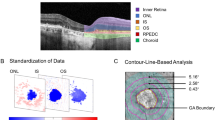
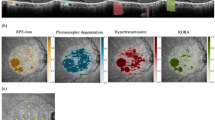
Abstract
Introduction
To evaluate the effect pegcetacoplan, a C3 and C3b inhibitor, on the rate of progression of geographic atrophy (GA) as assessed by spectral domain optical coherence tomography (SD-OCT) using a split-person study design and deep-learning quantification.
Methods
A post hoc analysis of phase 2 FILLY trial data comparing study (treated monthly, treated every other month and sham-treated) and fellow (untreated) eyes in a split-person study design was performed. This analysis included 288 eyes from 144 patients with bilateral GA from the FILLY phase 2 trial (Clinical Trials identifier: NCT02503332). Only patients with bilateral GA and without evidence of choroidal neovascularisation in either eye were included. Patient study eyes were treated with sham injections or with pegcetacoplan monthly (PM) or every other month (PEOM) for 12 months. SD-OCT scans of study and fellow eyes taken at baseline and 12 months were used for the analysis. The main outcomes were the annual change in the area of retinal pigment epithelial and outer retinal atrophy (RORA), its constituent features (photoreceptor degeneration [PRD], retinal pigment epithelium [RPE] loss, hypertransmission) and intact macula as compared to the untreated fellow eye.
Results
Annual GA growth was reduced in eyes treated with PM versus untreated fellow eyes for OCT features, including RORA (study eye 0.792 vs. fellow eye 1.13 mm2; P = 0.003), PRD (0.739 vs. 1.23 mm2; P = 0.015), RPE-loss (0.789 vs. 1.17 mm2; P = 0.007) and intact macula (− 0.735 vs. − 1.29 mm2; P = 0.011). Similar (but not statistically significant) trends were observed with the PEOM treatment or when GA was quantified with fundus autofluorescence (FAF). The sham treatment demonstrated no effect. Pearson correlation coefficients showed concordance in the enlargement rate of GA between the study and fellow eyes in the sham (R = 0.64) and PEOM (R = 0.68) groups, but not in the PM group (R = 0.21).
Conclusions
Pegcetacoplan-treated eyes demonstrated a reduction in spatial GA progression compared to their untreated counterparts. This effect was more evident on OCT than with FAF.
Trial Registration
Clinical Trials identifier: NCT02503332.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Geographic atrophy (GA) is a major vision-threatening manifestation of age-related macular degeneration. It is one of the leading causes of blindness globally and without a treatment in clinical use. |
FILLY is a phase 2 trial evaluating efficacy of intravitreal pegcetacoplan complement C3 and C3b inhibitor on slowing GA progression; only one eye per participant was randomised to a treatment group, and outcomes reporting have focused on the study eyes to date. |
As most participants had bilateral GA, patient-level analysis is possible. Under the twin assumptions of relative intra-individual GA symmetry and beneficial effect of pegcetacoplan, we hypothesised that eyes in groups with the C3 and C3b inhibitor treatment would progress at a slower rate than their fellow eyes. |
What was learned from the study? |
Monthly pegcetacoplan treatment was associated with slower growth of retinal pigment epithelium and outer retinal atrophy (RORA) and its features (retinal pigment epithelium loss, photoreceptor degeneration) when comparing study and untreated fellow eyes using spectral domain optical coherence tomography imaging. |
The treatment effect of pegcetacoplan was most pronounced with monthly intravitreal treatment. Similar but not statistically significant associations were seen in the case of pegcetacoplan treatment every other month or when the effect was analysed using fundus autofluorescence imaging. |
Introduction
Geographic atrophy (GA) is the defining lesion of late non-neovascular age-related macular degeneration (AMD), characterised by the progressive and irreversible loss of photoreceptors, retinal pigment epithelium (RPE) and underlying choriocapillaris. It can affect over 3.5% of those aged > 75, of which 40% report vision below 6/60 [1, 2]. Involvement of one eye has been hypothesised to increase the chance of GA development in the fellow eye. Indeed, up to 65% of patients with GA have bilateral involvement [3, 4]. The pathophysiology underlying GA is a complex interplay of environmental, physical and genetic factors, which explains the high observed concordance in the rate of GA progression within an individual but significant variability between individuals [4, 5]. A high level of bilateral involvement and similar rates of progression make it an amenable disease model for split-body studies.
