalus, degeneration diseases); (4) uncontrolled hypertension and diabetes mellitus which have well-established pathogenesis that can affect the retinal structural integrity; (5) medical ailment requiring concomitant corticosteroids or immunosuppressant therapy. Controls were individuals who attended our hospital for an annual checkup and had no history of any neurological or ophthalmological diseases.
The Ethics Committee of West China Hospital, Sichuan University, China (No. 2020 [922]) approved the study and followed the tenets of the Declaration of Helsinki. Each participant provided written informed consent before enrolling in our study.
Intracranial Pressure, Frisen Score, and Visual Acuity Assessment
An open pressure reading was taken at the L3–4 intervertebral space, left lateral position, after the fluctuating liquid levels in the manometer were stabilized.
A Frisen score was used to clinically grade each eye from 0 (normal) to 5 (severe papilledema) using the modified Frisen scale [11]. A single rater blinded to the clinical information and OCT/OCTA data assessed the Frisen score by fundus imaging of each participant. A second rater evaluated a random sample of 30 patients (60 eyes) to assess inter-rater agreement (kappa 0.91, P < 0.001).
The visual acuity (VA) of each eye of our participants was examined using the Snellen chart and later converted to a logarithm of the minimum angle of resolution (logMAR).
Fundus photography was performed on all participants to evaluate the ONH and fundus of all participants. Participants with abnormalities such as hemorrhages, microaneurysms, and hard exudates were excluded from our study. Two ophthalmologists (Guina Liu and Ruilin Wang) examined all images. Patients with IH also underwent intraocular pressure (IOP) examination. Patients with IOP > 21 mmHg or a previous diagnosis of glaucoma were excluded from our study.
Optic Nerve Head Imaging
The SS-OCT/OCTA tool (VG200S; SVision Imaging, Henan, China; version 2.1.016) contained a swept source laser with a central wavelength of 1050 nm and a scan rate of 200,000 A-scans per second. The tool is integrated with an eye tracker to compensate for the artifacts caused by blinking. The SS-OCT ONH scan covers an area of 6 × 6 mm focused on the optic disc. Ganglion cell and inner plexiform layer (GCIPL) was automatically segmented. In the ONH Angio mode, GCIPL, peripapillary retinal nerve fiber layer (pRNFL), and ONH rim data were obtained from the OCT tool. The GCIPL was the mean thickness of these layers in a 4.0-mm circle around the ONH. ONH rim was automatically evaluated by the OCT tool. ONH rim area was defined as the disc area minus the cup area. Automated placement of a circle with a diameter of 3.4 mm, centered on the disc, was used to measure the mean pRNFL as shown in Fig. 1. Manual adjustment of circle placement was performed if necessary.
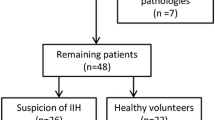
Flowchart of inclusion and exclusion criteria of participants and segmentation of OCT/OCTA images. a Flowchart of inclusion and exclusion criteria of participants. b Segmentation of structure thickness and ONH angiography imaging were performed in an area of 6 × 6 mm focused on the optic disc. The ONH rim area and mean peripapillary retinal nerve fiber layer (pRNFL) were then measured by automated placement of a circle 3.4 mm in diameter. Ganglion cell and inner plexiform layer (GCIPL) was automatically segmented by the OCT tool. En face angiograms of the nerve fiber layer plexus (NFLP), superficial vascular plexus (SVP), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) were generated by the OCTA tool
ONH angiography imaging was conducted using 6 × 6 mm scans. En face angiograms of the nerve fiber layer plexus (NFLP), superficial vascular plexus (SVP), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) were generated by the OCTA tool. NFLP was defined as the microvasculature found 5 µm below the inner limiting membrane (ILM) border to the nerve fiber layer (NFL) and ganglion cell layer (GCL) border. The SVP was defined as the microvasculature between the NFL/GCL border and the junction between the inner plexiform layer (IPL) and inner nuclear layer (INL); ICP was defined as the microvasculature between the IPL/INL border and the junction between INL and outer plexiform layer (OPL); DCP was defined as the microvasculature between the INL/OPL border and 25 µm below it as shown in Fig. 1. Average microvascular density was used to evaluate the ONH microvasculature which was generated by the OCTA tool. OCT/OCTA images with ophthalmic disorders such as age-macular degeneration, severe cataracts, optic neuritis, diabetic retinopathy, and optic neuritis were excluded. Individuals with low signal quality (SQ) on OCT/OCTA images (SQ < 6) were excluded from the study. The OSCAR-IB quality criteria [12] and APOSTEL recommendation [13] were followed in OCT/OCTA data.
