Abstract
Introduction
Anti-vascular endothelial growth factor (VEGF) therapy is the first-line treatment for diabetic macular edema (DME). We investigated the effect of initial glycosylated hemoglobin (HbA1c) level and glomerular filtration rate (GFR) on treatment outcomes in patients with DME receiving anti-VEGF injections in routine clinical practice.
Methods
A retrospective analysis of data from the prospective, multi-center, observational Fight Retinal Blindness! registry was performed. A total of 178 eyes with DME treated with anti-VEGF agents (ranibizumab or aflibercept) from 1 January 2010 to 31 March 2019 were enrolled in the analysis, with the long study period to allow for up to 24 months of follow-up. Data for eyes were tracked in the Fight Retinal Blindness! registry, and clinical parameters were collected by using local software. Changes in visual (best-corrected visual acuity [BCVA], in letters) and anatomic outcomes (central subfield thickness [CST], in microns) between subgroups of patients according to baseline HbA1c level (≤ 7% vs. > 7%) and GFR (> vs. ≤ 60 ml/min/m2 at 24 months were assessed.
Results
The multivariate adjusted mean improvement in BCVA at 24 months of treatment was + 5.2 and + 6.8 letters in subgroups with baseline HbA1c level ≤ 7% and > 7%, respectively (p = 0.541), and + 6.9 and + 6.4 letters in subgroups with GFR > 60 and < 60 ml/min/1.73 m2, respectively (p = 0.852). The multivariate adjusted mean CST reduction was − 89.9 and − 76.4 µm in subgroups with baseline HbA1c level ≤ 7% and > 7%, respectively (p = 0.505), and − 85 and − 115 µm in subgroups with baseline GFR > 60 and ≤ 60 ml/min/1.73 m2, respectively (p = 0.130).
Conclusion
These results seem to indicate that visual and anatomical improvement in patients receiving intravitreal VEGF inhibitors for DME are independent of baseline HbA1c level and GFR, leading to the conclusion that high HbA1c levels or low GFR should not dictate injection timing in routine clinical practice. This study offers valuable insights for ophthalmologists, enabling a personalized treatment approach and optimizing DME patient outcomes.
Plain Language Summary
Our study investigated how initial levels of glycosylated hemoglobin (HbA1c) and glomerular filtration rate (GFR) influence the treatment outcomes of diabetic macular edema (DME). DME is a complication of diabetes characterized by retinal swelling and vision problems. We analyzed data from a registry of DME patients who received intravitreal injections of medication to reduce swelling. Our study included 178 eyes receiving anti-vascular endothelial growth factor (anti-VEGF) injections in routine clinical practice. The results indicated that the initial HbA1c levels and GFR at baseline did not demonstrate a significant influence on the visual and anatomical improvements observed in patients with DME after 24 months of treatment, suggesting that HbA1c levels and kidney function should not be the primary factors taken into consideration in determining the timing of injections in routine clinical practice. These findings emphasize the importance of a personalized treatment approach that considers individual patient factors beyond HbA1c levels and kidney function to optimize outcomes for DME patients. This information can guide ophthalmologists in making informed decisions on the timing and frequency of injections for their patients with DME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Diabetic macular edema (DME), a common complication of diabetes, is effectively treated with anti-vascular endothelial growth factor (anti-VEGF) therapies, which have shown significant efficacy in improving visual function. |
The medical literature lacks consensus on the predictive value of impaired renal function and elevated glycosylated hemoglobin (HbA1c) levels for the response to intravitreal anti-VEGF in the treatment of DME. |
The aim of this study was to investigate the impact of baseline HbA1c levels and glomerular filtration rate (GFR) on treatment outcomes in patients with DME receiving anti-VEGF injections in routine clinical practice. |
What was learned from the study? |
The visual and anatomical improvement in patients receiving intravitreal anti-VEGF for DME seems to be independent of baseline HbA1c level and GFR. |
The study revealed that there is no correlation between baseline HbA1c level and GFR with the functional and anatomical outcomes in patients with DME receiving intravitreal anti-VEGF. |
The findings highlight the importance of recognizing the independence of treatment response from these factors and provide valuable guidance for clinicians in determining the frequency and timing of anti-VEGF reinjections in DME patients. |
Introduction
The global prevalence of diabetic macular edema (DME) is estimated to be 7.5%, with approximately 21 million people affected worldwide [1]. DME is the leading cause of vision loss in people with diabetes [2, 3]. Intravitreal VEGF inhibitors (anti-VEGF) have become the first-line treatment for DME [4,5,6,7], and injections of anti-VEGF agents have provided excellent outcomes in a large number of patients [8]. However, the benefit of these therapies is variable, and some patients remain with substantial visual disability even after treatment [8, 9]. Factors affecting therapeutic response are age at DME onset, initial visual acuity (VA), integrity of the EZ photoreceptor line, presence of disorganized inner retinal layers, foveolar atrophy and macular vessel density [10,11,12,13,14].
