Abstract
Introduction
The primary objective of this study is to assess whether the combination of intense pulsed light (IPL) with 3% diquafosol (DQS) ophthalmic solution is more effective than intense pulsed light in alleviating signs and symptoms of dry eye disease (DED).
Methods
This randomized study included 66 participants with evaporative dry eye (EDE) who received IPL + DQS therapy (n = 44 eyes), IPL therapy (n = 44 eyes), or sham therapy (n = 44 eyes). All participants were examined at baseline (D0), day 14 (D14), and day 28 (D28) for non-invasive break-up time (NITBUT), tear-film lipid layer (TFLL), corneal conjunctival staining (CS), meibomian gland quality (MGQ), meibomian gland expression (MGEx), and ocular surface disease index (OSDI).
Results
At day 28, comparison among the IPL + DQS therapy, IPL therapy, and sham therapy found significant differences in the mean NITBUT (12.03 ± 1.27 versus 10.47 ± 3.48 versus 4.57 ± 0.46; p < 0.001), TFLL (2.09 ± 0.29 versus 2.27 ± 0.45 versus 2.89 ± 0.65; p < 0.001), CS (1.43 ± 0.82 versus 1.93 ± 1.32 versus 3.52 ± 1.00; p < 0.001), MGQ (1.55 ± 0.66 versus 1.91 ± 0.77 versus 2.66 ± 0.53; p < 0.001), MGEx (1.27 ± 0.45 versus 1.75 ± 0.44 versus 2.41 ± 0.50; p < 0.001), and OSDI score (19.36 ± 7.01 versus 24.77 ± 4.68 versus 42.61 ± 7.49; p < 0.001); significant improvements in NITBUT, TFLL, CS, MGQ, MGEx, and OSDI were found in the IPL + DQS therapy and IPL therapy, while the sham therapy had no significant improvements.
Conclusion
Combining 3% diquafosol ophthalmic solution with intense pulsed light was superior to IPL therapy alone in relieving the signs and symptoms of patients with severe evaporative DED.
Trial registration
Clinical Trials Identifier: NCT05694026
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Diquafosol (DQS), a P2Y2 receptor agonist, is clinically proven to improve the signs and symptoms of dry eye disease (DED). |
Intense pulsed light (IPL) is an effective therapy for dermatological conditions. However, it was serendipitously discovered to improve evaporative dry eye and meibomian gland dysfunction in patients. |
The aim of this study was to explore the synergetic effects of 3% DQS ophthalmic solution and IPL therapy on the objective and subjective ocular surface dry eye parameters in patients with DED. |
What was learned from the study? |
During the follow-up period and final 28 days of the clinical study, no serious adverse events (AEs) were reported in all participants with IPL combined with DQS; furthermore, significant improvements in subjective and objective dry eye symptoms were found in these patients. |
The synergetic effects of 3% DQS ophthalmic solution with IPL therapy were found to be significantly better subjectively and objectively in comparison with IPL-only therapy. |
Introduction
It has been observed that evaporative dry eye (EDE) is the most common form of dry eye disease (DED) [1, 2], which is primarily caused by meibomian gland hypofunction or dysfunction (MGD) [3,4,5]. The International Workshop on MGD defines MGD as “a chronic, diffuse abnormality of the meibomian glands, frequently characterized by terminal duct obstruction and/or qualitative/quantitative abnormalities in glandular secretion” [6]. These glands are modified sebaceous glands that secrete meibum directly onto the ocular surface. By improving the quality and quantity of meibum secretion, signs and symptoms of EDE and MGD can be alleviated [7].
Diquafosol ophthalmic sodium (DQS) is a P2Y2 receptor agonist that may stimulate mucin and lipid production. Moreover, it enhances tear film composition and stability [8,9,10]. It has a corneal-epithelial-repairing effect and can be utilized to treat ocular surface damage caused by dry eye [3, 11]. It may decrease the expression of inflammatory pathways and inflammatory factors implicated in the pathophysiology of dry eye by targeting inflammation [12, 13].
Intense pulsed light (IPL) is widely used to treat dermatological conditions [14], and its noncoherent polychromatic light source, with a wide wavelength range of 500–1200 nm, has been reported to stimulate facial sebaceous glands [15, 16]. The photothermal effect of IPL is postulated to relieve inflammation by removing aberrant surface microvasculature and enhancing meibomian gland function [17, 18]. In addition, the application of IPL to the skin has been related to an increase in fibroblast proliferation, collagen production, and local blood flow [19, 20]. Numerous studies have shown the efficacy of IPL in reducing the signs and symptoms of DED on the periocular skin [21, 22] and have paired it with other treatments such as heated eye mask (HEM) [23,24,25], 0.1% sodium hyaluronate eye drops [17, 26], and blood extract eye drops [27]. Hence, a randomized controlled trial was conducted to evaluate the safety and effectiveness of combining IPL with DQS for individuals with DED.
