Abstract
Introduction
In current clinical practice, several optical coherence tomography (OCT) biomarkers have been proposed for the assessment of severity and prognosis of different retinal diseases. Subretinal pseudocysts are subretinal cystoid spaces with hyperreflective borders and only a few single cases have been reported thus far. The aim of the study was to characterize and investigate this novel OCT finding, exploring its clinical outcome.
Methods
Patients were evaluated retrospectively across different centers. The inclusion criterion was the presence of subretinal cystoid space on OCT scans, regardless of concurrent retinal diseases. Baseline examination was set as the first time the subretinal pseudocyst was identified by OCT. Medical and ophthalmological histories were collected at baseline. OCT and OCT-angiography were performed at baseline and at each follow-up examination.
Results
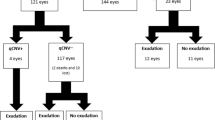
Twenty-eight eyes were included in the study and 31 subretinal pseudocysts were characterized. Out of 28 eyes, 16 were diagnosed with neovascular age-related macular degeneration (AMD), 7 with central serous chorioretinopathy, 4 with diabetic retinopathy, and 1 with angioid streaks. Subretinal and intraretinal fluid were present in 25 and 13 eyes, respectively. Mean distance of the subretinal pseudocyst from the fovea was 686 µm. The diameter of the pseudocyst was positively associated with the height of the subretinal fluid (r = 0.46; p = 0.018) and central macular thickness (r = 0.612; p = 0.001). At follow-up, subretinal pseudocysts disappeared in most of the reimaged eyes (16 out of 17). Of these, two patients presented retinal atrophy at baseline examination and eight patients (47%) developed retinal atrophy at follow-up. Conversely, seven eyes (41%) did not develop retinal atrophy.
Conclusion
Subretinal pseudocysts are precarious OCT findings, usually disclosed in a context of subretinal fluid, and are probably transient alterations within the photoreceptor outer segments and retinal pigment epithelium (RPE) layer. Despite their nature, subretinal pseudocysts have been associated with photoreceptor loss and incomplete RPE definition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Different optical coherence tomography (OCT) biomarkers have been proposed in the management of different retinal diseases. |
This study aimed to characterize and explore subretinal pseudocysts as a novel OCT finding and to distinguish them from outer retinal tubulations. |
Subretinal pseudocysts are transient OCT findings, involving different retinal diseases and usually located inside subretinal fluid. |
Subretinal pseudocyst resolution has been associated with photoreceptor loss and signs of retinal atrophy. |
Introduction
In the current clinical practice of retinal diseases, the severity and prognosis of neovascular age-related macular degeneration (AMD) are mainly assessed by defining disease type, identifying several biomarkers that may be present at clinical evaluation and treatment response of the patients to intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections [1,2,3]. The outstanding improvements in optical coherence tomography (OCT) and the introduction of OCT-angiography (OCT-A) provided groundbreaking advances to further understand the various types of macular neovascularization (MNV) and accompanying biomarkers, which encourages the use of multimodal imaging as an essential approach in neovascular AMD [2, 4,5,6,7,8,9,10]. Of these biomarkers, there is particular interest in retinal cystoid lesions.
Intraretinal cystoid-like spaces were previously defined as pseudocysts that could emerge as a result of Müller cell-related degeneration in eyes affected by AMD [11]. Although not strictly caused by an active exudation, the presence of intraretinal pseudocysts might be predictive of poorer visual prognosis in type 1 and 2 MNV, but they are not good predictors of outcome in type 3 MNV [7, 12]. Recently, subretinal pseudocyst lesions were detected as new OCT findings in AMD and diabetic macular edema [13, 14]. Subretinal pseudocyst lesions are defined as a subretinal cystoid space surrounded by hyperreflective edges. As subretinal Müller cell migration was previously shown by Edwards et al. in eyes with geographic atrophy (GA) [15], it is speculated that subretinal pseudocyst structures might be due to migrated Müller cell activity [13, 14].
In addition, few preliminary studies could be found in the literature in terms of the clinical outcome of subretinal hyporeflective spaces in AMD. Namely, the presence of subretinal optically empty spaces, without any hyperreflectivity at their borders with at least one concave or straight border, along with intraretinal cysts, has been associated with lower visual gains after therapy [16]. However, most of those subretinal spaces were probably just subretinal fluid, since no hyperreflective edge was considered and explored. As a matter of fact, subretinal pseudocysts should be distinguished from subretinal hyporeflective spaces since they are localized very close to photoreceptor outer segments or inside subretinal fluid, and they are surrounded by hyperreflective borders with cystoid appearance.