Multiple large epidemiologic studies have shown that systemic factors such as glycosylated hemoglobin (HbA1c) level or estimated glomerular filtration rate (eGFR) could affect vision by increasing the risk of DME [15]. However, only a few studies have assessed the impact of these metabolic parameters on clinical response to anti-VEGF treatment [16,17,18,19], and these have yielded inconclusive results. Knowing the effect of these factors on the response to anti-VEGF could improve the clinical management of DME by adjusting the delay of injection therapy in this subgroup of patients.
The purpose of our study was to investigate the effect of baseline HbA1c level and GFR on visual and anatomic outcomes after anti-VEGF treatment (ranibizumab or aflibercept). Our main hypothesis is that patients with DME and lower baseline HbA1c level or better GFR may have better visual and anatomic outcomes with anti-VEGF treatment than those with higher baseline HbA1c level or worse GFR.
Methods and Design
Study Design and Setting
We conducted a retrospective multi-centric observational study that included the Montpellier University Hospital (France), Clinique Beausoleil (Montpellier, France), Dijon University Hospital (France) and a private practice in Strasbourg (France).
Patients were tracked by the Fight Retinal Blindness! (FRB!) outcomes database [20]. The FRB! registry was designed to collect data from each clinical visit. Physicians who participated in the FRB! Project agreed to report 80% of their patients to avoid reporting biases. The treating physician decided the treatment and visit schedules in consultation with the patient, which reflects real-world practice.
The trial was designed and conducted in accordance with the principles of the Declaration of Helsinki and its later amendments. This study was approved by the institutional review boards and ethics committees of each participating center. All patients provided oral informed consent before being enrolled in the study.
Study Population
Both pre-treated and treatment-naïve patients with decreased vision due to DME, as documented on spectral-domain optical coherence tomography (SD-OCT), were eligible for enrollment in the study. All patients had received at least one injection of an anti-VEGF agent (ranibizumab or aflibercept) between 1 January 2010 and 31 March 2019, and data on HbA1c level or GFR at baseline were available.
For patients with bilateral disease, we included the eye with the worse baseline VA. If the baseline VA was equal, the eye treated first was used in the analysis. Exclusion criteria were DME treated with corticosteroids and macular edema resulting from other cause than diabetes.
Study Measurements
Data were recorded at each visit (baseline and follow-up visits). For each visit, we used the FRB! registry to collect information on the number of letters read on the logarithm of the minimum angle of (VA) resolution (logMAR) chart, the treatment used, central subfield thickness (CST, in microns) measured with OCT, DME activity (center-involving clinically significant macular edema [CSME], non-center involving CSME or no CSME; if not completed, DME activity was defined as “unknown”) and ocular adverse events. Age, duration and type of diabetes, grading of diabetic retinopathy (DR), smoking status and previous treatments for DME were recorded at the baseline visit, defined as the first visit recorded in the FRB! registry. No data prior to the baseline visit apart from previous treatments received were available. All treatment decisions, including choice of treatment and frequency of visits, were based on VA and OCT results at the discretion of the practitioner, thereby reflecting real-world practice. As our aim was to study the influence of biological parameters on visual outcomes after treatment, data on HbA1c level (%) and GFR (ml/min/1.73 m2) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) were registered at the baseline visit.
Outcome Measures
The main outcomes were mean change in VA (letters) and CST (in microns, using SD-OCT) at 24 months between subgroups of patients classified according to HbA1c level (≤ 7% or > 7%) and GFR (≤ 60 or > 60 ml/min/1.73 m2). These criteria were chosen according to the American Diabetes and Renal Association position statement on the generally accepted threshold of diabetic and renal control [21, 22].