Methods
Study Design
This is a prospective, randomized, controlled trial performed at He Eye Specialist Hospital, Shenyang, China (HESH). This study was conducted in compliance with the tenets of the Declaration of Helsinki and the Institutional Review Board of He Eye Specialist Hospital [ethics approval number: IRB (2023) K002.01] and was registered with the Registry of Clinical Trials (trial registration number: NCT05694026). This study was conducted between 1 November 2022 and 1 February 2023, and participants were recruited at the Department of Ophthalmology, HESH. Specific study information sheets were provided to patients prior to taking consent. The CONSORT checklist can be found in the Supplementary Materials.
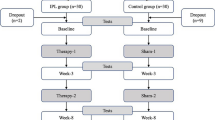
This research involved a total of 66 participants (132 eyes) who were diagnosed with dry eye and were at least 18 years of age. A custom computer randomization program was used to randomly (1:1:1) allocate participants to three study groups: (a) IPL + DQS group (study group), (b) IPL-only group (active control group), and (c) sham group (non-treatment control group) (Fig. 1).
Inclusion and Exclusion Criteria
Inclusion criteria were: (i) age ≥ 18 years; (ii) Fitzpatrick skin types I–IV; (iii) able and willing to comply with the treatment and follow-up schedule; (iv) bilateral diagnosis at any stages of MGD, according to the International Workshop on MGD [28]; and (v) bilateral signs and symptoms of dry eye disease: (a) the ocular surface disease index (OSDI) questionnaire ≥ 13, [29] and (b) a noninvasive tear film break-up time (NITBUT) of ≤ 5 seconds or a conjunctivocorneal staining score (CS) of ≥ 3 points. The presence of two or more criteria was used to establish a positive dry eye (DE) diagnosis on the basis of the 2016 Asia Dry Eye Society criteria [30].
Exclusion criteria were: (i) existing ocular trauma, infectious diseases, recent surgical history; (ii) skin defects, pigmentation, moles, scars in the treatment area, skin cancer; (iii) autoimmune diseases, skin allergies; (iv) pregnancy or lactation; (v) Fitzpatrick skin type IV and V; and (vi) patients with corneal refractive surgery such as laser assisted in situ keratomileusis (LASIK).
Treatment
Treatment consisted of one drop of 3% DQS (Diquas; Santen Pharmaceutical Co., Ltd., Osaka, Japan) six times per day for 4 weeks (28 days). Two IPL therapy sessions of M22 (Lumenis Ltd., Yokneam, Israel) IPL system or sham IPL therapy 2 weeks apart were administered. IPL treatment utilized a noncoherent polychromatic light source with a wavelength spectrum of 500–1200 nm on the cutaneous facial sebaceous glands.
Experimental Design
The study design is depicted in Fig. 1. Random allocations were conducted after enrollment. Random numbers with corresponding participants were determined in the order of the time of the visit and divided into three groups.
Group A: IPL + DQS group (study group); participants underwent IPL treatment with 12 uniformly spaced pulses of light to both eyes on day 0 and day 14. DQS was used six times daily for 28 days.
Group B: IPL-only group (active control group); participants received IPL treatment only with 12 homogeneously spaced pulses of light to both eyes at day 0 and day 14.
Group C: Sham group (non-treatment control group); opaque goggles and ultrasound gel were applied, and a non-active IPL device was placed on the periocular region and moved 12 times to simulate treating different areas around the eyes.
The researcher who collected data on day 0 (D0), day 14 (D14), and day 28 (D28) was unaware of the therapeutic assignments of the patients. The study investigator and data collectors were unaware of which group the participants were enrolled in.
Clinical Assessment
Before each IPL therapy, the following measurements were performed: ocular surface disease index (OSDI), noninvasive break-up time (NITBUT), tear film lipid layer (TFLL), meibomian gland (MG), and corneal and conjunctival staining (CS) at day 0 (D0), day 14 (D14), and day 28 (D28). We used primary and secondary outcomes measures, symptoms, and corneal and meibomian gland improvement for comparisons between the three groups. An expert physician gave the IPL and sham treatment. Adverse events also were monitored and documented.
Primary Outcome Measures
Ocular Surface Disease Index (OSDI): OSDI is a questionnaire consisting of 12 questions for evaluating the effects of dry eye syndrome on vision, ocular symptoms, and any condition associated with DED [29]. The patient will answer each question on a scale ranging from 0 to 4, with 0 indicating none of the time and 4 indicating all of the time. If a particular question is deemed irrelevant, it will be marked as not applicable (N/A) and excluded from the analysis. The OSDI total score is calculated according to the following formula. The total score ranges from 0 to 100, with higher scores representing more severe cases of dry eye disease symptoms.