Statistical Analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD) or expressed as the median and interquartile range (IQR) for skewed distributions; categorical variables were presented as frequencies and percentages. A comparison of demographic and clinical characteristics between patients with IH and controls was performed using the Fisher exact test for categorical variables and a t test for continuous variables. The differences in OCT/OCTA parameters between patients with IH and controls were performed by generalized estimating equations (GEE). GEE were also used to evaluate the correlations between OCT/OCTA data and clinical information. Covariates for GEE were set as age and gender while the correlation matrix in GEE was set as exchangeable. The added variable plot was used to show the partial correlation between clinical manifestations and OCT/OCTA parameters. The X-axis and Y-axis of added variable plots were the residuals of the dependent variable and the independent variable when both of these variables were regressed on the covariates (risk factors). P values less than 0.05 were considered statistically significant. All statistical analysis and plotting were conducted using R 4.2.3.
Results
A total of 121 eyes from 61 patients with IH (28 male, 33 female; mean age 34.51 years) and 130 eyes from 65 controls (23 male, 42 female; mean age 40.52 years) were included in our final analysis. Out of 121 eyes from patients with IH, 69 eyes had no or mild papilledema while 52 eyes had moderate or severe papilledema. The median Frisen score of patients with IH was 2 (IQR 1–4). The mean intracranial pressure was 291 mmH2O in patients with IH. Table 1 shows the demographics and clinical information of our study participants.
Comparison of OCT/OCTA Parameters Between Patients with IH and Controls
Figure 2 shows the differences in OCT/OCTA parameters between IH and controls. pRNFL and GCIPL structures of patients with IH were thicker when compared with controls (P < 0.001). Microvascular densities in SVP, ICP, and DCP of patients with IH were reduced (P < 0.001) while NFLP was increased significantly (P < 0.001) when compared with controls. Compared with controls, the ONH rim area was increased significantly (P < 0.001).
We stratified patients with IH into two groups (no/mild papilledema, Frisen score 0–2; moderate/severe papilledema, Frisen score 3–5) according to their Frisen scores. The ONH structure of eyes in the no/mild papilledema group was thinner than that in the moderate/severe group (P < 0.001, Supplementary Fig. 1). Microvascular densities were decreased in SVP, ICP, and DCP while NFLP was increased in the moderate/severe papilledema group (P < 0.001, Supplementary Fig. 1).
Association Between OCT/OCTA Parameters and Clinical Data
Structural thicknesses, ONH rim area, and microvascular densities showed a significant correlation with Frisen scores and intracranial pressure (P < 0.05, Figs. 3 and 4). No association was found between OCT/OCTA measures of ONH in patients with IH and visual acuity (P > 0.05, data not shown).
Correlation between OCT/OCTA parameters and intracranial pressure in patients with IH. pRNFL, GCIPL, ONH rim area, and NFLP positively correlated with intracranial pressure of patients with IH. SVP, ICP, and DCP densities inversely correlated with intracranial pressure of patients with IH. *P < 0.05
Discussion
The present study assessed the ONH structural and microvascular changes in patients with IH compared to controls. While comparable analyses of the structural alterations in the optic disc have been carried out, to the best of our knowledge, we provided the first study to focus on the ONH microvascular changes in patients with IH compared to controls. We showed that patients with IH had thicker ONH structures and larger ONH rim areas compared to controls. Besides, patients with IH had increased NFLP density and reduced SVP, ICP, and DCP densities in the ONH when compared to controls. Importantly we showed that thicker ONH structures and decreased microvascular densities in the ONH were associated with their intracranial pressure and Frisen score, highlighting the potential clinical relevance of these parameters.
