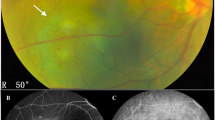
Abstract
Introduction
In an effort to replace ultrasonography-based thickness measurements, we investigated whether choroidal melanoma characteristics are related to progression-free survival (PFS) in patients monitored after linear accelerator (LINAC)-based hypofractionated stereotactic photon radiotherapy.
Methods
In a retrospective dataset, we used a linear mixed model to assess the associations between PFS and tumor characteristics; in particular, thickness, largest basal diameter (LBD), base area and volume.
Results
Thickness and LBD are statistically significantly associated with PFS. In a subgroup of 16 patients undergoing enucleation due to melanoma recurrence or progression, there were statistically significant changes in mean thickness and LBD before surgery. Mean PFS was 42 ± 30 months.
Conclusion
Ultrasonography-based thickness measurements may not be necessary for treated choroidal melanoma monitoring; instead, we propose sequential LBD assessments, which should preferably be performed using fundus photography in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
So far, choroidal melanoma follow-up after treatment has been based in particular on ultrasonography, but this is only available in sparse centers of expertise in many health care systems. |
This study links tumor characteristics to progression-free survival (PFS) in order to identify parameters which are accessible by a large number of ophthalmologists. |
What was learned from the study? |
Thickness and largest basal diameter (LBD) are statistically significantly associated with PFS. |
Ultrasonography-based thickness measurements may not be necessary for treated choroidal melanoma monitoring; instead, the assessment of largest basal diameter of the tumor—e.g., by fundus photography (a tool abundantly available to ophthalmologists)—may be used in future. |
Introduction
Choroidal melanoma is a relatively uncommon neoplasm originating from uveal melanocytes [1]. However, it is the most prevalent primary intraocular malignancy in adults. Risk factors for choroidal melanoma include fair skin and inability to tan as well as a light eye color [2, 3]. Associations between choroidal melanoma and oculodermal malanocytosis, choroidal nevus, and genetic risk factors are well documented [1, 4, 5].
Annual prevalence rates vary between two and eight per million in southern and northern Europe, respectively [6]. The majority of patients are white (94–98%), approximately 5% are Hispanic, and 1% are of Asian descent [7, 8]. Uveal melanoma develops from melanocytes of the choroid, the ciliary body, and the iris in approximately 90%, 7%, and 3% of patients, respectively [9, 10].
Choroidal melanoma carcinogenesis is still not fully understood today. So far, studies have shown that most choroidal melanomas are characterized by mutations in GNA11, GNAQ, CYSTLTR2 and PLCB4. Genetic alterations in additional genes may affect BAP1, SRSF2, SF3B1 or EIF1AX [11, 12]. Additionally cytogenetic alterations occur in choroidal melanoma, with diverse effects on the probability of metastasis. Most commonly, monosomy 3 is present in choroidal melanoma, but chromosome 8q and 6q gain as well as 6q, 16q, and 1q loss have also been described [13]. In 2–4% of cases, clinically evident metastases are detected at the time of diagnosis [14, 15]. The most common sites of metastasis are the liver (91%), due to hematogenous spread, followed by the lungs, bones and skin [16]. Despite treatment, approximately 30% of patients develop metastases within 10 years [17]. This may be attributed to the presence of circulating tumor cells, which can be found in the bloodstreams of patients with no clinical sign of metastatic spread [18].
When it comes to the detection and monitoring of choroidal melanoma, thickness as assessed by ultrasonography is an integral part [19]. Lesion thickness has historically been regarded as a reliable indicator of malignancy [20], but today it is also considered an essential criterion regarding the transformation of a nevus to choroidal melanoma [19]. The importance of this factor is mirrored by the mnemonic ‘To Find Small Ocular Melanoma Doing Imaging’ [19], but also by its integration into numerous classification and therapy guidance schemes such as AJCC 8 [21], NCCN [22], the COMS Revised Classification [23, 24], and many more [25]. However, the question arises as to whether ultrasonography and consequently lesion thickness measurements are necessary for progression detection and monitoring after the initial diagnosis and treatment.