In trials, randomisation can be used to overcome patient heterogeneity and confounding, which is inherently accounted for in split-body experiments [6]. Eyes present a unique setting in which there is a direct comparator against treatment that is less affected by the complex interplay of genetic or environmental factors that act on the population level, especially in conditions that are frequently bilateral. Key ophthalmic examples include trials in gene therapy for choroideremia or Leber’s hereditary optic neuropathy (LHON), surgical treatments of conditions such as myopia and explorations of the association between cataract surgery and AMD [7,8,9].
Intravitreal pegcetacoplan, a complement C3 and C3b inhibitor, has demonstrated safety and efficacy in slowing GA progression in the FILLY phase 2 trial [10]. In that trial, only one eye per enrolled participant was randomised to a treatment group, and outcomes reporting have focused on the study eyes to date. Yet most participants had bilateral GA and longitudinal imaging of both eyes, thus enabling patient-level analysis. Under the twin assumptions of relative intra-individual GA symmetry and beneficial effect of pegcetacoplan, we have hypothesised that eyes in groups with the C3 and C3b inhibitor treatment would progress at a slower rate than their fellow eyes. Conversely, no difference would be expected between study and fellow eyes in the sham treatment group. This study sets out to be the first to compare both study and fellow eyes in reporting pegcetacoplan efficacy free of inter-individual heterogeneity.
This post hoc analysis applies an externally validated deep learning-based model to optical coherence tomography (OCT) data captured in FILLY, automatically quantifying GA and its constituent features [11]. Moreover, novel OCT biomarkers of GA growth were considered, including photoreceptor degeneration (PRD) in isolation and macula free of GA changes (i.e. ‘intact macula’) [12]. To this end we assessed the effect of an intravitreal complement inhibitor by comparing rates of GA progression between treated eyes and their untreated counterparts across the treatment groups randomised to either pegcetacoplan or sham.
Methods
Subjects and Data Source
The FILLY phase 2 trial (Clinical Trials identifier: NCT02503332) reported the effect of pegcetacoplan on GA as evaluated using fundus autofluorescence [13]. This trial included 246 study subjects, with a single eye per person randomised into one of three treatment arms (intravitreal pegcetacoplan monthly [PM] or every other month [PEOM] and a pooled sham group including monthly and PEOM sham injections). This study involves human participants who were enrolled as patients at 46 sites in the USA (New England Institutional Review Board, University of Miami, Mayo Clinic, Institutional Review Board of the Cleveland Clinic Foundation, Duke University Health System Institutional Review Board, and Research Compliance Office Stanford University), Australia (Bellberry Ltd), and New Zealand (Northern A Health and Disability Ethics Committee, Health and Disability Ethics Committees, and Ministry of Health). The study was performed in accordance with the tenets of the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines and all applicable regulations. Institutional review board or ethics committee approval was obtained at each site. All patients provided written informed consent.
The present analysis only considered patients with spectral domain (SD)-OCT imaging acquired using the Spectralis ophthalmic imaging platform (Heidelberg Engineering, Heidelberg, Germany) with an acquisition density of at least 25 B-scans per volume at both baseline and 12 months post-baseline. In addition, patients with either unilateral GA or choroidal neovascularisation (CNV), an exudative complication of AMD characterised by growth of aberrant vessels, were excluded from the analysis (Electronic Supplementary Material [ESM] Fig. S1), leaving a total of 144 patients (1 study eye and 1 fellow eye per patient).
Outcomes
The primary outcome of the analysis was a comparison between study eyes and their untreated counterparts in terms of mean change of RPE and outer retinal atrophy (RORA) from baseline to 12 months (in mm2). Secondary outcomes included change from baseline to 12 months for the constituent OCT features of RORA as per the CAM (Classification of Atrophy Meetings) definitions (defined as homogeneous choroidal hypertransmission and absence of RPE measuring at least 250 μm with overlying outer retinal thinning and photoreceptor loss), PRD in isolation (defined as PRD without overlapping RPE-loss or hypertransmission) and macula completely absent of any GA changes (intact macula). A non-OCT-based secondary outcome of interest was the area of GA quantified by manual segmentation of fundus autofluorescence (FAF) images [11]. Finally, Pearson correlation coefficients were used to assess the symmetry of baseline areas of GA as measured by both OCT and FAF, as well as concordance in growth rate by treatment group (RORA only).