The aim of the study was to improve our knowledge of subretinal pseudocysts by investigating their structural and functional characteristics through multimodal imaging and evaluating their clinical outcomes.
Methods
This was a retrospective study enrolling patients from four retina referral centers (the Medical Retina and Imaging Unit of the Department of Ophthalmology of San Raffaele Scientific Institute, Milan, Italy; the Medical Retina Service of University Hospital “Maggiore della Carità”, Novara, Italy; the Retinal Disorders and Ophthalmic Genetics Division, Stein Eye Institute, University of California, Los Angeles, California, USA; the Department of Ophthalmology of University Paris Est, in Creteil, France) between June 2016 and June 2022. This retrospective study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study and publication of the information. The ethics committee of IRCCS Ospedale San Raffaele was notified about this study. According to Italian law, retrospective studies require the ethics committee to be notified, but do not require its approval.
The inclusion criterion was the presence of subretinal cystoid space on OCT scans, according to the previous reported cases of subretinal pseudocysts in the literature, regardless of concurrent ocular diseases. Subretinal pseudocyst has been defined as a hyporeflective structure at either immediate border of photoreceptor outer segments or inside subretinal fluid, which is surrounded by a relatively hyperreflective border, giving it a cystoid appearance. Patients with significant optic media opacities limiting image quality were excluded from the study.
Baseline was set as the first time the subretinal pseudocyst was identified. Past medical history and ongoing systemic therapies were collected at baseline. All patients underwent a complete ophthalmological examination, including best-corrected visual acuity (BCVA) on Snellen chart, slit-lamp biomicroscopy, intraocular pressure measurement, and indirect fundus examination at baseline and at each follow-up. BCVA was expressed as the logarithm of the minimum angle of resolution (logMAR) for statistical analyses. MultiColor imaging, infrared reflectance (IR), fundus autofluorescence (FAF), and structural spectral-domain OCT (SD-OCT) were acquired at each ophthalmological examination. OCT-A was performed in a substantial number of patients. MultiColor imaging, IR, FAF, and SD-OCT were performed using Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). Central macular thickness (CMT) in the central 1-mm-diameter circle of the Early Treatment Diabetic Retinopathy Study (ETDRS) thickness map was recorded with Spectralis software (Heidelberg Eye Explorer, Version 1.9.11.0; Heidelberg Engineering). All OCT-A examinations were acquired with Plex Elite 9000 Swept-Source OCT-A (Zeiss Meditech, Inc, Dublin, California, USA). Whenever performed, OCT-A considered a scanning area centered on the lesion. OCTA slabs were automatically segmented by OCT-A software and manually adjusted by a retinal expert ophthalmologist (RS).
Statistical analyses were performed using SPSS Statistics Software version 27.0 (IBM, Armonk, New York, USA). We set a threshold for statistical significance at p < 0.05. Categorical variables were expressed as absolute count and percentages. Continuous variables were summarized as mean ± standard deviation if normally distributed. If not, median and interquartile range (IQR) were provided. Normal distribution of continuous variables was tested using the Kolmogorov–Smirnov test. We explored correlations between OCT and OCT-A quantitative metrics using Pearson’s correlation and we reported correlation coefficients (r) and their 95% confidence interval (CI). We longitudinally explored BCVA and CMT using Student’s paired samples t test. Cross-sectional comparison of quantitative data in different subgroups was performed using Student’s independent samples t test.
Results
Twenty-eight eyes of 28 patients (11 female, 39%; 17 male, 61%; mean age 73 ± 14 years) were included in the study. A total of 31 subretinal pseudocysts were identified: 25 out of 28 eyes (89%) presented single subretinal pseudocysts; 3 eyes (11%) presented two subretinal pseudocysts each. All 28 patients were Caucasian. Eight out of 28 patients (29%) were affected by type 2 diabetes mellitus (T2DM). Medically controlled arterial hypertension was the most common systemic disease (14 out of 28 patients, 50%). Six patients (21%) did not have any prior known systemic disease during the medical history collection. Systemic diseases and ongoing therapies are summarized in Table 1 for each patient.