Statistical Analysis
Data were summarized using the mean (standard deviation [SD]), median (Q1, Q3) or number (%) as appropriate. Baseline characteristics and unadjusted outcomes between HbA1c and GFR groups at 24 months were compared using the two-sample t-test for continuous variables and the Chi-square (χ2) or Fisher’s exact test for categorical variables. Adjusted outcomes and p-values were calculated by using linear regression for VA change and CST change, generalized Poisson regression for injections and visits and Cox proportional-hazards models for non-persistence rate. Regression models were adjusted for baseline VA, age and CST; pre-treatment status; and practice. p < 0.05 was considered to indicate statistical significance. All analyses were conducted with R 4.0.5 with the glmmTMB package (V 1.0.2.1) for linear and generalized Poisson regression and the survival package (3.2–10) for Cox proportional-hazards models ® Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
A total of 178 patients who met the inclusion criteria were included in the study. Mean (SD) diabetes duration was 17.3 (10.9) years; 93% of patients had type 2 diabetes mellitus. Mean baseline VA was 53.9 (21.9) letters. HbA1c data were available for 173 (97%) patients and GFR data for 142 (80%). Baseline characteristics are summarized in Table 1.
Outcomes by HbA1c
This analysis focused on 173 eyes treated with anti-VEGF agents for which data were available on baseline HbA1c (HbA1c ≤ 7%, n = 65; > 7%, n = 108). Baseline VA significantly differed between the subgroups HbA1c level ≤ 7% and > 7% (58.7 vs. 51.9 letters, respectively; p = 0.034) while baseline CST was similar (421.6 vs. 422.2 µm, respectively; p = 0.980). All baseline characteristics and outcomes stratified by baseline HbA1c are summarized in Table 2.
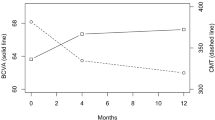
The 24-month VA was 63.1 letters for the subgroup of patients with HbA1c ≤ 7% versus 61.7 letters for the subgroup with HbA1c > 7% (p = 0.675; Fig. 1a). The multivariate adjusted mean (95% CI) change in VA from baseline was 5.2 (0.9, 9.5) letters in the Hba1c ≤ 7% subgroup compared with 6.8 (3.6, 10.1) letters (95% CI 3.6, 10.1) in the HbA1c > 7% subgroup (p = 0.541). At 24 months, 15% and 33% of patients with baseline HbA1c ≤ 7% and > 7% gained ≥ 15 letters, respectively (p = 0.390). The median number of injections was 10 and 8.5 in patients with baseline HbA1c ≤ 7% and > 7%, respectively (p = 0.842).
The mean (95% CI) adjusted CST reduction from baseline was − 85 (− 112.8, − 57.1) µm and − 77.1 (− 98.2, -56) µm for the baseline HbA1c ≤ 7% and > 7% subgroups, respectively (p = 0.656). The final mean CST at 24 months did not significantly differ between the two HbA1c subgroups (331.7 and 345.8 µm, respectively; p = 0.452; Fig. 1c). The proportion of patients with CST ≤ 250 µm at 24 months was also similar between two subgroups (29% and 27% with baseline HbA1c level ≤ 7% and > 7%, respectively; p = 0.939). The non-persistent DME rate was also similar in the two subgroups (37%; p = 0.566).
Outcomes by GFR
This analysis focused on 142 eyes treated with anti-VEGF agents with available data on baseline GFR (GFR ≤ 60 ml/min/1.73 m2, n = 57; > 60 ml/min/1.73 m2, n = 85). Baseline VA did not significantly differ between the two GFR subgroups GFR ≤ 60 and > 60 ml/min/1.73 m2 (51.4 and 57.2 letters; p = 0.124). Baseline mean CST was significantly thicker in patients with baseline GFR > 60 ml/min/1.73 m2 versus those with baseline GFR ≤ 60 (460.8 and 401.9 µm; p = 0.014). Patients with lower baseline GFR were older and diabetes duration was longer than those with higher baseline GFR (p = 0.015 and p = 0.039, respectively). All baseline characteristics and outcomes stratified by baseline GFR are summarized in Table 3.