Automatic Segmentation of GA Features from OCT Images
The model used to automatically quantify GA features from OCT images has been previously published [14]. The area included in this analysis was restricted to the macula, as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) region, a circle measuring 6 mm in diameter centred around the fovea [15]. This allowed for accurate and near-instantaneous quantification of area from available OCT volumes.
Statistical Analysis
All data analyses were carried out with R (https://www.r-project.org/) [16]. As a rule, the characteristic of interest could not be approximated using the normal curve, and non-parametric tests (Wilcoxon rank test) were used for comparisons. Statistical tests were two-sided with alpha = 0.05. The Pearson correlation coefficient was used to compare change in treated and their paired untreated eyes.
Results
Baseline Characteristics of Study Cohort
Of the 246 patients enrolled in the FILLY study, 144 had bilateral GA and therefore were taken forward for analysis (ESM Fig. S1). In the present analysis, these patients with bilateral GA were on average (SD) 79.4 (7.54) years of age, predominantly female (63%), and Caucasian (96%). This was also observed when sub-stratified by treatment group (Table 1). These demographic parameters of patients with bilateral GA did not observably differ from patients with unilateral GA or CNV (Table 1).
GA Lesion Characteristics are Symmetrical at the Patient Level
The GA lesions were characterised using both FAF and OCT imaging at baseline (Table 1c; Fig. 1). The total areas of GA lesions between study and fellow eyes were compared to query individual-level differences (i.e. inter-eye lesion symmetry) (Fig. 1; ESM Table S1); a strong positive concordance in baseline GA size was demonstrated when measured by either FAF (Pearson correlation coefficient = 0.67, P < 0.001) or OCT (RORA taken to be GA; Pearson correlation coefficient = 0.71, P < 0.001) (Fig. 1a). Indeed, the absolute difference between the study and fellow eyes was right-skewed with a median difference of 2.45 mm2 (mean 3.30 mm2, SD 2.87) when using FAF to query GA (Fig. 1b;ESM Table S1). A lower median difference at the individual level was observed when using OCT to quantify GA: 1.92 mm2 (mean 2.48 mm2, SD 2.13; Pearson correlation coefficient = 0.71, P < 0.001). Similarly, each of the constituent features of GA at the OCT level was also symmetrical at the patient level, including PRD (median 2.01 mm2; Pearson correlation coefficient = 0.73, P < 0.001); PRD in isolation (median 1.37 mm2; Pearson correlation coefficient = 0.72, P < 0.001); and RPE-loss (median 1.90 mm2; Pearson correlation coefficient = 0.71, P < 0.001).
Inter-eye correlation of GA lesion size at baseline. A Scatterplot of GA lesion size between study eye (x-axis) and fellow eye (y-axis) as measured by FAF (blue) and OCT (RORA taken to signify GA; purple). Constituent features of GA were also considered: PRD (yellow), PRD isolated (green), RPE-loss (black) and HTR (red). Pearson correlation coefficients and P values are shown. B Box and whiskers plot depicting the distribution of absolute differences in feature area between study and fellow eyes for each of the features. FAF fundus autofluorescence, GA geographic atrophy, HTR hypertransmission, OCT optical coherence tomography, PRD photoreceptor degeneration, RORA retinal pigment epithelial and outer retinal atrophy, RPE retinal pigment epithelium, GA geographic atrophy
Asymmetrical GA Growth Suggests Monthly C3 and C3b Inhibition Slows GA Growth
The growth of GA at 12 months following baseline was compared for study eyes and untreated fellow eyes across each of the treatment groups (Table 2; Fig. 2; ESM Fig. S2). Nearly all OCT features of the GA growth rate were demonstrably slower in the study eyes of the PM treatment group when compared to corresponding untreated fellow eyes, including: RORA (study eye 0.792 mm2 vs. fellow eye 1.13 mm2, P = 0.003), PRD (0.739 mm2 vs. 1.23 mm2, P = 0.015), RPE-loss (0.789 mm2 vs. 1.17 mm2, P = 0.007) and intact macula (− 0.735 mm2 vs. − 1.29 mm2, P = 0.011); the exception was hypertransmission (HRT; 1.18 mm2 vs. 1.04 mm2, P = 0.186) (Table 2). In the PEOM treatment group, mean growth of GA was nominally lower in the study eyes than in the fellow eyes for any of the OCT features (RORA: study eye 1.14 mm2 vs. fellow eye 1.44 mm2, P = 0.353; PRD: 1.10 mm2 vs. 2.08 mm2, P = 0.104; RPE-loss: study eye 1.16 mm2 vs. fellow eye 1.58 mm2, P = 0.286; intact macula: − 1.22 mm2 vs. − 2.17 mm2, P = 0.121). When comparing study and fellow eyes in the sham group, average growth rates for OCT correlates of GA were not consistently lower in study eyes and a statistically significant difference was not observed. The annual growth rate of GA was also considered with FAF imaging (Table 2;Fig. 2). Here, a statistically significant difference was not detected between study and fellow eyes for any of the treatment groups.