Previous reports [7, 14, 15] using SD-OCT have shown the morphometric changes in and around the ONH and therefore our approach is not entirely new. Previous reports [7, 16] showed that patients with IH had larger ONH volumes compared to controls. SD-OCT uses a low-coherence light source and produces an interference spectrum by spectral splitting, while SS-OCT uses a broadband swept source whose wavelength varies over time [17]. With a longer wavelength and higher speed, the SS-OCT is a more recent advancement of the SD-OCT that allows for better and more detailed visualization of deeper microvasculature [18, 19]. Therefore, at deeper scanning depths, the SS-OCT is more sensitive and has a lower signal-to-noise ratio compared to the SD-OCT. Using SS-OCT we showed that patients with IH had thicker pRNFL and GCIPL thicknesses when compared to controls. We also showed that ONH rim area was larger in patients with IH compared to controls. We suggest that the thickening of the ONH structural parameters may be due to the increased intracranial pressure which may be transmitted to the expandable leptomeningeal sheath surrounding the optic nerve via passage of the excess cerebrospinal fluid into the tiny rim of the subarachnoid space between the sheath and the nerve as previously reported [20]. Taken together, since these structural parameters of the ONH were reflections of their neuroaxonal integrity, thicker pRNFL and GCIPL thicknesses, and ONH rim area may reflect swelling of the neuroaxons due to increased intracranial pressure.
By compressing the microvasculature due to swollen axons and extracellular fluid buildup as a result of edema, IH is thought to increase the risk of ischemia and decrease blood flow. This increases vascular resistance, venous congestion, and stasis, which in turn increases venous pressure and decreases perfusion pressure [21, 22]. This makes the ONH susceptible to ischemia in IH. Using SS-OCTA we showed that patients with IH had increased NFLP microvascular density and reduced SVP, ICP, and DCP densities when compared to controls. The NFLP contains capillaries, arterioles, and venules that reside in the NFL which extends beyond the peripapillary region [23]; it perfuses the NFL and forms a microvascular network in the peripapillary region making it sensitive to ischemic changes. The increased NFLP density seen in IH may be due to compression of capillaries, extracellular fluid accumulation in edema, and venous congestion. The SVP, ICP, and DCP are found beneath the NFLP; thus, reduced microvascular densities in these plexuses may be due to reduced blood flow from the NFLP to these microvascular plexuses.
We further divided the IH group according to their Frisen scores using the modified Frisen scale. We found that patients with moderate/severe papilledema IH had increased NLFP density, reduced SVP, ICP, and DCP density, and thicker ONH structural thicknesses compared to patients with no/mild papilledema IH. Clinical hallmarks of elevated intracranial pressure that affect the ONH include papilledema, the absence of spontaneous retinal venous pulsations, and peripapillary retinal vein engorgement [22]. We suggest that the severity of papilledema affects the ONH microvasculature and structure.
The main clinical method for evaluating papilledema in patients with IH is the Frisen score. It is proposed that in patients with newly diagnosed IH, assessments of papilledema utilizing more modern technologies, such as fundus photography and OCT, are associated with Frisen scores [11, 24]. Even though OCT/OCTA was used in clinical practice to evaluate structural and microvascular changes in ONH, we showed that Frisen score correlated with structural and microvascular changes in and around the ONH in patients with IH. Our findings suggest that imaging with the OCT/OCTA tool can be useful in the assessment of papilledema by confirming or complementing a clinician’s examination in patients with IH.
Previous reports [7, 25] showed a weak correlation between global ONH volume and intracranial pressure in patients with IH. We showed a strong correlation between intracranial pressure and structural and microvascular changes of ONH in patients with IH. Thus, it is plausible to suggest that OCT/OCTA measures may be sensitive indicators of increased intracranial pressure in patients with IH.