The VA at 24 months was 60.7 and 65.1 letters for baseline GFR ≤ 60 and > 60 ml/min/1.73 m2 (p = 0.201), respectively (Fig. 1b). The adjusted mean (95% confidence interval [CI]) change in VA from baseline was 6.4 (2.4, 10.4) letters for patients with baseline GFR ≤ 60 ml/min/1.73 m2 versus 6.9 (3.7, 10.0) letters for patients with baseline GFR > 60 ml/min/1.73 m2 (p = 0.852). At 24 months, 30% and 26% of patients with baseline GFR ≤ 60 and > 60 ml/min/1.73 m2, respectively, gained ≥ 15 letters (p = 0.772). The median number of injections was 7 and ten injections for patients with baseline GFR ≤ 60 and > 60 ml/min/1.73 m2, respectively (p = 0.042).
The mean (95% CI) adjusted CST reduction from baseline was − 115 µm (− 144.0, − 85.2) and − 85 µm (− 109.0, − 61.3) for patients with baseline GFR ≤ 60 and > 60 ml/min/1.73 m2, respectively (p = 0.130). The 24-month CST was significantly different between the two subgroups (307.1 and 364 µm, respectively; p = 0.008). The proportion of patients with CST < 250 µm at 24 months was significantly different between the GFR ≤ 60 and GFR > 60 ml/min/1.73 m2 subgroups (43% and 14%, respectively; p = 0.003), as was the non-persistent DME rate (49% and 28%, respectively; p = 0.010).
Correlation Between Baseline HbA1c Level and GFR with VA at 24 months
The HbA1c level was negatively correlated with VA at 24 months (r = − 0.18; p = 0.017), whereas GFR was not correlated with the final VA (r = 0.07; p = 0.413) (Fig. 2). However, the correlation between HbA1c and 24-month VA was likely driven by the significant difference in initial VA between the groups (baseline VA between HbA1c level ≤ 7% and > 7%: 58.7 and 51.9, respectively; p = 0.034).
Discussion
We investigated the effect of HbA1c level and GFR on treatment outcomes in patients with DME receiving anti-VEGF treatment. The multivariate adjusted mean improvement in VA at 24 months did not significantly differ between subgroups with baseline HbA1c level ≤ 7% versus > 7% (5.2 vs. 6.8 letters, respectively) or between those with GFR ≤ 60 versus > 60 ml/min/1.73 m2 (6.4 vs. 6.9 letters, respectively). Nor did the adjusted mean CST reduction differ between subgroups with baseline HbA1c level ≤ 7% versus > 7% (− 85 vs. − 77.1, respectively) or between those with baseline GFR ≤ 60 versus > 60 ml/min/1.73 m2 (− 115 vs. − 85 µm, respectively). Thus, the improvement in VA and CST after anti-VEGF treatment would seem to be independent of baseline HbA1c level and GFR in patients with DME.
Identifying factors associated with good or poor outcomes can inform ophthalmologists and patients of what outcomes they can expect, on average, when choosing intravitreal injections as a treatment for DME. The effect of systemic factors on the incidence of DR and other micro- and macrovascular complications has been well studied. Tight control of blood sugar and other associated systemic factors, such as hypertension, serum cholesterol level and kidney function, can significantly delay the onset of DR and DME [23]. However, little information is currently available on whether these systemic factors affect the anatomical and visual response of patients to anti-VEGF treatments.
Do et al. [24] reported that patients with higher HbA1c level had more severe and recurrent macular edema than those with better HbA1c level, which may suggest that strict glycemic control may work in favor of anti-VEGF therapy. Similarly, diabetic nephropathy is a complicating factor for DR and DME, which has been investigated in a few studies but with contrasting conclusions drawn by the respective authors. For example, studies using proteinuria as a marker of renal disease failed to associate DME and renal pathology [25] while a study based on data from a German/Austrian database found that macroalbuminuria increased the risk of DME by 177% [26]. Because of these differences in results using albuminuria as a marker, we chose GFR as a marker of renal failure (using the CKD–EPI formula [27]). In the study by Romero et al. [28], the severity of renal damage was correlated with the development of DME. Knudsen et al. showed a correlation between the measurement of macular thickness in OCT and the urinary albumin excretion rate in patients with type 2 diabetes mellitus and DME [29, 30]. Finally, renal dialysis is likely to rapidly alleviate DME in patients with end-stage renal disease.