In this study, we investigate whether tumor characteristics are related to progression-free survival (PFS). We further seek to investigate if thickness measurements may be replaced by other abundantly available tumor characteristics, in particular largest basal diameter (LBD), which could be assessed using fundus photography in future.
Methods
The Ethics Committee of the Medical University of Vienna, Vienna, Austria, approved the protocol of the present study (EK 1888/2016; ClinicalTrials.gov: NCT05733728), which was conducted in adherence to the Declaration of Helsinki (including current revisions) and the Good Clinical Practice (GCP) guidelines. Written informed consent was not obtained as this study was of a retrospective nature. We reviewed all cases of choroidal melanoma treated at the Medical University of Vienna with linear accelerator (LINAC)-based hypofractionated stereotactic photon radiotherapy.
In this disease, death usually occurs 1 to 3 years after treatment [26, 27]. We therefore included patients only if they had at least 6 months of follow-up to detect progression. Progression-free survival (PFS) was defined as the time until progression. The following events were defined as progression: metastatic spread, death, or enucleation due to progression in size, pigmentation or, e.g., orange pigment. Data describing tumor dimensions were extracted form ultrasound B-echography. The tumor area was calculated by applying the rectangular area approach based on ultrasonography measurements, as previously devised [28, 29]. Tumor volumes were calculated using the ellipsoidal solid model (π/6 × length × width × height) [30].
Statistical Statement
Descriptive statistics are reported as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS Statistics (version 26.0; IBM Corp., Armonk, NY). Distribution normality was evaluated using boxplots and the Shapiro–Wilk test. We calculated a linear mixed model including a random effect for the patient to assess the associations between PFS and the following tumor-associated factors at each visit: area, volume, LBD and thickness as well as time till LINAC therapy, age and gender. A random intercept for each subject was used to address correlated data. A dependent samples t-test was used to assess change over time. The level of significance was set to α = 0.05.
Results
We identified 350 patients treated with LINAC-based hypofractionated stereotactic photon radiotherapy due to choroidal melanoma. At the time of treatment, mean patient age was 61 ± 13 years (range 21–89 years); 54.3% (190) of patients were male and 45.7% (160) were female. Mean time until LINAC therapy was 39 ± 67 days. 36.9% (129) of patients reached progression after therapy; death: 56 (16%), metastatic spread: 57 (16.3%), enucleation due to tumor progression: 16 (4.6%). Mean PFS (see Fig. 1) was 42 ± 30 months.
Mean choroidal melanoma characteristics at initial presentation were 5.1 ± 1.9 mm in thickness, LBD of 11.3 ± 2.6 mm, 77.9 ± 49.7 mm2 in area, and 331 ± 261 mm3 in volume. Figure 2 shows the mean tumor dimensions during the follow-up time.
The linear mixed model showed that thickness (p > 0.001) and LBD (p = 0.008) as well as time until therapy (p = 0.04) are statistically significantly associated with PFS. This was not the case for melanoma base area (mm2, p = 0.6) and tumor volume (mm3, p = 0.6). Residuals of the linear mixed model were normally distributed in histogram view.
In a subgroup of 16 (4.6%) patients undergoing enucleation due to melanoma recurrence or progression after LINAC therapy, there was a statistically significant increase in mean thickness and LBD (p = 0.05 and p > 0.005, respectively, see Fig. 3A, B). In absolute numbers, the increase was 0.95 and 0.90 mm, respectively, after a median time until progression of 13.5 (range 7–18) months. Tumor base area and volume (p = 0.72 and 0.53, respectively, see Fig. 3C, D) did not show a statistically significant change.
