Abstract
Introduction
Retinal microvasculature is known to be altered in patients with Fabry disease (FD). We aimed to investigate the long-term changes in macular microvasculature and explore a reliable retinal biomarker for treatment monitoring in FD.
Methods
Prospective study of 26 eyes with FD followed up to 48 months (mean 24, range 8–48). OCT angiography (OCTA) images (2.9 × 2.9 mm) were obtained using Heidelberg Spectralis II at baseline and follow-up. Macular vessel area density (VAD, %) was measured in three layers: superficial vascular plexus (SVP), intermediate capillary plexus (ICP) and deep capillary plexus (DCP) in three peri-macular circular sectors (c1, c2, c3). Additionally, foveal avascular zone (FAZ) area (mm2) and horizontal and vertical diameters (µm) were assessed.
Results
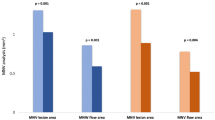
VAD decreased over time in SVP, ICP (in sectors c2 and c3) and DCP (all sectors) (p < 0.04). VAD reduction was predominantly seen in treated FD patients. FAZ and horizontal diameters increased at follow-up in FD patients compared to baseline (p ≤ 0.025). Correlation analysis showed a moderate to strong negative correlation between VAD of SVP and DCP in the innermost circle and FAZ in treated patients (r = − 0.6; p < 0.0001).
Conclusions
This is the first long-term follow-up OCTA study in FD to our knowledge. A decrease in VAD, pronounced in the peripheral circle and deeper layers, as well as an enlargement of the FAZ could be observed over time. These changes reflect the vascular remodelling during the course of the disease. Interestingly, the reduction of VAD was more pronounced in treated patients. This could be a result of enzyme replacement therapy and could be potentially used as a reliable biomarker for monitoring the treatment of the disease. A baseline examination of VAD and FAZ before treatment initiation is meaningful. Larger studies are needed to establish the use of VAD and FAZ as biomarkers for treatment monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We present a first longitudinal study on retinal microvasculature, assessed as macular vessel area density (VAD) and foveal avascular zone (FAZ), in Fabry disease (FD) using optical coherence tomography angiography (OCTA). |
We hypothesized that in the course of FD—without and under treatment—the retinal microvasculature underlies changes and remodelling. The aim of the study was to explore if these changes in the retinal microvasculature could be used as a prognostic or treatment- monitoring retinal biomarker. |
The VAD and FAZ findings of our study indicate microvascular changes in Fabry disease over time and support our hypothesis of vascular remodelling during the course of the disease. |
The retinal microcirculation seems to be applicable as a prognostic or treatment monitoring biomarker in this inherited metabolic disorder. OCTA could be used to observe the untreated FD patients and to monitor the FD patients under anti-enzymatic replacement therapy. |
Introduction
Fabry disease is a hereditary X-linked lysosomal, multisystemic disorder caused by deficiency of the enzyme α-galactosidase A due to abnormalities in the GLA gene on Xq22 [1]. This leads to accumulation of glycosphingolipids, particularly globotriaosylceramide (Gb3) and galactosylceramide, within several tissues and organs.
The incidence of Fabry disease has been estimated at 1/40,000 to 1/117,000 live births for males [2, 3]. Women are also affected but may express normal residual enzyme activity, which impedes diagnosis [4]. Predominantly affected organs are kidney and heart in which accumulation of glycosphingolipids in the vascular endothelium occur and severe kidney as well as heart failure develops [5]. A severe manifestation of Fabry disease is cerebrovascular disease in which the accumulation of glycosphingolipids in the neuronal tissue can lead to stroke, with a particularly high incidence in young men (aged 25–44 years) [6]. The manifestations of Fabry disease often vary in different patients; therefore, treatment is individualized depending on patient characteristics, disease variant and stage [7]. Enzyme replacement therapy (ERT) is the only specific treatment for Fabry disease to delay or slow the disease progression. Additionally, non-specific adjunctive therapies are necessary to address organ damage and to improve long-term prognosis [7].
Ophthalmological findings in Fabry disease include cornea verticillata (corneal whorl-like opacities), retinal vessel tortuosity (abnormal curvature of the vessels and 'corkscrew' appearance), posterior spoke shaped lens opacities and conjunctival vessel tortuosity [8, 9]. Especially the presence of cornea verticillata in younger patients and/or in the absence of medication known to cause cornea verticillata, amiodarone, chloroquine can be considered as pathognomonic for eye involvement in Fabry disease. These changes in ocular anatomy can lead to reversible (cornea verticillata) or irreversible loss of visual acuity, the most severe complications—potentially leading to blindness—being retinal vein or retinal artery occlusion with a relatively high incidence of up to 12% over time in the literature [10].