Change in GA area (mm2) from baseline. The change in area for study eyes (black dotted line) and fellow eyes (blue solid line) from each treatment arm are plotted over time with their corresponding confidence intervals at 6 and 12-month timepoints. This is shown by treatment arm (SHAM, PEOM and PM) and by imaging modality (FAF as well as OCT features: RORA, PRD, PRD [isolated], RPE-loss, HTR and intact macula). Peg EOM pegcetacoplan every other month, PM pegcetacoplan monthly, SHAM sham injected, FAF Fundus autofluorescence, PRD photoreceptor degeneration, RORA retinal pigment epithelial and outer retinal atrophy, RPE retinal pigment epithelium, SD standard deviation, PRD photoreceptor degeneration, OCT optical coherence tomography, GA geographic atrophy, HTR hypertransmission
An alternate way of visualising treatment effect on GA growth is represented in Fig. 3, which plots the distribution of patient-level RORA growth at 12 months around a line of no effect. In the case of no treatment effect, one would expect differences in growth between the study and fellow eyes to be normally distributed with the line as its origin and approximately half the values on either side. A treatment effect would be observed as asymmetrical growth, with slower growth observed in the treated eyes than in the untreated fellow eyes. In the sham group, approximately half of its participants fell on each side of the line of no effect. The correlation of enlargement rates between eyes was high (R = 0.64), suggesting high concordance of GA growth. Individual-level GA growth was higher in the fellow eye than in the study eye for 55% (32/58) versus 45% (26/58) of patients, respectively. The average (SD) distance to the line of no effect was 0.1 (1.2) mm2. Conversely, the mean (SD) difference in GA area between the fellow and study eye in the PEOM group was 0.42 (1.2) mm2, with 69% (36/52) of patients having faster GA growth in their fellow eye. Overall, concordance between fellow and study eye remained high, with a correlation coefficient of R = 0.68. In the PM group, however, a significant correlation of GA growth was no longer observed between fellow and study eyes (R = 0.21; P = 0.23). In the PM group, 75% (42/56) of patients had a higher GA growth rate in the fellow eye at 12 months, and the mean (SD) difference between eyes was 0.45 (1.2) mm2. Assuming that GA growth is symmetrical at the individual level, these data suggest that a mean 0.45 mm2 and 0.42 mm2 of RORA over 1 year was prevented by monthly and every other monthly pegcetacoplan therapy, respectively, with the caveat of preserved inter-eye concordance and lack of statistical significance for GA area growth comparisons in the PEOM group.