NITBUT was assessed using the Keratograph 5M (Oculus, Germany) topographer. Three consecutive measurements were taken, and the median value was entered as the final reading.
Secondary Outcome Measures
Meibomian gland function and secretion quality: meibum quality was assessed under a slit-lamp [31]: eight meibomian glands in the middle parts of the eyelid were assessed using a scale of 1 to 4 for each gland (1 represented clear meibum; 2 represented cloudy meibum; 3 represented cloudy and granular meibum; and 4 represented thick meibum with toothpaste-like consistency) [32].
Tear Film Lipid Layer Score (TFLL): tear film lipid layer interferometry was assessed using DR-1 (Kowa, Nagoya, Japan). The results were graded as follows: grade 1, somewhat gray color, uniform distribution; grade 2, somewhat gray color, nonuniform distribution; grade 3, a few colors, nonuniform distribution; grade 4, many colors, nonuniform distribution; grade 5, corneal surface partially exposed [21, 33].
Fluorescein and lissamine conjunctival and cornea staining (CFS): fluorescein and lissamine staining of the ocular surface was divided into three zones consisting of nasal conjunctival, corneal, and temporal conjunctival areas. The staining score ranged from 0 to 3 for each zone, yielding a total score of 0–9 for the ocular surface [30, 34].
Statistical Analysis
Statistical Analysis in Social Sciences (SPSS) for MacOS software was used to analyze the data (version 26, IBM Corp.). Data from both eyes were taken at baseline, week 2 of the first follow-up, and week 4 of the second follow-up for all patients receiving the therapy. Repeated measures analysis allowed for comparisons across time periods, while paired analyses allowed for comparisons of pre- and post-treatment data at specific time periods. The Kolmogorov–Smirnoff test will be used to determine the normality of variables. The background of the study’s subjects will be tabulated by calculating the mean and standard deviation for continuous variables and the frequency and percentage for categorical variables. Analysis of variance (ANOVA) will be used to analyze ordinal variables and those having non-normal distributions. The primary outcome measures for this study are NITBUT and OSDI scores before and after treatment. For the primary endpoint, between-group comparisons using baseline as a covariate and an analysis of covariance were made to produce the adjusted mean, its 95% confidence interval, and the p-value.
Results
Patient Disposition
Figure 1 summarizes the distribution of the 66 adult participants (132 eyes) who were randomly assigned to receive (a) IPL + DQS, (b) IPL, or (c) sham IPL treatment at an equal ratio. The final analysis included 22 participants (44 eyes) in the IPL + DQS, 22 participants (44 eyes) in the IPL, and 22 participants (44 eyes) in the sham IPL. Table 1 displays the demographic information regarding the IPL + DQS group, IPL group, and sham IPL group. The background characteristics of patients in the three groups were similar.
Efficacy Evaluation
The mean NITBUT at baseline assessment was not significantly different (p = 0.323) among the three groups. Significant improvement was shown in the IPL + DQS group from 4.98 ± 0.36 seconds at baseline to 12.03 ± 1.27 seconds at day 28 (p < 0.001), while gradual improvement was shown in the IPL-only group from 4.60 ± 0.48 seconds at baseline to 10.47 ± 3.48 seconds at day 28 (p < 0.001). The sham group had no statically significant difference in the NITBUT measurements (baseline: 4.74 ± 0.45 seconds, D28: 4.57 ± 0.46 seconds, p > 0.05). Intergroup comparison of NITBUT found a significant difference between the three groups at D14 (p < 0.001) and D28 (p < 0.001). ΔNITBUT (baseline minus D28) was found to be greatest in the IPL + DQS group (−7.43), followed by the IPL-only group (−5.88) and the sham group (0.85) (Table 2).
The mean TFLL score was not statistically different among the IPL + DQS group, IPL-only group, and sham group at baseline (p = 0.099). A significant difference in mean TFLL score among the IPL + DQS group, IPL-only group, and sham group was found at D14 (p < 0.001) and D28 (p < 0.001) (Table 2). TFLL score for the IPL + DQS group improved from 2.70 ± 0.59 at baseline to 2.09 ± 0.29 at D28, and the IPL-only group improved from 2.80 ± 0.48 to 2.27 ± 0.45, while the sham group had no significant changes at D28 (p = 0.106). ΔTFLL (baseline minus D28) for the IPL + DQS group, IPL-only group, and sham group was 0.61, 0.52, and −0.05, respectively (Table 2).