Various studies of sensitive and specific systemic biomarkers for systemic and organ involvement are being used for screening, supporting diagnosis and monitoring response to treatment [11]. Lyso Gb3 has been proposed as systemic biomarker; urinary Gb3 is a biomarker in renal disease; endothelial nitric oxide synthase (eNOS) and 3-nitrotyrosine (3-NT) act as biomarkers in vascular and cardiac involvement. Several proteins and cytokines such as tumour necrosis factor (TNF), interleukin-6 (IL-6) and tumour necrosis factor receptor (TNFR) 1 and 2 have been explored as inflammatory biomarkers, while matrix metalloprotease (MMP) 2 and 9, brain natriuretic peptide (BNP) and mid-regional pro atrial natriuretic peptide (MR-proANP) serve as biomarkers for cardiac remodelling. In addition to lyso Gb3, MMP9 has been evaluated as fibrosis marker and the N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) as imaging marker [11].
Recently, assessment of the retinal blood flow with OCT angiography (OCTA) has been established in several retinal diseases. This is a non-invasive technology that provides depth-resolved visualization of the central retinal, choroidal as well as peripapillary microvasculature independent of the invasive use of fluorescein dye [12, 13]. The OCTA has been also used to evaluate different ocular conditions of the anterior segment of the eye [14].
Various studies have previously evaluated the retinal blood flow with OCTA in Fabry patients and have shown variable OCTA and FAZ changes of retinal blood flow in different retinal layers compared to healthy controls [15,16,17,18,19,20,21,22,23].
The present study aims to investigate the long-term OCTA changes of macular vessel area density (VAD) in three microvascular layers: superficial vascular plexus (SVP), intermediate capillary plexus (ICP) and deep capillary plexus (DCP) followed up to 48 months. The second aim of the study was to analyse the long-term follow-up changes of foveal avascular zone (FAZ) area (mm2) and horizontal and vertical diameters (µm) in whole retinal thickness scans. Pearson’s correlation analyses were applied to explore any linear association between FAZ and VAD in this disease. We focused on investigation of the long-term changes in macular microvasculature by exploring a prognostic and treatment- monitoring retinal biomarker with the aim of further expanding the knowledge of ocular involvement in FD. To our knowledge, this is the first long-term follow-up OCTA study on retinal microcirculation in FD patients.
Methods
The study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of Hannover Medical School, Germany, Ethics approval no. 7687. Written informed consent was obtained from each patient before any examination procedures were performed.
Study Design and Patient Selection
We conducted a prospective study of 26 eyes with Fabry disease (13 patients, 9 treated, 4 untreated) followed up to 48 months (mean 24, range 8–48). Patients who obtained a baseline ophthalmological examination > 6 months before were recruited from our Fabry Database for a follow-up examination (January 2017 to December 2021). All patients were recruited through the nephrology department of Hannover Medical School, Germany, and had been diagnosed with FD before enrolment, including diagnosis confirmation by genetic testing. The treatment criteria were discussed with the patient and adjusted according to respective organ involvement [7]. All participants underwent a complete ophthalmological examination including best corrected visual acuity, slit-lamp biomicroscopy and fundus examination for ocular involvement of FD. Exclusion criteria were age-related macular degeneration (AMD), diabetic retinopathy, glaucoma, dense cataract, retinal and venous alteration such as occlusions and uveitis. Many retinal conditions are known to present impaired retinal microvasculature identified by OCTA [24].
To minimise the magnification errors, patients with refractive error > 3.5 diopters, as well as uncooperative patients and those who were not able to give an informed consent were also excluded from the study.
OCTA Image Processing and Measurement of Vessel Area Density (VAD) in the Macular Area
OCTA images were obtained from both eyes using Heidelberg Spectralis II (Heidelberg Engineering GmbH, Heidelberg, Germany; Acquisition Software Version 6.12.4.0). En face OCTA scans consist of 512 A-scans per B-scan and 512 consecutive B scans, (scan angle of 15°; lateral resolution 5.7 µm/pixel) measuring 2.9 × 2.9 mm, which correspond to a size of 8.41 mm2 of human retina. To ensure correct image interpretation, quality of the segmentation in horizontal foveal B scan OCT and en face OCTA maps and in OCTA B scans was proved by two experienced examiners. The obtained scans were exported and analysed using the Erlangen Angio Tool (EA-Tool coded in MATLAB (The MathWorks, Inc., Natick, MA, USA, R2017b), as previously reported [13, 25, 26]. EA-Tool is an application with high reliability and comparability of repeated macular scans [27].
A semi-automatic algorithm using Frangi vessenless filter and Otsu thresholding algorithm was applied to the en face OCTA images of SVP, ICP and DCP to generate binarized images, as previously published [27]. After segmentation, the centre of the macular region, being the centre of the circle, which is defined as the region of interest (ROI, diameter of 2.9 mm, resulting in a total ROI area of 6.1 mm2), was marked by the user. The ROI was defined as an annulus, which itself was sub-divided into three circular sectors, c1, c2, c3. The first circle had a radius of 0.40 mm, while radius increment of each circular sector was 0.35 mm, as previously reported [26].