Patient-level growth of GA at 12 months. A Patient-level change in RORA at 12 months. Dotted line through origin with gradient of 1 is taken to be line of no treatment effect. Pearson correlation coefficients and P values for the concordance in enlargement rate between eyes are shown. B Individual-level GA growth in study versus fellow eyes, presenting the number of patients in each treatment arm with greater progression in study versus fellow eye. C Distance to no effect line by treatment arm, representing the mean (SD) change in RORA in each treatment arm. EOM Every other month, SD standard deviation, GA geographic atrophy, RORA retinal pigment epithelial and outer retinal atrophy, PEOM pegcetacoplan every other month, PM pegcetacoplan monthly
Discussion
In sham-controlled randomised phase 2 and 3 trials, intravitreal C3 and C3b inhibition resulted in significantly slowed mean growth rates of GA in the pegcetacoplan treatment groups when compared to sham treatment [10, 17, 18]. In the present study, we demonstrate that in a split-body post hoc analysis of the phase 2 FILLY study cohort, the treatment effect of pegcetacoplan can also be observed in treated study eyes compared to their treatment-naïve fellow eyes. The effect on mean GA growth rate was significant for monthly pegcetacoplan treatment. A similar trend was observed on an every-other-month treatment pattern, but not in the sham control group. On an individual patient level, changes in GA area for the fellow eye exceeded those in the study eye for most patients in the pegcetacoplan treatment groups, while changes in the sham group approached randomness between study and fellow eyes.
In our study we used FAF as well as OCT-based segmentation to assess change in GA and its constituent features. Bilateral GA concordance has previously been demonstrated for FAF- [3, 5] and CFP (colour fundus photography)-based GA imaging [4, 19, 20] (ESM Table S2). One study using OCT-based GA detection found no statistical difference in GA progression between eyes if bilateral GA was present at any specific location measured [3]. Our data are consistent with these reports, as all OCT-based microstructural markers of GA, including PRD, RPE-loss and full RORA, showed inter-eye correlation coefficients of > 0.7 at baseline. For the eye pairs in the sham treatment group, the correlation observed at baseline was further maintained over time, and on average, there was a similar change observed versus baseline between fellow and study eye at the 12 month observation time point. Conversely, monthly pegcetacoplan treatment disrupted concordant GA growth rates, in favour of the study eye.
FAF has been used in clinical trials, however OCT-based imaging provides an opportunity to examine other biomarkers of GA progression and the latter modality is likely to be preferred, as noted by the Classification of Atrophy Meetings (CAM) Consensus group [21]. Our post-hoc analysis of FILLY data suggests that FAF can underestimate treatment efficacy if used as a sole diagnostic tool. For FAF, statistically significant differences in GA growth rate were not detected between study and fellow eyes for any of the analysis groups; in contrast, significant differences were seen with OCT-based analysis of GA area growth as well as change in the microstructural markers of GA in the monthly pegcetacoplan treatment group (PM). OCT-based methods have the additional advantage of visualising and quantifying the microstructural changes within the retinal layers, allowing additional insights into the pathophysiology of GA. It is critical to note that the data presented here are of a sub-cohort of the FILLY trial—those with bilateral GA and without CNV in fellow eyes imaged on the Heidelberg platform; as such, our results are not directly comparable to the FILLY results.
We observed no statistically significant difference between fellow and study eyes for rates of growth in areas of RPE-loss or PRD in the sham treatment arm, suggesting that without treatment not only RORA but also its individual components progress at similar rates in paired eyes. The finding that monthly pegcetacoplan can slow RPE-loss and PRD as well as RORA may serve as further evidence that complement inhibition is a disease-modifying pathway in GA.
Adequately powered parallel-group randomised trial designs are the gold standard for efficacy and safety studies. Yet, the eyes are a prominent example of a complex physiological system, in which split body experiments are not only possible, but have been successfully applied in cases where parallel-group trials are not feasible or desirable [22, 23]. In the context of GA, fellow eye analysis can serve as an additional control for the specificity of observed changes [24] because split-body experiments inherently control for many patient factors, reducing the effects of inter-patient heterogeneity. The present posthoc analysis corroborates findings from the FILLY, OAKS (Clinical Trials identifier: NCT03525613), and DERBY (Clinical Trials identifier: NCT03525600) trials, and adds to the slowly growing body of evidence to support that intravitreal C3 inhibition slows mean growth rates of GA [10, 24].
This retrospective post-hoc study was limited by the number of eyes with appropriate pathology as well as relevant imaging with Heidelberg Spectralis, which reduced the numbers that could be enrolled. Furthermore, intravitreal therapies in AMD, namely anti-vascular endothelial growth factor therapies, have previously been shown to likely cross the blood-retina barrier and exert an effect on the other eye [25, 26]. Despite the local administration this is a possibility and may be considered in follow up studies.