At baseline, the mean CS score was 2.84 ± 1.55, 2.95 ± 1.57, and 3.27 ± 1.40 in the IPL + DQS group, IPL-only group, and sham group, respectively (p = 0.075). At D14, the CS score for the IPL + DQS group, IPL-only group, and sham group was 2.23 ± 1.01, 2.80 ± 1.52, and 3.57 ± 1.00, respectively (p < 0.001). At D28, the CS score for the IPL + DQS group, IPL-only group, and sham group was 1.43 ± 0.82, 1.93 ± 1.32, and 3.52 ± 1.00, respectively (p < 0.001). Significant differences among the groups were found at D14 and D28. ΔCS (baseline minus D28) was found to be greatest in the IPL + DQS (1.41), followed by the IPL-only (1.02) and sham group (− 0.25) (Table 2).
The MGQ score can range from 1 to 4. A score of one represents the best score and a score of four represents the worst score. The mean MGQ score at baseline was 2.57 ± 0.59, 2.59 ± 0.58, and 2.57 ± 0.55 for the IPL + DQS group, IPL-only group, and sham group, respectively (p = 0.916) and improved to 1.55 ± 0.66, 1.91 ± 0.77 and 2.66 ± 0.53 at D28, respectively (p < 0.001). A significant difference between the groups was found at D14 and D28. ΔMGQ (baseline minus D28) for the IPL + DQS group, IPL-only group, and sham group was 0.970, 0.690, and −0.090, respectively (Table 2).
The MGEx score can range from 1 to 4. A score of one represents the best score and a score of four represents the worst score. Significant improvement was shown in the IPL + DQS group from 2.41 ± 0.50 at baseline to 1.27 ± 0.45 at D28 (p < 0.001), while improvement was demonstrated in the IPL-only group from 2.48 ± 0.51 at baseline to 1.75 ± 0.44 at D28 (p < 0.001). The sham group had no statistically different in the MGEx (baseline: 2.36 ± 0.49, D28: 2.41 ± 0.50, p = 0.376). ΔMGEx (baseline minus D28) for the IPL + DQS group, IPL-only group, and sham group was 1.13, 0.74, and −0.05, respectively (Table 2).
The mean OSDI score at baseline was recorded as 43.57 ± 10.20, 42.97 ± 8.78, and 42.36 ± 7.10 for the IPL + DQS group, IPL-only group, and sham group, respectively (p = 0.596). Significant differences among the groups in the OSDI score were found at D14 and D28. OSDI scores for the IPL + DQS group, IPL-only group, and sham group improved to 19.36 ± 7.01, 24.77 ± 4.68, and 42.61 ± 7.49 at D28, respectively. ΔOSDI (baseline minus D28) for the IPL + DQS group, IPL-only group, and sham group was 24.20, 18.91, and −0.25, respectively (Table 2).
Adverse Events
No systemic adverse events were observed during the study. After treatment, in rare cases eye irritation, conjunctival hyperemia, eye pain, and the skin around the eye becoming sensitive and fragile may occur. No special treatment was required for these to relieve and subside within a few hours.
Discussion
Signs and symptoms associated with DED may be unpleasant for both patients and physicians due to its association with a number of intrinsic and extrinsic factors, including eyelid abnormalities, blink rate, lacrimal glands, meibomian glands, ocular surface cells, and corneal nerve fibers [35, 36]. In certain situations, these issues appear as symptoms such as grittiness, pain, burning sensation, hyperemia, and secondary epiphora at the interface between the tear film and the ocular surface [22, 37].
Assessing DQS in combination with IPL therapy for evaporative DED, we found that the subjective symptoms of dryness ratings in the DQS combination groups were lower than in the IPL group and control group. The difference between the IPL + DQS, IPL-only, and control groups was statistically significant; furthermore, the IPL + DQS performed the best on all subjective and objective dry eye criteria evaluated in this study. It was discovered that combination therapy with DQS accelerated the healing of dry eye signs and symptoms. The NITBUT value was considerably greater in the combination group, indicating that topical instillation of DQS promoted the recovery of tear function. Numerous studies have shown the efficacy of IPL therapy in the treatment of various types of DED [16, 22, 32], however, a handful of studies have explored combining IPL therapy with topical ophthalmic agents [17, 27].
The current findings indicate that combining the synergistic mechanisms of action of DQS and IPL treatment significantly improves tear stability, lipid layer quality, and meibomian gland health, as well as provides symptomatic relief [38]. It has been shown that DQS enhances the health of the ocular surface and the stability of the tear film by stimulating water and mucin production from conjunctival cells through the P2Y2 receptor.