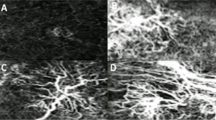
The perfusion density, defined as the total length of perfused vasculature per unit area in a region of interest (ROI), was calculated as previously described [27, 28]. We used the term “vessel area density (VAD)” in conformity with the recommendation for standardisation of OCTA metrics [29]. After correct segmentation and checking for artefacts, the VAD was measured in all three microvascular layers: superficial vascular plexus (SVP, thickness: 80 µm), measured from RNFL (retinal nerve fibre layer) to inner border of inner plexiform layer (IPL), intermediate capillary plexus (ICP, thickness: 50 µm), measured from inner border of IPL to outer border of IPL and deep capillary plexus (DCP, thickness: 40 µm), measured from outer border of IPL to outer border of OPL (outer plexiform layer). Figure 1 presents the analysis of VAD in a binary image done in the three peri-macular single circular sectors, c1, c2, c3, as well as overall (full scan as a sum of c1 + c2 + c3) and calculated in percentage (%).
Binarized images of OCTA scans analysed with the Erlangen Angio Tool. a Superficial vascular plexus (SVP), b intermediate capillary plexus (ICP) and c deep capillary plexus (DCP). Region of interest: Macula with circular segmentation of the three peri-macular sectors, c1, c2, c3. The first circle has a radius of 0.40 mm. Radius increment of each circular sector is 0.35 mm
Measurement of the Foveal Avascular Zone
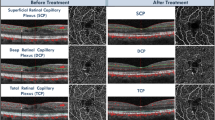
According to histological findings and evidence suggesting that the retinal plexuses merge at the edge of the FAZ [30], the FAZ was measured in a single whole retinal thickness scan. The FAZ was delineated manually (ML and approved by KH) by interconnecting the most inward-reaching vessel tails. Afterwards, the application custom software provided by Heidelberg Spectralis was used, which automatically calculated the total size of the FAZ area (mm2), as previously reported [27]. In Fig. 2, the measurement of FAZ area (mm2) as well as horizontal and vertical diameters (µm) at baseline and at follow-up is presented.
Statistical Analysis
Data were obtained from both eyes and analysed using Statistical Package for Social Sciences for Windows (SPSS), version 27.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics, including arithmetic mean and standard deviation (SD) and median (min, max, range), were calculated. The conformity of data to normal distribution was assessed with the Shapiro-Wilk test. Student's t-test for paired samples was used to compare the mean of numerical variables between baseline and follow-up and unpaired Student's t-test for testing between treated and untreated groups. For the not normally distributed data, the Wilcoxon rank test was used to evaluate differences between baseline and follow-up. Pearson's correlation analysis was performed to estimate the linear relationship between continuous variables. Macula VAD and FAZ data are presented as mean and standard deviation (SD). The statistical significance level (α) for all tests was set at 0.05. p < 0.05 was considered statistically significant. For multiple testing, the level of significance was adjusted according to the Bonferroni method considering multiple comparisons of VAD.
Results
Demographic Data
A total of 26 eyes of 13 FD patients (9 treated, 4 untreated, 5 males, 8 female) were included in the study. The mean age of the FD patients was 42.2 ± 17.1 years. The patients were followed up for up to 48 (mean 24, range 8–48) months. A summary of the demographic, genetic and clinical data is shown in Table 1.
Vessel Area Density (VAD, %)
Mean VAD in full scan (c1 + c2 + c3) of SVP was 51.25 ± 10.53 at baseline and decreased significantly to 48.27 ± 12.36 at follow-up (p = 0.043). In circular sector c3 of this layer, the VAD decreased from 60.80 ± 10.54 at baseline to 56.66 ± 12.88 at follow-up, the reduction reaching statistical significance (p = 0.009).
In ICP, the mean VAD in full scan decreased from 32.05 ± 7.46 at baseline to 27.83 ± 8.80 at follow-up (p = 0.005). The decrease of VAD in circular sectors c2 (32.15 ± 7.58 at baseline vs. 27.82 ± 8.90 at follow-up, p = 0.005 t-test) and c3 (33.77 ± 7.65 at baseline vs. 28.53 ± 9.19 at follow-up, p = 0.002 Wilcoxon rank test) of ICP was also statistically significant.