Our study is the first real-world study using precisely recognized thresholds for diabetes and chronic renal failure (7% for HbA1c and 60 ml/min/1.73 m2 for GFR). However, we could not demonstrate a significant association between HbA1c level, GFR and final VA or CST at 24 months. Our results are similar to prior studies using these metabolic factors to investigate the effects of anti-VEGF treatment.
Effect of HbA1c Level on Visual and Anatomic Outcomes
We identified a few studies that analyzed the impact of HbA1c level on visual parameters. In a small prospective study of 38 patients, Warid al Laftah et al. [31] demonstrated that a greater proportion of patients with HbA1c level < 7% showed an improvement in VA compared with those with HbA1c level > 7%, which suggests that better glycemic control improves visual prognosis. In contrast, a prospective study of 52 patients by Macky and Mahgoub [32] did not find any difference at 6 months between patients with HbA1c levels > 7% versus < 7% in VA or CST after three intravitreal injections. More recently, in a retrospective analysis of 124 patients, Matsuda et al. [16] demonstrated that patients with initial HbA1c < 7% had a better 12-month VA outcome than those with initial HbA1c level > 7%. The authors concluded that “patients with more optimal HbA1c had better final BCVA after one-year anti-VEGF treatment”. However, in this study [16], baseline vision was worse in patients with HbA1c level > 7% than in those with HbA1c < 7%, which may have confounded the results. Wong et al. reported the same results [33] but with a small sample size (35 patients). A recent analysis by Alok et al. [19] found no significant differences at 36 months in vision, change in vision or CST between patients with baseline HbA1c < 7% and > 7%. Our results are similar to those of Ozurk et al.[34]: after 24 months of anti-VEGF treatment, we found no difference in VA or CST in patients with HbA1c level ≤ 7% versus > 7% on multivariate analysis which adjusted for the significantly worse baseline vision in patients presenting with HbA1c > 7%. Our univariate analysis found a negative correlation between baseline HbA1c and VA at 24 months, which was very likely due to the worse baseline vision in eyes in patients with higher HbA1c. Indeed, previous studies [31] that have shown a significant correlation between HbA1c level and VA after treatment did not adjust for this key factor, which may confound the results. Thus, higher HbA1c may impact outcomes indirectly via worse presenting VA, but otherwise does not directly impact patient response to anti-VEGF treatment.
Effect of GFR on Visual and Anatomic Outcomes
We did not find a significant difference in final VA between the GFR groups. However, we did find a significant difference in final macular thickness (greater thickness with GFR > 60 than ≤ 60 ml/min/1.73 m2), likely because of the difference in initial CST between the subgroups (baseline mean CST significantly thicker with baseline GFR > 60 ml/min/1.73 m2 than with baseline GFR ≤ 60 ml/min/1.73 m2: 460.8 and 401.9 µm, respectively; p = 0.014). Why patients with better GFR have a higher initial CST is unclear. Of note, patients with thicker CST (GFR > 60 ml/min/1.73 m2) had better visual outcomes; this is reflection that many factors are involved in the visual prognosis and that anatomic edema is not the only factor.
Older retrospective analyses tended to find a significant correlation between renal failure and visual prognosis after anti-VEGF treatment, but these were low-power studies with few patients [31]. Our results agree with those of recent high-powered studies which found no relation between GFR and anti-VEGF efficacy [16]. One finding of interest, reported by Raafay et al. [17], was the poorer visual prognosis for untreated patients with DME and renal insufficiency compared to that for those without renal pathology. Renal disease results in a lower clearance of urea and other toxins from plasma, so plasma may be more damaged when it leaks into interstitial spaces in the macula.
Study Limitations
We analyzed the impact of HbA1c and GFR at baseline, but an exploration of the longitudinal fluctuations in HbA1c level and GFR over time and their correlation with VA and macular thickness at the 24-month time point would have provided valuable insights. However, recent studies [35] did not show any significant differences using this technique. Nevertheless, investigating the effects of these fluctuations could provide valuable insights for optimizing patient management in future studies.