DED is an inflammatory illness that affects the surface of the eye [39, 40] and includes inflammatory components [41,42,43] and inflammatory mediators [44, 45]. Inflammation is the fundamental process that plays a crucial role in the pathogenesis of DED, as shown by tissue culture, animal models, and human subject research [40]. A vicious cycle of inflammation may be caused by tear film instability, tear hyperpermeability, corneal/conjunctival apoptosis, and inflammation of the ocular surface [1]. The ubiquitous distribution of purines in ocular tissues such as the cornea, conjunctiva, and lacrimal gland, which play a role in regulating their physiology and pathology, reflects the link between purine receptors and DED [46]. Inflammation is unquestionably one of the most fundamental processes behind DED, and purinergic receptors are essential therapeutic targets for treating inflammation. DQS stimulates mucin and lipid production; in addition, it is an antiinflammatory drug that simultaneously promotes tear formation by means of the pro-secretory agent route, and its treatment strategy focuses on targeting the underlying pathologic pathways, yielding superior outcomes [47].
The mechanisms of action of IPL in the treatment of evaporative dry eye are still being elucidated, and nine hypotheses have been proposed thus far: (i) abnormal blood vessel thrombosis; (ii) meibum heating and liquefaction; (iii) epithelial turnover reduction; (iv) photomodulation; (v) fibroblast activation; (vi) Demodex eradication; (vii) modulation and secretion of proinflammatory and antiinflammatory molecules; (viii) suppression of matrix metalloproteases; and (ix) release of reactive oxidative species [48, 49]. The most commonly recognized theory explains thrombosis of aberrant blood vessels by converting light received by hemoglobin into heat, hence decreasing the number of inflammatory mediators in the eyelid and meibomian glands [49]. As photomodulation has been shown to promote fibroblast proliferation and increase collagen concentration in the periorbital area, better collagen fibers may induce accelerated meibomian pumping, resulting in an extended tear film breakdown time [19]. Regarding adverse events, our present investigation yielded no occurrences of adverse events or complexities throughout the entirety of the research. The majority of research suggests that the participants did not manifest any notable adverse events, with the exception of transient instances of erythema, edema, and discomfort. However, it is important to note that there is a possibility of hyperpigmentation, blistering, and a sensation of burning in specific cases, particularly in patients with darker skin types [25, 50].
The trial’s low sample size and data collection at a single site are limitations of the research. The fact that statistical significance was attained with such a small sample size for both indications and symptoms suggests the efficacy of the mechanism of action and the amount of therapeutic alleviation. Thus, the first outcomes of the research appear positive. A further problem is the average age of the patients is too young; future research will concentrate on a larger sample size and a randomized controlled study to maximize the study’s statistical power and cover a broader age range. More study is required to clarify the underlying mechanism and evaluate the combined efficacy of DQS and IPL treatment on evaporative dry eye in animal models. Furthermore, the Schirmer’s test is very important to quantify the tear volume in pre- and post-treatment, but due to the invasive nature of the test and its possibility of causing mild discomfort to the participants, we opted not to use it for this study; however, future studies will include the Schirmer’s test. Critical assays, such as tear film osmolarity and MMP-9 evaluations, were not conducted, which was another weakness of the research. Future investigations will be planned to measure tear film osmolarity, tear film inflammatory markers, corneal sensitivity, and corneal confocal microscopy alterations since the goal of this research was limited to assessing only tear film changes and DED symptoms.
Conclusion
Combining 3% diquafosol ophthalmic solution with intense pulsed light for the treatment of evaporative dry eye is safe and well tolerated. Overall, the combination of 3% diquafosol ophthalmic solution with IPL treatment was shown to be superior to IPL therapy alone in relieving the signs and symptoms of patients with severe evaporative DED.
Change history
15 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40123-023-00806-w
References
Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf [Internet]. 2017 [cited 2023 Jul 6];15:438–510. https://pubmed.ncbi.nlm.nih.gov/28736340/.
Ma J, Pazo EE, Zou Z, Jin F. Prevalence of symptomatic dry eye in breast cancer patients undergoing systemic adjuvant treatment: a cross-sectional study. Breast [Internet]. 2020 [cited 2023 Jul 6];53:164–71. https://pubmed.ncbi.nlm.nih.gov/32836200/.
Georgiev GA, Eftimov P, Yokoi N. Contribution of mucins towards the physical properties of the tear film: a modern update. Int J Mol Sci [Internet]. 2019 [cited 2023 Jul 6];20. https://pubmed.ncbi.nlm.nih.gov/31817367/.
Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology [Internet]. 2017 [cited 2023 Jul 6];124:S20–6. https://pubmed.ncbi.nlm.nih.gov/29055358/.
Zhang X, Vimalin Jeyalatha M, Qu Y, He X, Ou S, Bu J, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci [Internet]. 2017 [cited 2023 Jul 6];18. https://pubmed.ncbi.nlm.nih.gov/28661456/.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf [Internet]. 2017 [cited 2023 Jul 6];15:276–83. https://pubmed.ncbi.nlm.nih.gov/28736335/.