The most significant decrease of mean VAD was observed in full scan and all circular sectors of DCP. VAD in full scan decreased statistically significantly from 35.75 ± 8.36 at baseline to 31.11 ± 8.74 at follow-up (p = 0.006). The VAD in circular sectors c1 (21.43 ± 8.01 at baseline vs. 17.63 ± 7.95 at follow-up, p = 0.002), c2 (36.84 ± 8.75 at baseline vs. 31.91 ± 9.24 at follow-up, p = 0.005) and c3 (41.38 ± 9.46 at baseline vs. 36.55 ± 9.78 at follow-up, p = 0.017 Wilcoxon rank test) of DCP decreased over time.
The overall measurements of VAD at baseline and follow-up for the SVP, ICP and DCP are presented in Table 2
We further calculated the difference of reduction in VAD in all statistically significant layers and sectors as percentage %. The mean reduction of VAD in full thickness scans in SVP, ICP and DCP was 6% vs. 10% vs. 9%, respectively. Within the sectors (c1–c3), the mean reduction of VAD in SVP was 10% in c3 and much greater in ICP c2 (26%), ICP c3 (29%), DCP c1 (38%), DCP c2 (23%) and DCP c3 (21%).
Foveal Avascular Zone (FAZ mm2; Horizontal and Vertical diameter, µm)
The average area of FAZ in whole retinal thickness scans of all patients was 0.21 ± 0.09 mm2 at baseline. It enlarged significantly to 0.23 ± 0.11 mm2 at follow-up (p = 0.023). Also, the horizontal diameter of the FAZ enlarged significantly from 524.42 ± 123.78 µm at baseline to 554.08 ± 135.82 µm over time (p = 0.025). The vertical measurement of the FAZ was 535.83 ± 92.85 µm at baseline and 547.54 ± 107.87 µm at follow-up. These changes were not statistically significant (p = 0.33). In Table 2, the summary of FAZ measurements is presented.
FAZ and VAD in Treated vs. Untreated FD Groups
When comparing the FAZ in treated and untreated FD patient groups, there was significant enlargement in horizontal measurements of FAZ in ICP layer in the treated group (p = 0.015). Table 3 presents the extended results of the comparison between both groups.
The mean VAD in full scans of SVP in treated patients decreased statistically significantly from 51.15 ± 11.77 at baseline to 47.62 ± 12.99 at follow-up (p = 0.048). In circular sector c3 of this layer, the VAD decreased from 60.16 ± 11.67 at baseline to 54.98 ± 12.83 at follow-up in treated patients and reached statistical significance (p = 0.004).
In ICP, mean VAD in full scan also decreased significantly from 30.70 ± 8.26 at baseline to 26.01 ± 7.38 at follow-up (p = 0.013) in treated patients. In circular sector c2 and c3, the decrease of VAD was also statistically significant (c2: 30.89 ± 8.43 at baseline to. 25.92 ± 7.46 at follow-up, p = 0.011 and c3: 32.26 ± 8.43 at baseline to. 26.45 ± 7.49 at follow-up p = 0.005).
The mean VAD in full scan of DCP also decreased statistically significantly in treated patients from 34.14 ± 9.28 at baseline to 29.78 ± 7.65 at follow-up (p = 0.034). In circular sector c1 und c2, the decrease of VAD in DCP was statistically significant (c1: 22.16 ± 9.30 at baseline to 17.69 ± 8.68 at follow-up p = 0.006 and c2: 34.89 ± 9.50 at baseline to 30.12 ± 7.93 at follow-up p = 0.027) in treated patients.
Correlation Analyses of FAZ and VAD
Pearsons’s correlation analysis showed a strong negative correlation between VAD in the innermost circle of SVP and DCP with FAZ in treated FD patients at baseline (Pearson coefficient r = − 0.67; p < 0.0001 and r = − 0.65; p < 0.0001, respectively) and between VAD in the innermost circle of SVP and FAZ in untreated FD patients at baseline (Pearson coefficient r = − 0.73; p < 0.001).
Similarly to correlation analysis at baseline, a moderate to strong negative correlation between VAD in the innermost circle (c1) of SVP and DCP with FAZ in treated FD patients was found at follow-up (Pearson coefficient r = − 0.62; p = 0.01 and r = − 0.53; p = 0.036, respectively).
When comparing VAD in the outermost circle (c3) of ICP and FAZ in untreated FD patients, we also found a moderate but not significant positive correlation at follow-up (Pearson coefficient r = 0.54; p = 0.17). There were no significant correlations within other layers and FAZ.
Discussion
Previous studies reported a great variability of vascular density in the superficial and deep vascular plexus in Fabry patients [15,16,17,18,19,20,21,22]. It is unclear whether these changes are specific to Fabry disease or can be found in various small vessel diseases [31]. The use of different OCTA devices and variety of methods for quantitative evaluation make the comparison of the published results extremely difficult.