Structure–function correlations are important, in that moving from visual acuity examinations to objective clinical tools, such as OCT/OCTA, would be advantageous for patients and clinicians. Macular structural measurements are accurate and reliable indicators of visual acuity changes [26, 27]. Besides, previous reports [28,29,30,31] showed that structural macular thickness changes in patients with IH correlated with reduced visual acuity. However, our current study did not find an association between OCT/OCTA measures of ONH in patients with IH and their visual acuity. This may suggest that the structural and microvascular changes in and around the ONH of patients with IH may not be linked with visual acuity.
We would like to acknowledge some limitations. Firstly, assessment of the intracranial pressure was not done in our controls and thus changes seen in our patients with IH may not be predominantly from increased intracranial pressure as speculated. Future studies may be needed to evaluate how changes in the intracranial pressure may affect OCT/OCTA metrics. Additionally, the relatively small sample size and the cross-sectional design of our study limit the interpretation of the cause and effect of the results shown in our report. Longitudinal studies with larger sample sizes are needed to validate our findings. Moreover, participants with ocular comorbidities and inability to undergo OCT/OCTA imaging were excluded which might introduce selection bias. The strengths of this study include an assessment of the structural and microvascular parameters in ONH of patients with IH and a cohort spanning varying degrees of papilledema.
Conclusion
The ONH structure and microvasculature were significantly different in patients with IH compared to controls. Importantly, we showed that structural and microvascular changes in the ONH were correlated with their Frisen score and intracranial pressure in patients with IH. Patients with IH may benefit from monitoring ONH structural and microvascular changes with the OCT/OCTA tool.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Nusbaum DM, Wu SM, Frankfort BJ. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res. 2015;136:38–44.
Zhu Z, Waxman S, Wang B, et al. Interplay between intraocular and intracranial pressure effects on the optic nerve head in vivo. Exp Eye Res. 2021;213:108809.
Reier L, Fowler JB, Arshad M, et al. Optic disc edema and elevated intracranial pressure (ICP): a comprehensive review of papilledema. Cureus. 2022;14(5):e24915.
Wall M. Update on idiopathic intracranial hypertension. Neurol Clin. 2017;35(1):45–57.
Virdee J, Larcombe S, Vijay V, Sinclair AJ, Dayan M, Mollan SP. Reviewing the recent developments in idiopathic intracranial hypertension. Ophthalmol Ther. 2020;9(4):767–81.
Sinclair AJ, Burdon MA, Nightingale PG, et al. Rating papilloedema: an evaluation of the Frisen classification in idiopathic intracranial hypertension. J Neurol. 2012;259(7):1406–12.
Dreesbach M, Joachimsen L, Kuchlin S, et al. Optic nerve head volumetry by optical coherence tomography in papilledema related to idiopathic intracranial hypertension. Transl Vis Sci Technol. 2020;9(3):24.
Albrecht P, Blasberg C, Ringelstein M, et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J Neurol. 2017;264(7):1370–80.
Vijay V, Mollan SP, Mitchell JL, et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol. 2020;138(12):1264–71.
Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–65.
Fischer WS, Wall M, McDermott MP, Kupersmith MJ, Feldon SE, NORDIC Idiopathic Intracranial Hypertension Study Group. Photographic Reading Center of the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT): methods and baseline results. Invest Ophthalmol Vis Sci. 2015;56(5):3292–303.
Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE. 2012;7(4):e34823.
Aytulun A, Cruz-Herranz A, Aktas O, et al. APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2021;97(2):68–79.
Huang-Link YM, Al-Hawasi A, Oberwahrenbrock T, Jin YP. OCT measurements of optic nerve head changes in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2015;130:122–7.
Hoffmann J, Kreutz KM, Csapo-Schmidt C, et al. The effect of CSF drain on the optic nerve in idiopathic intracranial hypertension. J Headache Pain. 2019;20(1):59.
Malhotra K, Padungkiatsagul T, Moss HE. Optical coherence tomography use in idiopathic intracranial hypertension. Ann Eye Sci. 2020;5:7. https://doi.org/10.21037/aes.2019.12.06.
Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499–505.