It would also have been appropriate to analyze the combination of HbA1c level and GFR, that is, analyze patients with high HbA1c level but low GFR and those with low HbA1c level but high GFR. We found sample sizes were small when stratified by both parameters, but the results of this analysis are reported in Electronic Supplementary Material Table 1.
The main limitation of our study is the small sample size considering the high prevalence of the disease. However, despite this constraint, our study provides a meaningful portrayal of real-world circumstances and potentially informs the development of treatment algorithms.
Another limitation is our focus solely on the initial macular thickness as a baseline characteristic, without considering other confounding OCT parameters, such as disorganization of retinal inner layers (DRIL) or atrophic/degenerative changes. These additional parameters could have provided valuable insights, as they potentially impact VA and central macular thickness. It is crucial to acknowledge that this limitation introduces a potential bias in our findings.
As this was a real-world study, treatment decisions were made at the discretion of the practitioner in consultation with the patient without a guided study protocol. We therefore do not know the treatment regimen nor the re-treatment criteria. While there was no difference in the number of injections between the HbA1c subgroups, patients with GFR > 60 ml/min/1.73 m2 received significantly more injections, likely due to these presenting with a significantly higher CST. While this presents a possible bias, it reflects real-world practice.
Finally, we analyzed only two groups according to the proposed variable. While several cut-offs for stratifying HbA1c and GFR could have been explored, the aim of our study was to provide concrete and easy-to-use elements to practitioners (and also patients) and the figures 7% and 60 ml/min/1.73 m2 are elements recognized in the literature as thresholds of clinical aggravation for these pathologies.
Conclusions
We found no association of baseline HbA1c level and GFR with the functional and anatomical outcomes in DME patients receiving intravitreal VEGF inhibitor. Recognizing the independence of treatment response from baseline HbA1c level and GFR empowers clinicians to make informed decisions regarding the frequency and timing of anti-VEGF re-injections in this patient population.
References
Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003.
Kiire CA, Porta M, Chong V. Medical management for the prevention and treatment of diabetic macular edema. Surv Ophthalmol. 2013;58:459–65.
Heier JS, Korobelnik J-F, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376–85.
Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405.
Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Bhandari S, Nguyen V, Fraser-Bell S, et al. Ranibizumab or aflibercept for diabetic macular edema: comparison of 1-year outcomes from the Fight Retinal Blindness! registry. Ophthalmology. 2020;127:608–15.
Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257–69.
Campos A, Campos EJ, do Carmo A, et al. Evaluation of markers of outcome in real-world treatment of diabetic macular edema. Eye Vis (Lond). 2018;5:27.
Santos AR, Costa MÂ, Schwartz C, et al. Optical coherence tomography baseline predictors for initial best-corrected visual acuity response to intravitreal anti-vascular endothelial growth factor treatment in eyes with diabetic macular edema: the CHARTRES Study. Retina. 2018;38:1110–9.
Busch C, Okada M, Zur D, et al. Baseline predictors for visual acuity loss during observation in diabetic macular oedema with good baseline visual acuity. Acta Ophthalmol. 2020;98:e801–6.
Moreira RO, Trujillo FR, Meirelles RM, Ellinger VC, Zagury L. Use of optical coherence tomography (OCT) and indirect ophthalmoscopy in the diagnosis of macular edema in diabetic patients. Int Ophthalmol. 2001;24:331–6.
Hsieh Y-T, Alam MN, Le D, et al. OCT angiography biomarkers for predicting visual outcomes after ranibizumab treatment for diabetic macular edema. Ophthalmol Retina. 2019;3:826–34.
Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334–40.
Matsuda S, Tam T, Singh RP, et al. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J Diabetes Complic. 2014;28:166–70.
Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015;122:1395–401.
Shalchi Z, Okada M, Bruynseels A, et al. Effect of glycosylated hemoglobin on response to ranibizumab therapy in diabetic macular edema: real-world outcomes in 312 patients. Can J Ophthalmol. 2018;53:415–9.
Bansal AS, Khurana RN, Wieland MR, et al. Influence of glycosylated hemoglobin on the efficacy of ranibizumab for diabetic macular edema: A post hoc analysis of the RIDE/RISE trials. Ophthalmology. 2015;122(8):1573–9.