It has been previously hypothesized that the triad of endothelial dysfunction, cerebral hyperperfusion and prothrombotic state with likely increased synthesis of reactive oxygen species contributes to vascular dysfunction of the cerebral circulation in FD [32]. At the level of the retinal microcirculation, there might be several mechanisms which can lead to alteration of the retinal capillary plexus in Fabry disease. The primary mechanism might be an obstructive microvasculopathy due to accumulation of glycolipid, with a secondary mechanism being consecutive tissue alteration due to inflammation. These factors can then separately or synergistically lead to endothelial dysfunction, a fact previously observed with accumulation of cholesterol-laden lipids during atherosclerosis [33].
Additionally, hypoxia and blood pressure play an important role in retinal microcirculation. The oxygen distribution and oxygen consumption within the distinctive retinal layers are different. In animal models, Yu and Cringle have observed a relatively high oxygen consumption in inner and outer plexiform layers possibly related to synaptic activity in these layers. Moreover, they found that the oxygen level in the choriocapillaris and deep capillary layer varied linearly with blood pressure changes, but the oxygen levels in the deeper choroid and superficial capillary bed were much better regulated [34].
Whatever mechanism is responsible in Fabry disease, the effects on retinal microcirculation seem to be more obvious in the deeper capillary layers, namely ICP and DCP. Our results also demonstrate the greatest changes in mean vessel density in the intermediate and deep capillary layer over time. The reduction of the ICP was 27.5% (ICP c2 26%, ICP c3 29%) and 27.3% in DCP (DCP c1 38%, DCP c2 23%, DCP c3 21%). The greatest difference of mean vascular density was found in the innermost circle of the DCP c1 with 38%. We hypothesized that increased VAD is due to increased retinal flow at baseline, which remodels or normalizes during the treatment.
Cerebral vasculature has been well studied in vivo in FD, and previous studies have reported an increased cerebral blood flow in FD [35, 36]. Shen et al. reported that lysosomal accumulations of Gb3 induce oxidative stress and promote the formation of reactive oxygen species [37], causing sustained dilation of the cerebral vessels, which increases the vulnerability of cerebral vasculature and promotes the development of endothelial dysfunction [32]. Moore et al. have reported that chronic nitric oxide pathway dysregulation in FD can generate peroxynitrite, which induces cerebral vasodilation and resists vasoconstriction by humoral mediators, increasing cerebral blood flow [38]. This finding was reversible with enzyme replacement therapy [38]. On the other hand, increased cerebral blood flow can damage the vessel walls, stimulating endothelial cells to convert the mechanical stimulus into a biochemical signal that promotes vascular remodelling by upregulation of growth factors [39]. This reaction could increase the incidence of emboli or thrombosis [38, 40]. The pathophysiological mechanisms by which the accumulation of phosphosphingolipids leads to vascular remodelling seem to be multi-complex and stage-dependent.
Complementary to the cerebral vasculature, it can be speculated that the retinal microcirculation is involved in a similar pattern. In the beginning of the disease, the accumulation of phosphosphingolipids, initiating the previous mentioned multi-complex mechanisms, might lead to vascular hyperperfusion of the retinal blood flow, which then mimics an initial increase of mean vascular density. We found an increased VAD in SVP, ICP and DCP in Fabry patients at baseline compared to normal. This is possibly related to hyperperfusion or increased vascular tortuosity at baseline (manuscript under review OJRD-D-22-00805). However, enzyme replacement therapy (ERT) may contribute to the reduction of phosphosphingolipids in the blood vessels and can reduce or rather “normalize” the mean vascular density in the course of the disease. Moore et al. showed that the generation of peroxynitrite, which induced cerebral vasodilation, was reversible with enzyme replacement therapy [38]. This could explain our findings of reduced VAD in the deeper capillary layers at follow-up. We cannot finally exclude an iscahemic aetiology of the reduction in VAD. An increased incidence of emboli or thrombosis in the cerebral vasculopathy in FD is known [39]. Moreover, chronic kidney disease accelerates atherosclerosis and worsens vasculopathy in the course of Fabry disease [41]. Therefore, an additional ischaemic component within the retinal circulation in the course of the disease is also possible.
VAD and FAZ might depend on the stage and severity of the disease, which could explain the heterogeneity of OCTA results observed in previous studies [15, 17,18,19,20,21,22]. Also, the variety of manifestations of FD and inter-patient differences in disease severity, natural history and treatment may have an influence on the retinal microvasculature. Larger numbers of FD patients with longer follow-up can hopefully help distinguish and explain how much the retinal capillaries are affected in different stages of the disease. Therefore, we think that VAD and FAZ changes are more suitable as biomarkers for progression or regression of FD than they are as diagnostic biomarkers.
In a sub-analysis comparing treated vs. untreated patients, we found that VAD reduced significantly over time, predominantly in patients with ERT under treatment. This finding could again support our hypothesis that improvement in microcirculation might occur within the fovea during the treatment course of the disease. We think it could be helpful to monitor the disease over time and verify the effect of ERT. However, these results may be biased because of small patient numbers and varying time points of follow-up. Also, the non-specific adjunctive therapies were not sub-analysed because of the variety in their administration. Here, having a larger dataset and more frequent follow-up intervals would offer further insight into the progression of the disease.