Twenty-one patients were enrolled from the Department of Ophthalmology of San Raffaele Scientific Institute, four patients from the Medical Retina Service of University Hospital “Maggiore della Carità”, two patients from the Retinal Disorders and Ophthalmic Genetics Division, Stein Eye Institute, and one patient from the Department of Ophthalmology of University Paris Est.
Sixteen out of 28 eyes (57%) were diagnosed with AMD, 7 (25%) with central serous chorioretinopathy (CSC), 4 (14%) with diabetic retinopathy, and 1 eye with angioid streaks complicated by choroidal neovascularization (CNV). Twenty-four (86%) out of 28 eyes underwent previous ocular treatment before pseudocyst presented. Namely, 18 eyes were treated with intravitreal injection of anti-VEGF agents. All previous ocular treatments were recorded and are summarized in Table 1.
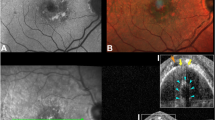
At baseline, mean BCVA was approximately 20/50 Snellen equivalent (0.40 ± 0.26 logMAR). Out of 28 eyes, subretinal and intraretinal fluid were disclosed in 25 (89%) and 13 (46%) eyes, respectively. Retinal pigment epithelium (RPE) elevation beneath the pseudocyst was observed in 15 eyes (54%). Out of 31 subretinal pseudocysts, 21 (65%) of them were located less than 500 µm from the fovea, 7 between 500 µm and 1500 µm, and 3 over 1500 µm. Concurrent subretinal fluid was present in 25 out of 28 eyes (89%), whereas intraretinal fluid was present in 13 eyes (46%). The mean distance of the subretinal pseudocyst from the fovea was 686 + 644 µm (median 450 µm; IQR 252–774 µm). Subretinal fluid, if any, was measured in its greatest height (158 ± 126 µm; median 136 µm, IQR 69–180 µm). The greatest diameter of the subretinal pseudocyst was measured and recorded. Mean value of the greatest diameter of the pseudocyst was 141 ± 132 µm. The diameter of the pseudocyst was positively associated with height of the subretinal fluid (r = 0.46; CI 0.08–0.73; p = 0.018) and the CMT (r = 0.612; CI 0.29–0.81; p < 0.01). Furthermore, we characterized the location of subretinal psudocysts within the subretinal space, particularly regarding RPE and neurosensory retina (Fig. 1). Eighteen (58%) out of 31 displayed connection both with the RPE and the neurosensory retina (Fig. 1a, b), 7 (23%) exclusively with the RPE (Fig. 1c), 3 (10%) exclusively with the neurosensory retina (Fig. 1d), and 3 (10%) did not display any connection and appeared as a floating cystoid space within the subretinal fluid (Fig. 1e).
Spectral-domain optical coherence tomography (SD-OCT) of subretinal pseudocysts. a Two subretinal pseudocysts without the presence of subretinal fluid and connected to both the retinal pigment epithelium (RPE) and the neurosensorial retina. b Single subretinal pseudocyst connected to both the RPE and the neurosensorial retina in the presence of subretinal fluid. c Single subretinal pseudocyst exclusively connected to RPE. d Single subretinal pseudocyst strictly connected to neurosensorial retina only. e Single subretinal pseudocyst appearing as floating cystoid space within the subretinal fluid
OCT-A was performed in 15 eyes. On B-scan, flow inside the cystoid space was present in six eyes out of 15 (40%) Subretinal pseudocysts with signs of flow on B-scan OCT-A presented a statistically significant greater diameter, compared to those pseudocysts which did not display flow on B-scan OCT-A (149 + 48 mm vs 78 + 49 mm; p = 0.018).