Conclusion
In conclusion, C3 and C3b inhibition protects eyes from spatial GA progression compared to their treatment-naive fellow eyes. Treatment effects are most pronounced with monthly intravitreal treatment. This study further supports the use of OCT data in documenting GA, as it affords additional insights into retinal morphological markers of GA pathophysiology and is more sensitive to GA progression than other imaging modalities.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to data sharing agreements with research collaborators however can be requested from Apellis Pharmaceuticals Inc.
References
Vingerling JR, Dielemans I, Hofman A et al. The prevalence of age-related maculopathy in the rotterdam study. Ophthalmology. 1995;102:205–10. https://doi.org/10.1016/s0161-6420(95)31034-2.
Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–91.
Fleckenstein M, Adrion C, Schmitz-Valckenberg S et al. Concordance of disease progression in bilateral geographic atrophy due to AMD. Invest Ophthalmol Vis Sci. 2010;51:637–42.
Sunness JS, Margalit E, Srikumaran D et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–7.
Bellmann C, Jorzik J, Spital G, Unnebrink K, PauleikhoffD. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Arch Ophthalmol. 2002;120:579. https://doi.org/10.1001/archopht.120.5.579.
Krummenauer F, Dick B, Schwenn O, Pfeiffer N. The determination of sample size in controlled clinical trials in ophthalmology. Br J Ophthalmol. 2002;86:946–7. https://doi.org/10.1136/bjo.86.9.946.
Malecaze FJ, Hulin H, Bierer P et al. A randomized paired eye comparison of two techniques for treating moderately high myopia: LASIK and artisan phakic lens. Ophthalmology. 2002;109:1622–30.
Wang JJ, Sze-Un Fong C, Rochtchina E et al. Risk of age-related macular degeneration 3 years after cataract surgery: paired eye comparisons. Ophthalmology. 2012;119:2298–303.
Guy J, Feuer WJ, Davis JL et al. Gene therapy for LHON: low and medium dose results (S26.004). Neurology. 2017; 88(16 Suppl).
Liao DS, Grossi FV, El Mehdi D et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–95.
Sadda SR, Guymer R, Holz FG et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125:537–48.
Fu DJ, Glinton S, Lipkova V et al. Deep-learning automated quantification of longitudinal OCT scans demonstrates reduced RPE loss rate, preservation of intact macular area and predictive value of isolated photoreceptor degeneration in geographic atrophy patients receiving C3 inhibition treatment. Br J Ophthalmol. 2023. https://doi.org/10.1136/bjo-2022-322672.
Apellis Pharmaceuticals, Inc. (sponsor) Study of pegcetacoplan (APL-2) therapy in patients with geographic atrophy (FILLY)—full text view. ClinicalTrials.gov. 2023. https://clinicaltrials.gov/ct2/show/NCT02503332. Accessed 07/09/2023.
Zhang G, Fu DJ, Liefers B et al. Clinically relevant deep learning for detection and quantification of geographic atrophy from optical coherence tomography: a model development and external validation study. Lancet Digit Health. 2021;3:e665–75. https://doi.org/10.1016/s2589-7500(21)00134-5.
Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 2020;127:S99–119.
RStudio Team. RStudio: integrated development environment for R. Boston: RStudio Inc.; 2020.
Apellis Pharmaceuticals, Inc. (sponsor). A study to compare the efficacy and safety of intravitreal APL-2 therapy with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration. 2023. https://clinicaltrials.gov/ct2/show/NCT03525613. Accessed 7 Sept 2023
Apellis Pharmaceuticals, Inc.(sponsor). Study to compare the efficacy and safety of intravitreal APL-2 therapy with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration. 2023. https://clinicaltrials.gov/ct2/show/NCT03525600. Accessed 7 Sept 2023
Klein R, Meuer SM, Knudtson MD, Klein BEK. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye study. Am J Ophthalmol. 2008;146:692–9.
Lindblad AS, Lloyd PC, Clemons TE et al. Change in area of geographic atrophy in the age-related eye disease study: AREDS report number 26. Arch Ophthalmol. 2009;127:1168–74.