Looking at the FAZ in the course of the disease, we found a statistically significant enlargement of FAZ area and horizontal diameter at follow-up in FD patients. Correlation analysis showed a moderate negative correlation between VAD in the innermost circle of SVP and DCP and FAZ in treated FD patients (r = − 0.6, p < 0.05), which might be a result of reduced VAD and remodelling of the foveal capillaries during treatment.
Previous studies also showed an increase of FAZ in Fabry patients compared to normal subjects, and this was thought to be related to disease progression or ischaemic changes [18, 20]. In contrast to these findings, two studies reported no significant difference in FAZ between Fabry and normal subjects [22, 42]. However, the size of FAZ is variable in the normal population and depends on axial length measurements. The initially smaller FAZ in our analysis could be due to hyperperfusion or tortuosity with consecutively increased VAD. Subsequently, enlargement of FAZ over time could then be a result of normalized perfusion or vascular remodelling represented by decreased VAD. However, an ischaemic component in these vascular changes might be possible and could not be finally excluded.
Special attention hast to be paid to a possible ischaemic component in Fabry disease. It is known that FD is an important cause of stroke at young age. Every suspected change in retinal circulation can be used as a mirror of cerebral circulation to prevent serious events in these patients. It has been previously reported that the retinal vasculature is more sensitive to injury compared to the cerebral vasculature. Hui et al. showed in an animal model that systemic disruption of blood-neural barriers causes vascular leakage in both tissues, but earlier in the retina, suggesting that pharmacological blood-neural barrier disruption may be detected earlier in the eye than in cortical vasculature [43]. Additionally, an impaired retinal microvasculature in patients with diabetic retinopathy correlates with impaired brain functioning before any structural changes occur [44]. Wong et al. showed in a study of 110 patients with stroke that impaired retinal vasculature was a predictive factor for stroke due to ischaemic injury and hypertension, with or without diabetes [45]. Looking at both studies, we hope that in the future the changes of the retinal microvasculature in Fabry patients over time will be used also as predictive biomarker of possible injury of cortical vasculature and stroke. Therefore, ophthalmologists, specialists in internal medicine, neurologists and paediatricians should work together on improving the knowledge in this multisystemic inherited disease.
Conclusions
In summary, VAD and FAZ findings in our study indicate vascular changes in Fabry disease over time and point to vascular remodelling during the course of the disease. The retinal microcirculation seems to be reliable as a prognostic or treatment monitoring biomarker in this inherited metabolic disorder. Therefore, OCTA can be used to observe the untreated FD patients and to monitor the FD patients under anti-enzymatic replacement therapy. Baseline examination of VAD and FAZ with OCTA is meaningful in all FD patients, particularly when treatment is initiated. Larger longitudinal studies are needed to establish OCTA hallmarks as retinal biomarkers correlating with disease progression and treatment effects. Beyond that, this biomarker could help predict patients' risk for complications like stroke.
Strengths and Limitations of the Study
The major strength of our study is the long-term follow-up. Other strengths are assessing only the high-quality images and manual determination of FAZ in all layers. However, our study has potential limitations. First, the number of participants was relatively small, and follow-up was heterogeneous because of the rarity of the disease, which limited the power for evaluating the effect of variables. Another limitation is the absence of a control group and absence of axial length measurements. None of the patients showed high myopia or hyperopia, however. Including both eyes in the analysis could also make our data less powerful. In general, we focused on analysing longitudinal changes and treatment effects within the same group in comparison to baseline. We also did not examine the repeatability of our measurements. However, Hosari et al. previously reported excellent repeatability and reproducibility of measurements of the SVP, ICP, DCP and FAZ with Spectralis OCT II in combination with the semiautomated vessel density software EA-Tool [27].
References
Fabry H. Angiokeratoma corporis diffusum–Fabry disease: historical review from the original description to the introduction of enzyme replacement therapy. Acta Paediatr Oslo Nor 1992 Suppl. 2002;91:3–5.
Desnick RJ, Ioannou YA, Eng CM, Desnick RJ, Ioannou YA, Eng CM. In the metabolic and molecular bases of inherited disease. In: Scriver CR, Beaudet AL, Sly WX, Valle D, editors. α-Galactosidase A deficiency; Fabry disease. New York: McGraw Hill; 1995. p. 2741–84.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54.
Michaud M, Mauhin W, Belmatoug N, Garnotel R, Bedreddine N, Catros F, et al. When and how to diagnose Fabry disease in clinical practice. Am J Med Sci. 2020;360:641–9.
Zarate YA, Hopkin RJ. Fabry’s disease. Lancet Lond Engl. 2008;372:1427–35.