Save Sight Registries. https://frbresearch.org/login. Accessed 1 Sep 2021
American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14-80.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52.
Bandello F, Battaglia Parodi M, Lanzetta P, Loewenstein A, Massin P, Menchini F, Veritti D. Diabetic macular edema. Dev Ophthalmol. 2017;58:102–38.
Persistent diabetic macular edema is associated with elevated hemoglobin A1c. https://pubmed-ncbi-nlm-nih-gov.ezpum.biu-montpellier.fr/15808156/. Accessed 21 Nov 2022
Aroca PR, Espeso Sentís O, Del Castillo DD. Prospective study of correlation between diabetic retinopathy and microalbuminuria in diabetes type 1 patients. Arch Soc Esp Oftalmol. 2000;75:307–14.
Hammes H-P, Welp R, Kempe H-P, et al. Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV database. PLoS ONE. 2015;10:e0132492.
Chen Y-W, Chen H-H, Wang T-E, et al. Difference between CKD-EPI and MDRD equations in calculating glomerular filtration rate in patients with cirrhosis. World J Gastroenterol. 2011;17:4532–8.
Romero-Aroca P, Reyes-Torres J, Baget-Bernaldiz M, Blasco-Suñe C. Laser treatment for diabetic macular edema in the 21st century. Curr Diabetes Rev. 2014;10:100–12.
Knudsen ST, Bek T, Poulsen PL, Hove MN, Rehling M, Mogensen CE. Macular edema reflects generalized vascular hyperpermeability in type 2 diabetic patients with retinopathy. Diabetes Care. 2002;25:2328–34.
Koo NK, Jin HC, Kim KS, Kim YC. Relationship between the morphology of diabetic macular edema and renal dysfunction in diabetes. Korean J Ophthalmol. 2013;27:98–102.
Warid Al-Laftah FA, Elshafie M, Alhashimi M, Pai A, Farouq M. Pretreatment clinical variables associated with the response to intravitreal bevacizumab (Avastin) injection in patients with persistent diabetic macular edema. Saudi J Ophthalmol. 2010;24:133–8.
Macky TA, Mahgoub MM. The effect of glycemic control on visual and anatomic outcomes in response to therapy for diabetic macular edema. Eur J Ophthalmol. 2012. 10.5301/ejo.5000179.
Wong WM, Chee C, Bhargava M, et al. Systemic factors associated with treatment response in diabetic macular edema. J Ophthalmol. 2020;2020:1875860.
Ozturk BT, Kerimoglu H, Adam M, Gunduz K, Okudan S. Glucose regulation influences treatment outcome in ranibizumab treatment for diabetic macular edema. J Diabetes Complic. 2011;25:298–302.
Singh RP, Habbu K, Ehlers JP, Lansang MC, Hill L, Stoilov I. The impact of systemic factors on clinical response to ranibizumab for diabetic macular edema. Ophthalmology. 2016;123:1581–7.
Acknowledgements
We thank the participants of the study
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing and/or Editorial Assistance
We would like to express our sincere gratitude to Hannah Crowdy, Clinical Research Associate, for her significant contributions to the compilation of data for this study. Her technical assistance and expertise have been invaluable to the completion of this research.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Vuong Nguyen. The first draft of the manuscript was written by Robin Medard, Vincent Daien and Eloi Debourdeau. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
This work was presented as a poster at the ARVO 2023 conference, New Orleans, 23–27 April, 2003.
Disclosures
The authors declare no competing interests or associations, whether commercial or otherwise, that may be perceived to pose a competing interest in connection with the submitted material.
Compliance with Ethics Guidelines
The trial was designed and conducted in accordance with the principles of the Declaration of Helsinki and its later amendments. This study was approved by the institutional review boards and ethics committees of each participating center. All patients provided oral informed consent before being enrolled in the study.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Debourdeau, E., Medard, R., Chamard, C. et al. Does HbA1c Level or Glomerular Filtration Rate Affect the Clinical Response to Endothelial Growth Factor Therapy (Ranibizumab or Aflibercept) in Diabetic Macular Edema? A Real-Life Experience. Ophthalmol Ther 12, 2657–2670 (2023). https://doi.org/10.1007/s40123-023-00758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00758-1