Haas AM, Ahmed D, Stattin M, Graf A, Krepler K, Ansari-Shahrezaei S. Comparison of macular neovascularization lesion size by the use of spectral-domain optical coherence tomography angiography and swept-source optical coherence tomography angiography versus indocyanine green angiography. Acta Ophthalmol. 2021;99(2):e260–6.
Ting DS, Cheung GC, Lim LS, Yeo IY. Comparison of swept source optical coherence tomography and spectral domain optical coherence tomography in polypoidal choroidal vasculopathy. Clin Exp Ophthalmol. 2015;43(9):815–9.
Toscano S, Lo Fermo S, Reggio E, Chisari CG, Patti F, Zappia M. An update on idiopathic intracranial hypertension in adults: a look at pathophysiology, diagnostic approach and management. J Neurol. 2021;268(9):3249–68.
Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001;20(5):595–624.
Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016;50:108–44.
Hormel TT, Jia Y, Jian Y, et al. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res. 2021;80:100878.
OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group, Auinger P, Durbin M, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014;55(12):8173–9.
Kaufhold F, Kadas EM, Schmidt C, et al. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS ONE. 2012;7(5):e36965.
Charbel Issa P, Troeger E, Finger R, Holz FG, Wilke R, Scholl HP. Structure-function correlation of the human central retina. PLoS ONE. 2010;5(9):e12864.
Poh S, Tham YC, Chee ML, et al. Association between macular thickness profiles and visual function in healthy eyes: the Singapore Epidemiology of Eye Diseases (SEED) study. Sci Rep. 2020;10(1):6142.
Monteiro ML, Afonso CL. Macular thickness measurements with frequency domain-OCT for quantification of axonal loss in chronic papilledema from pseudotumor cerebri syndrome. Eye (Lond). 2014;28(4):390–8.
Nogueira PF, Caiado GC, Gracitelli CPB, et al. Association between optical coherence tomography measurements and clinical parameters in idiopathic intracranial hypertension. J Ophthalmol. 2021;2021:1401609.
Banerjee M, Phuljhele S, Saluja G, et al. Optical coherence tomography features and correlation of functional and structural parameters in patients of idiopathic intracranial hypertension. Indian J Ophthalmol. 2022;70(4):1343–9.
Kwapong WR, Cao L, Pan R, et al. Retinal microvascular and structural changes in intracranial hypertension patients correlate with intracranial pressure. CNS Neurosci Ther. 2023. https://doi.org/10.1111/cns.14298.
Funding
This work, including the journal’s Rapid Service fee, was supported by the National Natural Science Foundation of China (82071320, 8601022), the 1.3.5 project for disciplines of excellence of West China Hospital, Sichuan University (ZYGD18009), Post Doctor Research Project, West China Hospital, Sichuan University (2021HXBH081), Sichuan Science and Technology Program (2023NSFSC1558), Medical-Engineering Integration Interdisciplinary Talent Training Fund Project of West China Hospital, Sichuan University and University of Electronic Science and Technology of China (HXDZ22011/ZYGX2022YGRH017).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hang Wang, Le Cao, William Robert Kwapong, Bo Wu. Methodology: Hang Wang, Le Cao, William Robert Kwapong. Formal analysis and investigation: Hang Wang, Le Cao. Writing—original draft preparation: Hang Wang, Le Cao, William Robert Kwapong. Writing—review, and editing: Hang Wang, Le Cao, William Robert Kwapong, Junfeng Liu, Bo Wu. Funding acquisition: Junfeng Liu, Bo Wu. Supervision: William Robert Kwapong, Junfeng Liu, Bo Wu.
Corresponding authors
Ethics declarations
Conflict of interest
All named authors declare that they have no conflicts of interest.
Ethical Approval
The Ethics Committee of West China Hospital, Sichuan University, China (No. 2020 [922]) approved the study and followed the tenets of the Declaration of Helsinki. Each participant provided written informed consent before enrolling in our study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, H., Cao, L., Kwapong, W.R. et al. Optic Nerve Head Changes Measured by Swept Source Optical Coherence Tomography and Angiography in Patients with Intracranial Hypertension. Ophthalmol Ther 12, 3295–3305 (2023). https://doi.org/10.1007/s40123-023-00822-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00822-w