Mishra V, Banerjee A, Gandhi AB, Kaleem I, Alexander J, Hisbulla M, et al. Stroke and Fabry disease: a review of literature. Cureus. 2020;12: e12083.
Wanner C, Arad M, Baron R, Burlina A, Elliott PM, Feldt-Rasmussen U, et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab. 2018;124:189–203.
Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A. Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Experiment Ophthalmol. 2005;33:164–8.
Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 2007;91:210–4.
Utsumi K, Yamamoto N, Kase R, Takata T, Okumiya T, Saito H, et al. High incidence of thrombosis in Fabry’s disease. Intern Med Tokyo Jpn. 1997;36:327–9.
Simonetta I, Tuttolomondo A, Daidone M, Pinto A. Biomarkers in Anderson–Fabry disease. Int J Mol Sci. 2020;21:E8080.
Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50.
Hohberger B, Lucio M, Schlick S, Wollborn A, Hosari S, Mardin C. OCT-angiography: regional reduced macula microcirculation in ocular hypertensive and pre-perimetric glaucoma patients. PLoS ONE. 2021;16: e0246469.
D’Aloisio R, Viggiano P, Borrelli E, Parravano M, Agbèanda A-G, Evangelista F, et al. Changes in Iris perfusion following scleral buckle surgery for rhegmatogenous retinal detachment: an Anterior Segment Optical Coherence Tomography Angiography (AS-OCTA) study. J Clin Med. 2020;9:1231.
Finocchio L, Sodi A, Bacherini D, Lenzetti C, Berni A, Mucciolo DP, et al. Optical coherence tomography angiography in Fabry disease. Invest Ophthalmol Vis Sci. 2018;59:2838.
Hufendiek K, Kaufeld J, Volkmann I, Brockmann D, Awe M, Kromer R, et al. Density of the retinal blood flow and thickness mapping by spectralis OCT in patients with Fabry disease. Invest Ophthalmol Vis Sci. 2018;59:5460.
Baur J, Al-Sheikh M, Barthelmes D, Nowak A, Böni C, Zweifel SA. Optical coherence tomography angiography findings in patients with Fabry’s disease. Invest Ophthalmol Vis Sci. 2018;59:4249.
Minnella AM, Barbano L, Verrecchia E, Martelli F, Pagliei V, Gambini G, et al. Macular impairment in Fabry disease: a morpho-functional assessment by swept-source OCT angiography and focal electroretinography. Invest Ophthalmol Vis Sci. 2019;60:2667–75.
Cennamo G, Di Maio LG, Montorio D, Tranfa F, Russo C, Pontillo G, et al. Optical coherence tomography angiography findings in Fabry disease. J Clin Med. 2019;8:E528.
Cakmak AI, Atalay E, Cankurtaran V, Yaşar E, Turgut FH. Optical coherence tomography angiography analysis of Fabry disease. Int Ophthalmol. 2020;40:3023–32.
Dogan C, Gonen B, Dincer MT, Mergen B, Kiykim E, Bakir A, et al. Evaluation of the reasons for the microvascular changes in patients with Fabry disease using optic coherence tomography angiography. Eur J Ophthalmol. 2021;31:3231–7.
Lin Z, Pan X, Mao K, Jiao Q, Chen Y, Zhong Y, et al. Quantitative evaluation of retinal and choroidal changes in Fabry disease using optical coherence tomography angiography. Lasers Med Sci. 2022;37:269–77.
Wiest MRJ, Toro MD, Nowak A, Bajka A, Fasler K, Al-Sheikh M, et al. Relationship of vessel density to vessel length density in patients with treated Fabry disease. Diagn Basel Switz. 2023;13:1227.
Kashani AH, Chen C-L, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100.
Szewczykowski C, Mardin C, Lucio M, Wallukat G, Hoffmanns J, Schröder T, et al. Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int J Mol Sci. 2022;23:7209.
Hohberger B, Ganslmayer M, Lucio M, Kruse F, Hoffmanns J, Moritz M, et al. Retinal microcirculation as a correlate of a systemic capillary impairment after severe acute respiratory syndrome coronavirus 2 infection. Front Med. 2021;8: 676554.
Hosari S, Hohberger B, Theelke L, Sari H, Lucio M, Mardin CY. OCT angiography: measurement of retinal macular microvasculature with spectralis II OCT angiography—reliability and reproducibility. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd. 2020;243:75–84.
Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135:370–6.
Sampson DM, Dubis AM, Chen FK, Zawadzki RJ, Sampson DD. Towards standardizing retinal optical coherence tomography angiography: a review. Light Sci Appl. 2022;11:63.
Gariano RF, Iruela-Arispe ML, Hendrickson AE. Vascular development in primate retina: comparison of laminar plexus formation in monkey and human. Invest Ophthalmol Vis Sci. 1994;35:3442–55.