Heidari M, Noorizadeh F, Wu K, Inomata T, Mashaghi A. Dry eye disease: emerging approaches to disease analysis and therapy. J Clin Med [Internet]. 2019 [cited 2023 Jul 6];8. https://pubmed.ncbi.nlm.nih.gov/31514344/.
Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther [Internet]. 2002 [cited 2023 Jul 6];18:363–70. https://pubmed.ncbi.nlm.nih.gov/12222766/.
Yokoi N, Kato H, Kinoshita S. The increase of aqueous tear volume by diquafosol sodium in dry-eye patients with Sjögren’s syndrome: a pilot study. Eye (Lond) [Internet]. 2016 [cited 2023 Jul 6];30:857–64. https://pubmed.ncbi.nlm.nih.gov/27055679/.
Dota A, Sakamoto A, Nagano T, Murakami T, Matsugi T. Effect of diquafosol ophthalmic solution on airflow-induced ocular surface disorder in diabetic rats. Clin Ophthalmol [Internet]. 2020 [cited 2023 Jul 6];14:1019–24. https://pubmed.ncbi.nlm.nih.gov/32280196/.
Yagi-Yaguchi Y, Kojima T, Higa K, Dogru M, Ibrahim OMA, Shimizu T, et al. The effects of 3% diquafosol sodium eye drops on tear function and the ocular surface of Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice treated with antiglaucoma eye medications. Diagnostics (Basel) [Internet]. 2020 [cited 2023 Jul 6];10. https://pubmed.ncbi.nlm.nih.gov/31906291/.
Zhang Q, Zhang H, Qin G, Wu Y, Song Y, Yang L, et al. Impact of diquafosol ophthalmic solution on tear film and dry eye symptom in type 2 diabetic dry eye: a pilot study. J Ocul Pharmacol Ther [Internet]. 2022 [cited 2023 Jul 6];38:133–40. https://pubmed.ncbi.nlm.nih.gov/35049373/.
Matsumoto Y, Ibrahim OMA, Kojima T, Dogru M, Shimazaki J, Tsubota K. Corneal in vivo laser-scanning confocal microscopy findings in dry eye patients with Sjögren’s syndrome. Diagnostics (Basel) [Internet]. 2020 [cited 2023 Jul 6];10. https://pubmed.ncbi.nlm.nih.gov/32698387/.
Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med [Internet]. 2003 [cited 2023 Jul 6];32:78–87. https://pubmed.ncbi.nlm.nih.gov/12561039/.
Heymann WR. Intense pulsed light. J Am Acad Dermatol [Internet]. 2007 [cited 2023 Jul 6];56:466–7. https://pubmed.ncbi.nlm.nih.gov/17317488/.
Qin G, Chen J, Li L, Xia Y, Zhang Q, Wu Y, et al. Managing severe evaporative dry eye with intense pulsed light therapy. Ophthalmol Ther [Internet]. 2023 [cited 2023 Jul 6];12. https://pubmed.ncbi.nlm.nih.gov/36693992/.
Wu Y, Xu L, Song Y, Zhang Q, Qin G, Yang L, et al. Management of post-LASIK dry eye with intense pulsed light in combination with 0.1% sodium hyaluronate and heated eye mask. Ophthalmol Ther [Internet]. 2022 [cited 2023 Jul 6];11:161–76. https://pubmed.ncbi.nlm.nih.gov/34741758/.
Liu R, Rong B, Tu P, Tang Y, Song W, Toyos R, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol [Internet]. 2017 [cited 2023 Jul 6];183:81–90. https://pubmed.ncbi.nlm.nih.gov/28887117/.
Cao Y, Huo R, Feng Y, Li Q, Wang F. Effects of intense pulsed light on the biological properties and ultrastructure of skin dermal fibroblasts: potential roles in photoaging. Photomed Laser Surg [Internet]. 2011 [cited 2023 Jul 6];29:327–32. https://pubmed.ncbi.nlm.nih.gov/21438701/.
Jeng SF, Chen JA, Chang LR, Chen CC, Shih HS, Chou TM, et al. Beneficial effect of intense pulsed light on the wound healing in diabetic rats. Lasers Surg Med [Internet]. 2020 [cited 2023 Jul 6];52:530–6. https://pubmed.ncbi.nlm.nih.gov/31763712/.
Song Y, Yu S, He X, Yang L, Wu Y, Qin G, et al. Tear film interferometry assessment after intense pulsed light in dry eye disease: a randomized, single masked, sham-controlled study. Cont Lens Anterior Eye [Internet]. 2022 [cited 2023 Jul 6];45. https://pubmed.ncbi.nlm.nih.gov/34433517/.