Discussion
Detecting progression is key for the diagnosis and follow-up of patients with choroidal melanoma. Today, sequential and multimodal imaging combined with clinical examination are abundantly used to watch for progression. In particular, assessing lesion thickness by sonography is included in numerous diagnostic and therapy guidance schemes [25]. As ultrasonography is not ubiquitously available in many health care systems or facilities, patients with choroidal nevi or melanoma have to be monitored and followed up in sparse centers of expertise, so the question arises as to whether measurements using ultrasonography, in particular thickness measurements, may be replaced by measurements of other parameters such as LBD or tumor base area to detect change, as these parameters may be extracted from, e.g., fundus photography. We have also included volume (although thickness measurements are necessary for volume calculations), as it was previously shown that gross tumor volume, thickness, LBD, and time till therapy are prognostic for local recurrence-free survival [31]. With respect to tele-medicine and virtual surveillance of patients living far from oncology centers, we focused the research question solely on patient follow-up following treatment for choroidal melanoma, excluding the process of diagnosis and therapy finding as well as choroidal nevi from this research approach.
We used a linear mixed model to assess the associations of tumor characteristics including thickness, LBD, melanoma base area, tumor volume and time until treatment with PFS. Melanoma thickness and LBD as well as time until treatment are statistically significantly associated with PFS. As melanoma base area and tumor volume are parameters calculated based on ultrasound measurements, we expected that these two parameters would also be statistically significantly associated with PFS. Interestingly, this was not the case (p = 0.6 for both parameters), which is in contrast to a previous study [25]. One can assume that the gross approximation of tumor area using a rectangular approach and tumor volume using an ellipsoidal approach does not adequately represent the true tumor scales. This should be readdressed in future studies by using the true tumor dimensions.
The percentage of eyes that were enucleated in this patient collective after they had received LINAC therapy was 16.6% (58) during a follow-up period of up to 15 years (6 months to 15 years), and enucleation was most commonly due to neovascular glaucoma, which is in line with the literature (6.9–14.7%) [31,32,33,34]. Sixteen (4.6%) of these patients underwent enucleation due to tumor progression. In this subgroup, we expected tumor progression to be mirrored in melanoma size. There were statistically significant changes over time in thickness and LBD. Tumor base area and volume did not show a statistically significant change. In order to assess if we would have missed melanoma progression in patients by omitting thickness measurements, we reviewed patient records. Growth was recorded in 13 patients, and in all of these cases growth was expressed by increases in both thickness and LBD. In three patients, funduscopic changes such as new or increasing tumor surface pigmentation or orange pigment were recorded. Consequently, we would not have missed progression in this patient collective by assessing LBD only.
A previous study by Roelofs et al. investigated whether ultrasonography is necessary for detecting progression of melanocytic choroidal tumors [35]. They found that in high- and low-risk nevi (MOLES 1 and 2), progression can be detected by fundus photography alone. Overall, the failure to detect progression without ultrasonography was 3% if photography alone was used in lesions classified as having a MOLES score ≥ 3. However, if photography is combined with OCT and AF, the failure to detect progression drops to 1% in patients who should already be under the care of an ocular oncologist according to the underlying MOLES protocol. However, this study cannot be compared with our approach, as we focused on treated patients with choroidal melanoma as opposed to attributing risk for progression in melanocytic choroidal lesions. A previous study with a different approach investigated whether ultrasonography is necessary in order to detect progression of treated choroidal melanomas [36]. Negretti et al. used wide-field fundus photography to detect 98.7% of local treatment failures. One patient showed extraocular extension which was only detected by ultrasonography. These findings are in accordance with our results, which show that both thickness and LBD are statistically significantly associated with PFS in a linear mixed model. Further, a study assessing prognostic factors for choroidal melanoma progression 5 years after enucleation shows that the factors influencing survival change with time. Five years thereafter, male gender and gain of 8q are the only independent predictors of poor prognosis; tumor characteristics such as thickness are not predictive anymore [37].
Time plays an important role in choroidal melanoma treatment. In this study, time until therapy was statistically significantly associated with PFS. The median time until progression after LINAC treatment was 13.5 (range 7–18) months. The corresponding time in a previous study was 17.9 (range 10.9–43.6) months [31]. This shows that the most vulnerable phase concerning progression after treatment may be between 1 and 2 years after LINAC therapy, which further supports omitting thickness measurements, especially in the long-term post-treatment monitoring phase.