Biffi E, Turple Z, Chung J, Biffi A. Retinal biomarkers of cerebral small vessel disease: a systematic review. PLoS ONE. 2022;17: e0266974.
Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;257:258–63.
Perrault LP, Mahlberg F, Breugnot C, Bidouard JP, Villeneuve N, Vilaine JP, et al. Hypercholesterolemia increases coronary endothelial dysfunction, lipid content, and accelerated atherosclerosis after heart transplantation. Arterioscler Thromb Vasc Biol. 2000;20:728–36.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208.
Moore DF, Ye F, Brennan M-L, Gupta S, Barshop BA, Steiner RD, et al. Ascorbate decreases Fabry cerebral hyperperfusion suggesting a reactive oxygen species abnormality: an arterial spin tagging study. J Magn Reson Imaging JMRI. 2004;20:674–83.
Phyu P, Merwick A, Davagnanam I, Bolsover F, Jichi F, Wheeler-Kingshott C, et al. Increased resting cerebral blood flow in adult Fabry disease. Neurology. 2018;90:e1379–85.
Shen J-S, Meng X-L, Moore DF, Quirk JM, Shayman JA, Schiffmann R, et al. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab. 2008;95:163–8.
Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, et al. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001;104:1506–12.
Kolodny E, Fellgiebel A, Hilz MJ, Sims K, Caruso P, Phan TG, et al. Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke. 2015;46:302–13.
Mendez MF, Stanley TM, Medel NM, Li Z, Tedesco DT. The vascular dementia of Fabry’s disease. Dement Geriatr Cogn Disord. 1997;8:252–7.
Navab KD, Elboudwarej O, Gharif M, Yu J, Hama SY, Safarpour S, et al. Chronic inflammatory disorders and accelerated atherosclerosis: chronic kidney disease. Curr Pharm Des. 2011;17:17–20.
Bacherini D, Vicini G, Nicolosi C, Tanini I, Lenzetti C, Finocchio L, et al. Optical coherence tomography angiography for the evaluation of retinal vasculature in Fabry disease: our experience and review of current knowledge. Front Neurol. 2021;12: 640719.
Hui F, Nguyen CTO, He Z, Vingrys AJ, Gurrell R, Fish RL, et al. Retinal and cortical blood flow dynamics following systemic blood-neural barrier disruption. Front Neurosci. 2017;11:568.
Wang Z, Lu Z, Li J, Pan C, Jia Z, Chen H, et al. Evaluation of apparent diffusion coefficient measurements of brain injury in type 2 diabetics with retinopathy by diffusion-weighted MRI at 3.0 T. NeuroReport. 2017;28:69–74.
Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet Lond Engl. 2001;358:1134–40.
Acknowledgements
The authors thank the German Ministry of Lower Saxony for its scientific and cultural organization to support our retinal imaging studies in the Ophthalmology Department of Hannover Medical School financed by the “Niedersachsen Vorab”.
Author Contribution
Conceptualization: Katerina Hufendiek and Jessica Kaufeld; methodology, Migle Lindziute, Jessica Kaufeld; Sami Hosari, Bettina Hohberger, Christian Mardin; software, Sami Hosari, Bettina Hohberger, Christian Mardin; validation, Katerina Hufendiek, Karsten Hufendiek, Carsten Framme, Jan Tode; formal analysis Katerina Hufendiek, Migle Lindziute; investigation Katerina Hufendiek, Migle Lindziute, Jessica Kaufeld, Karsten Hufendiek, Ingo Volkmann, Dorothee Brockman, Carsten Framme, Jan Tode; resources Christian Mardin, Carsten Framme; data curation, Katerina Hufendiek, Migle Lindziute, Jessica Kaufeld; and writing original draft preparation, Katerina Hufendiek and Migle Lindziute; writing—review and editing Katerina Hufendiek, Migle Lindziute, Jessica Kaufeld, Karsten Hufendiek, Ingo Volkmann, Dorothee Brockmann, Carsten Framme, Jan Tode; visualization, Katerina Hufendiek and Migle Lindziute; supervision, Christian Mardin, Carsten Framme, Katerina Hufendiek; project administration, Katerina Hufendiek, Migle Lindziute, Jessica Kaufeld. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The journal’s Rapid Service fee was funded by the authors.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hannover Medical School (Ethics Approval no. 7687; 13.12.2017). Informed consent was obtained from all subjects involved in the study.
Conflict of Interest
All named authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hufendiek, K., Lindziute, M., Kaufeld, J. et al. Investigation of OCTA Biomarkers in Fabry Disease: A Long Term Follow-Up of Macular Vessel Area Density and Foveal Avascular Zone Metrics. Ophthalmol Ther 12, 2713–2727 (2023). https://doi.org/10.1007/s40123-023-00776-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00776-z