Pazo EE, Huang H, Fan Q, Zhang C, Yue Y, Yang L, et al. Intense pulse light for treating post-lasik refractory dry eye. Photobiomodul Photomed Laser Surg [Internet]. 2021 [cited 2023 Jul 6];39:155–63. https://pubmed.ncbi.nlm.nih.gov/33296261/.
Vigo L, Pellegrini M, Carones F, Scorcia V, Giannaccare G. Outcomes of serial sessions of Activa mask combined with intense pulsed light therapy in patients with meibomian gland dysfunction. BMC Ophthalmol [Internet]. 2022 [cited 2023 Jul 6];22. https://pubmed.ncbi.nlm.nih.gov/35854254/.
Xu L, Wu Y, Song Y, Zhang Q, Qin G, Yang L, et al. Comparison between heated eye mask and intense pulsed light treatment for contact lens-related dry eye. Photobiomodul Photomed Laser Surg [Internet]. 2022 [cited 2023 Jul 6];40:189–97. https://pubmed.ncbi.nlm.nih.gov/35298282/.
Qin G, Chen J, Li L, Zhang Q, Xu L, Yu S, et al. Efficacy of intense pulsed light therapy on signs and symptoms of dry eye disease: a meta-analysis and systematic review. Indian J Ophthalmol [Internet]. 2023 [cited 2023 Jul 6];71:1316–25. https://pubmed.ncbi.nlm.nih.gov/37026263/.
Ang BCH, Sng JJ, Wang PXH, Htoon HM, Tong LHT. Sodium hyaluronate in the treatment of dry eye syndrome: a systematic review and meta-analysis. Sci Rep [Internet]. 2017 [cited 2023 Jul 6];7. https://pubmed.ncbi.nlm.nih.gov/28827614/.
Wu Y, Mou Y, Zhang Y, Han Y, Lin L, Huo Y, et al. Efficacy of intense pulsed light combined blood extract eye drops for treatment of nociceptive pain in dry eye patients. J Clin Med [Internet]. 2022 [cited 2023 Jul 6];11. https://pubmed.ncbi.nlm.nih.gov/35268405/.
Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Investig Ophthalmol Vis Sci [Internet]. 2011 [cited 2023 Jul 5];52:1922.
Zhang XM, Yang LT, Zhang Q, Fan QX, Zhang C, You Y, et al. Reliability of Chinese web-based ocular surface disease index questionnaire in dry eye patients: a randomized, crossover study. Int J Ophthalmol [Internet]. 2021 [cited 2023 Jul 6];14:834–43. https://pubmed.ncbi.nlm.nih.gov/34150537/.
Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf [Internet]. 2017 [cited 2023 Jul 6];15:65–76. https://pubmed.ncbi.nlm.nih.gov/27725302/.
Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol [Internet]. 1998 [cited 2023 Jul 6];438:349–60. https://pubmed.ncbi.nlm.nih.gov/9634908/.
Yang L, Pazo EE, Zhang Q, Wu Y, Song Y, Qin G, et al. Treatment of contact lens related dry eye with intense pulsed light. Cont Lens Anterior Eye [Internet]. 2022 [cited 2023 Jul 6];45. https://pubmed.ncbi.nlm.nih.gov/33933353/.
Hosaka E, Kawamorita T, Ogasawara Y, Nakayama N, Uozato H, Shimizu K, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol [Internet]. 2011 [cited 2023 Jul 6];151. https://pubmed.ncbi.nlm.nih.gov/20970770/.
Najafi L, Malek M, Valojerdi AE, Khamseh ME, Aghaei H. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: a diagnostic accuracy study using STARD standard. J Diabetes Metab Disord [Internet]. 2015 [cited 2023 Jul 6];14. https://pubmed.ncbi.nlm.nih.gov/26020035/.
Bamrolia NR, Arora R, Yadava U. Unusual presentation of a case of Sjogren’s syndrome with neurological and ocular manifestation. Cont Lens Anterior Eye [Internet]. 2012 [cited 2023 Jul 6];35:85–8. https://pubmed.ncbi.nlm.nih.gov/22098691/.
Vereertbrugghen A, Galletti JG. Corneal nerves and their role in dry eye pathophysiology. Exp Eye Res [Internet]. 2022 [cited 2023 Jul 6];222. https://pubmed.ncbi.nlm.nih.gov/35850173/.
Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther [Internet]. 2020 [cited 2023 Jul 6];9:877–88. https://pubmed.ncbi.nlm.nih.gov/33090327/.
Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology [Internet]. 2012 [cited 2023 Jul 6];119:1954–60. https://pubmed.ncbi.nlm.nih.gov/22739038/.