Holz FG, Sadda SR, Staurenghi G et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:464–78.
Newman NJ, Yu-Wai-Man P, Carelli V et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. 2021;128:649–60.
MacLaren RE, Groppe M, Barnard AR et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37.
Pfau M, Schmitz-Valckenberg S, Ribeiro R et al. Association of complement C3 inhibitor pegcetacoplan with photoreceptor degeneration beyond areas of geographic atrophy. Sci Rep. 2022;12(1):17870. https://doi.org/10.1101/2022.06.20.22276634.
IVAN Study Investigators, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Hanhart J, Tiosano L, Avenbukh E et al. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye. 2014;28:646–53.
Funding
Funding for the study and rapid service fee was provided by the NIHR Biomedical Research Center at Moorfields Eye Hospital NHS Foundation Trust.
Author information
Authors and Affiliations
Contributions
Konstantinos Balaskas, Dun Jack Fu: research design. Konstantinos Balaskas, Veronika Lipkova, Sophie Glinton, Livia Faes, Bart Liefers, Gongyu Zhang, Nikolas Pontikos, Alex McKeown, Lukas Scheibler, Pearse A Keane, Dun Jack Fu: data analysis, interpretation, research execution. Dun Jack Fu, Veronika Lipkova, Bart Liefers, Sophie Glinton, Livia Faes, Alex McKeown, Lukas Scheibler, Nikolas Pontikos, Praveen J Patel, Gongyu Zhang, Pearse A Keane and Konstantinos Balaskas all contributed towards the preparation of the manuscript and approved the final submitted version. The corresponding author is solely responsible for managing communication between co-authors, ensuring that all authors are included in the author list and that the order is that agreed upon all authors, and that all authors are aware that the paper was submitted.
Corresponding author
Ethics declarations
Conflict of Interest
Nikolas Pontikos: Moorfields Eye Charity Career Development Award (R190031A), equity owner, Phenopolis Ltd. Dun Jack Fu: Consulting for Abbvie, Allergan, DeepMind. Livia Faes: Nothing to declare. Gongyu Zhang: No conflicts of interest. Bart Liefers: No conflicts of interest. Sophie Glinton: Moorfields Eye Charity Grant (GR001003), Wellcome Trust Grant (206619_Z_17_Z). Veronika Lipkova: No conflicts of interest. Praveen J Patel: Consulting for Roche, Novartis, Bayer and Boeringher Ingelheim; speaker fees from Bayer and Roche. Pearse A Keane: Moorfields Eye Charity Career Development Award (R190028A), UK Research & Innovation Future Leaders Fellowship (MR/T019050/1); Consulting for DeepMind, Roche, Novartis, Apellis and BitFount; equity owner in Big Picture Medical; speaker fees from Heidelberg Engineering, Topcon, Allergan and Bayer. Konstantinos Balaskas: Speaker fees from Novartis, Bayer, Alimera, Allergan and Heidelberg; consulting fees from Novartis and Roche and research support from Apellis, Novartis and Bayer. Alex McKeown: Employee of Apellis. Lukas Scheibler: Employee of Apellis.
Ethical Approval
This study involves human participants who were enrolled as patients at 46 sites in the USA (New England Institutional Review Board, University of Miami, Mayo Clinic, Institutional Review Board of the Cleveland Clinic Foundation, Duke University Health System Institutional Review Board, and Research Compliance Office Stanford University), Australia (Bellberry Ltd), and New Zealand (Northern A Health and Disability Ethics Committee, Health and Disability Ethics Committees, and Ministry of Health). The study was performed in accordance with the tenets of the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines and all applicable regulations. Institutional review board or ethics committee approval was obtained at each site. All patients provided written informed consent. For more information please visit ClinicalTrials.gov using the following identifier: NCT02503332. This is a post hoc study and the full details of institutions involved is attached to the original FILLY trial. No additional data was collected for this post hoc analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fu, D.J., Lipkova, V., Liefers, B. et al. Evaluating the Effects of C3 Inhibition on Geographic Atrophy Progression from Deep-Learning OCT Quantification: A Split-Person Study. Ophthalmol Ther 12, 3143–3158 (2023). https://doi.org/10.1007/s40123-023-00798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00798-7