Limitations to be considered are the retrospective nature of this study and the fact that LBD was derived from ultrasonography rather than fundus photography serving as a surrogate parameter. We are consequently planning on getting general ophthalmologists and trained ocular oncologists to assess tumor dimensions in fundus photos in order to assure that this approach is not prone to erroneous progression detection, which may be the case in ultrasonography due to different measuring approaches (in/excluding sclera in the thickness measurement). Further studies are warranted to determine the performance of LBD assessment based on fundus photography in a non-ocular-oncologist setting or even in an artificial intelligence [38] setting.
Conclusion
In conclusion, this study shows that ultrasonography-based thickness measurements may not be necessary for treated choroidal melanoma monitoring; instead, we propose sequential LBD assessments, which should preferably be based on fundus photography in future, as it is abundantly available to ophthalmologists.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request in combination with ethics approval based on a specific research question.
References
Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). 2017;31(2):241–57.
Nayman T, Bostan C, Logan P, Burnier MN Jr. Uveal melanoma risk factors: a systematic review of meta-analyses. Curr Eye Res. 2017;42(8):1085–93.
Weis E, Shah CP, Lajous M, Shields JA, Shields CL. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol. 2006;124(1):54–60.
Abdel-Rahman MH, Sample KM, Pilarski R, Walsh T, Grosel T, Kinnamon D, et al. Whole exome sequencing identifies candidate genes associated with hereditary predisposition to uveal melanoma. Ophthalmology. 2020;127(5):668–78.
Derrien AC, Rodrigues M, Eeckhoutte A, Dayot S, Houy A, Mobuchon L, et al. Germline MBD4 mutations and predisposition to uveal melanoma. J Natl Cancer Inst. 2021;113(1):80–7.
Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6(7):1230–44.
Shields CL, Kaliki S, Cohen MN, Shields PW, Furuta M, Shields JA. Prognosis of uveal melanoma based on race in 8100 patients: the 2015 Doyne Lecture. Eye (Lond). 2015;29(8):1027–35.
Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140(4):612–7.
Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38(5):549–53.
Damato B. Treatment of primary intraocular melanoma. Expert Rev Anticancer Ther. 2006;6(4):493–506.
Smit KN, Jager MJ, de Klein A, Kiliҫ E. Uveal melanoma: towards a molecular understanding. Prog Retin Eye Res. 2020;75: 100800.
Pandiani C, Beranger GE, Leclerc J, Ballotti R, Bertolotto C. Focus on cutaneous and uveal melanoma specificities. Genes Dev. 2017;31(8):724–43.
Ortega MA, Fraile-Martinez O, Garcia-Honduvilla N, Coca S, Alvarez-Mon M, Bujan J, et al. Update on uveal melanoma: translational research from biology to clinical practice (review). Int J Oncol. 2020;57(6):1262–79.
Freton A, Chin KJ, Raut R, Tena LB, Kivela T, Finger PT. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur J Ophthalmol. 2012;22(2):236–43.
Finger PT, Kurli M, Reddy S, Tena LB, Pavlick AC. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol. 2005;89(10):1270–4.
Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004;22(12):2438–44.
Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–43.
Beasley AB, Chen FK, Isaacs TW, Gray ES. Future perspectives of uveal melanoma blood based biomarkers. Br J Cancer. 2022;126(11):1511–28.
Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK Jr, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39(10):1840–51.
Gass JD. Observation of suspected choroidal and ciliary body melanomas for evidence of growth prior to enucleation. Ophthalmology. 1980;87(6):523–8.
Baron ED, Nicola MD, Shields CL. Updated AJCC classification for posterior uveal melanoma A. Retina Today. 2018. https://retinatoday.com/articles/2018-may-june/updated-ajcc-classification-for-posterior-uveal-melanoma.
Rao PK, Barker C, Coit DG, Joseph RW, Materin M, Rengan R, et al. NCCN Guidelines Insights: Uveal melanoma, version 1.2019. J Natl Compr Canc Netw. 2020;18(2):120–31.
Collaborative Ocular Melanoma Study Group. Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report no. 1. Arch Ophthalmol. 1990;108(9):1268–73.
Margo CE. The Collaborative Ocular Melanoma Study: an overview. Cancer Control. 2004;11(5):304–9.