Wilson SE. Inflammation: a unifying theory for the origin of dry eye syndrome. Manag Care [Internet]. 2003 [cited 2023 Apr 4];12:14–9. https://europepmc.org/article/med/14723109.
Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens [Internet]. 2014 [cited 2023 Jul 6];40:248–56. https://pubmed.ncbi.nlm.nih.gov/25390549/.
Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig Ophthalmol Vis Sci [Internet]. 2004 [cited 2023 Jul 6];45:4293–301. https://pubmed.ncbi.nlm.nih.gov/15557435/.
Pisella PJ, Brignole F, Debbasch C, Lozato PA, Creuzot-Garcher C, Bara J, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology [Internet]. 2000 [cited 2023 Jul 6];107:1841–9. https://pubmed.ncbi.nlm.nih.gov/11013183/.
de Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol [Internet]. 2008 [cited 2023 Jul 6];71:89–95. https://pubmed.ncbi.nlm.nih.gov/19274418/.
Acera A, Rocha G, Vecino E, Lema I, Durán JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res [Internet]. 2008 [cited 2023 Jul 6];40:315–21. https://pubmed.ncbi.nlm.nih.gov/18688174/.
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci [Internet]. 2007 [cited 2023 Jul 6];48:2561–9. https://pubmed.ncbi.nlm.nih.gov/17525185/.
Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, et al. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res [Internet]. 2014 [cited 2023 Jul 6];127:270–9. https://pubmed.ncbi.nlm.nih.gov/25151301/.
Guzman-Aranguez A, Santano C, Martin-Gil A, Fonseca B, Pintor J. Nucleotides in the eye: focus on functional aspects and therapeutic perspectives. J Pharmacol Exp Ther [Internet]. 2013 [cited 2023 Jul 6];345:331–41. https://pubmed.ncbi.nlm.nih.gov/23504005/.
Fishman HA, Periman LM, Shah AA. Real-time video microscopy of in vitro demodex death by intense pulsed light. Photobiomodul Photomed Laser Surg [Internet]. 2020 [cited 2023 Jul 6];38:472–6. https://pubmed.ncbi.nlm.nih.gov/31985328/.
Zeng H, Gong L. A review of applications and intracellular mechanisms of intense pulsed light in eyelid inflammatory diseases. Photobiomodul Photomed Laser Surg [Internet]. 2023 [cited 2023 Jul 6];41:104–19. https://pubmed.ncbi.nlm.nih.gov/36927050/.
Jewsbury H, Morgan F. Uveitis and iris photoablation secondary to intense pulsed light therapy. Can J Ophthalmol [Internet]. 2012 [cited 2023 Jul 6];47. https://pubmed.ncbi.nlm.nih.gov/22883855/.
Acknowledgements
We appreciate all participation in this research.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contribution
Conceptualization: Jiayan Chen, Sile Yu, Xingru He, Emmanuel Eric Pazo. Formal analysis and investigation: Jiayan Chen, Sile Yu, Xingru He, and Emmanuel Pazo. Writing—original draft preparation: Jiayan Chen. Writing—review and editing: Guanghao Qin, Liangzhe Li, Yifan Qi, Yang Xia, Qing Zhang, Yi Wu, Yue You, Lanting Yang, Naici Guo, Salissou Moutari, Jonathan E Moore, Shaochong Bu, Ling Xu, Wei He. Funding acquisition: Sile Yu and Xingru He. Resources: Sile Yu, Xingru He. Supervision: Sile Yu, Xingru He, and Emmanuel Eric Pazo.
Funding
This study was entirely funded by He Eye Specialist Hospital, Shenyang, China. No support was received for the publication of this article. The journal's Rapid Service Fees was funded by the authors.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
This study was conducted in compliance with the tenets of the Declaration of Helsinki and the Institutional Review Board of He Eye Specialist Hospital [ethics approval number: IRB (2023) K002.01] and was registered with the Registry of Clinical Trials (Trial registration number: NCT05694026). Specific study information sheets were provided to patients prior to taking consent.
Conflict of Interest
The authors: Jiayan Chen, Guanghao Qin, Liangzhe Li, Yifan Qi, Yang Xia, Qing Zhang, Yi Wu, Yue You, Lanting Yang, Naici Guo, Salissou Moutari, Jonathan E Moore, Shaochong Bu, Ling Xu, Wei He, Sile Yu, Emmanuel Eric Pazo, and Xingru He confirm that they have nothing to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The original online version of this article was revised to correct few values in the text and in Table 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, J., Qin, G., Li, L. et al. The Combined Impact of Intense Pulsed Light Combined and 3% Diquafosol Ophthalmic Solution on Evaporative Dry Eye: A Randomized Control Study. Ophthalmol Ther 12, 2959–2971 (2023). https://doi.org/10.1007/s40123-023-00784-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00784-z