Skinner CC, Augsburger JJ, Augsburger BD, Correa ZM. Comparison of alternative tumor size classifications for posterior uveal melanomas. Invest Ophthalmol Vis Sci. 2017;58(9):3335–42.
Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol. 1978;62(6):420–5.
Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern MH, et al. Uveal melanoma. Nat Rev Dis Primers. 2020;6(1):24.
Jensen OA. Malignant melanomas of the human uvea: 25-year follow-up of cases in Denmark, 1943–1952. Acta Ophthalmol (Copenh). 1982;60(2):161–82.
Davidorf FH, Lang JR. The natural history of malignant melanoma of the choroid: small vs large tumors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1975;79(2):OP310–20.
Gass JD. Comparison of uveal melanoma growth rates with mitotic index and mortality. Arch Ophthalmol. 1985;103(7):924–31.
Sreenivasa S, Wosle M, Gager Y, Vordermark D, Grajewski L, Krause L, et al. Impact of tumour volume and treatment delay on the outcome after linear accelerator-based fractionated stereotactic radiosurgery of uveal melanoma. Br J Ophthalmol. 2023. https://doi.org/10.1136/bjo-2022-322750.
Zahorjanova P, Sekac J, Babal P, Stubna M. Enucleation after stereotactic radiosurgery in patients with uveal melanoma. Cesk Slov Oftalmol. 2020;76(1):46–51.
Muller K, Naus N, Nowak PJ, Schmitz PI, de Pan C, van Santen CA, et al. Fractionated stereotactic radiotherapy for uveal melanoma, late clinical results. Radiother Oncol. 2012;102(2):219–24.
Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62(5):1405–11.
Roelofs KA, O’Day R, Al Harby L, Hay G, Arora AK, Cohen VML, et al. Detecting progression of melanocytic choroidal tumors by sequential imaging: is ultrasonography necessary? Cancers (Basel). 2020;12(7):1856.
Negretti GS, Harley U, Arora AK, Hay G, Sagoo MS, Damato BE. Detecting progression of treated choroidal melanomas: is ultrasonography necessary? Cancers (Basel). 2021;13(22):5832.
Dogrusoz M, Brouwer NJ, de Geus SJR, Ly LV, Bohringer S, van Duinen SG, et al. Prognostic factors five years after enucleation for uveal melanoma. Invest Ophthalmol Vis Sci. 2020;61(3):31.
Shields CL, Lally SE, Dalvin LA, Sagoo MS, Pellegrini M, Kaliki S, et al. White paper on ophthalmic imaging for choroidal nevus identification and transformation into melanoma. Transl Vis Sci Technol. 2021;10(2):24.
Funding
No funding or sponsorship was received for the study. The journal’s Rapid Service fees are covered by the Department of Ophthalmology and Optometry, Medical University of Vienna, Austria.
Author information
Authors and Affiliations
Contributions
Reinhard Told: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization. Judith Kreminger: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization. Ursula Schmidt-Erfurth: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—review and editing, visualization. Roman Dunavoelgyi: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—review and editing, visualization. Adrian Reumueller: conceptualization, methodology, validation, supervision, formal analysis, investigation, resources, data curation, writing—review and editing, visualization.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict or financial interest to declare regarding this publication. Prof. U. Schmidt-Erfurth is a consultant for Genentech, Kodiak, Novartis, Roche, and RetInSight.
Ethical Approval
The Ethics Committee of the Medical University of Vienna, Vienna, Austria, approved the protocol of the present study (EK 1888/2016; ClinicalTrials.gov: NCT05733728), which was conducted in adherence to the Declaration of Helsinki (including current revisions) and the Good Clinical Practice (GCP) guidelines. Written informed consent was not obtained as this study was of a retrospective nature.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Told, R., Kreminger, J., Schmidt-Erfurth, U. et al. Impact of Choroidal Melanoma Characteristics on Progression-Free Survival in Patients Undergoing Hypofractionated Stereotactic Photon Radiotherapy. Ophthalmol Ther 12, 3039–3046 (2023). https://doi.org/10.1007/s40123-023-00790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00790-1