Seventeen out of 28 patients (61%) were re-examined with a median follow-up time of 2 months (IQR 1–5 months). The subretinal pseudocysts disappeared in most of the examined eyes (16 out of 17, 94%). Only one pseudocyst was present at a 4-month follow-up after a single intravitreal injection of ranibizumab, with no remarkable changes in its diameter and in the greatest height of subretinal fluid (respectively 86 µm vs 68 µm and 449 µm vs 433 µm). Fourteen out of 17 patients (82%) were treated with anti-VEGF intravitreal therapy in the eye with subretinal pseudocyst before follow-up examination. The remaining three patients did not receive any treatment prior to follow-up examination. At follow-up examination BCVA did not change significantly compared to the baseline (follow-up BCVA 0.52 ± 0.36 logMAR, median 0.52 logMAR, IQR 0.19–0.70 logMAR, p = 0.35). Similarly, follow-up CMT did not show significant changes (medium value 342 µm + 109 µm, median 331 µm, IQR 253–414 µm, p = 0.245). Subretinal fluid displayed a significant change in its greatest height at follow-up compared to the baseline (baseline height 154 ± 30 µm; follow-up 104 ± 30 µm; p = 0.049).
Out of 17 re-examined patients, 2 (11%) presented retinal atrophy at baseline examination and both of eyes presented subretinal pseudocysts above a fibrotic scar. Eight patients (47%) developed different stages of retinal atrophy at a median follow-up of 5.5 months (IQR 5–8 months) (Fig. 3). Conversely, seven eyes (41%) did not develop retinal atrophy during a median follow-up of 21 months (IQR 16–26 months). Out of eight patients who developed retinal atrophy at follow-up, two (25%) presented a large fibrovascular atrophic scar and six (75%) showed signs of loss of inner segment–outer segment (IS/OS) junction and signal hypertransmission in the site of the previous subretinal pseudocyst. In particular, the segment of retinal atrophy covered an area larger than the preceding subretinal pseudocyst in all patients. Both eyes with nAMD and CSC presented atrophy at follow-up (six and two eyes, respectively).
Discussion
Subretinal pseudocysts were first reported by Sacconi et al. as a novel OCT finding in a patient affected by diabetic retinopathy and were described as a cystoid structure with hyperreflective edges occupying the subretinal space. The current multicenter study retrospectively investigates structural characteristics of subretinal pseudocysts in 28 eyes and provides a comprehensive overview of their characteristics including the most frequently associated ocular conditions and treatment response [13].
Subretinal pseudocysts were identified in different retinal diseases (AMD, CSC, DR, and angioid streaks complicated by CNV), with heterogeneous pathogenesis. Retinal dynamic changes are shared by different diseases and can explain subretinal pseudocyst formation. Most of them (87%) were associated with subretinal fluid, and they did not show any preferential localization within the macula. They rather localized inside or nearby subretinal fluid, and this supports the idea that subretinal pseudocysts could be disclosed in a general setting of retinal pathological changes. As previously presented, subretinal pseudocysts presented different depth of location with respect to the neurosensory retina and the RPE. Although the majority (21 out of 31) presented a connection to photoreceptors, three of them appeared as floating cystoid structures within the subretinal space and seven were strictly connected to the RPE only. Despite the heterogeneity of findings, the presence of connection to the neurosensorial retina for most of the lesions could support the theoretical role of the outer retinal structures, such as photoreceptor outer segments, and Müller cell processes in the pathogenesis of subretinal pseudocysts. In addition, the association with the loss of definition of photoreceptor layer and hypertransmission could support the role of subretinal pseudocyst as a marker of retinal stress, commonly shared in sever retinal conditions in our cohort of patients.
Less than half of the subretinal pseudocysts presented flow inside the cystoid space on B-scan OCT-A. Specifically, whenever present, flow signal was weak (Fig. 2), a phenomenon which could be attributable to a suspended scattering particles in motion (SSPinM) effect [17]. Subretinal pseudocysts with flow inside the cystoid space were larger in their maximum diameter, compared to those which did not display flow on B-scan OCT-A. However, the presence of flow inside the pseudocyst did not show any association with concurrent ocular disease, the location of the pseudocyst, or the presence of subretinal fluid. On the basis of this data, we speculated that a chaotic Brownian motion of particles could be present inside the cystoid space, probably enhanced by saccadic movement. It could be argued that a certain width of space is needed to obtain an SSPinM-related signal from cystoid lesions. Similarly, we hypothesized that the size and volume of the pseudocyst could have a limit for OCT-A to detect the low signal inside due to chaotic motion of particles. Of note, as Kashani et al. [17] have largely discussed, Brownian motion presumes the presence of suspended particles inside the cystoid space; they also postulated about the lipidic and lipoproteinaceous nature of the intracystoid fluid, which should be related to the exudative activity and rupture of blood-retinal barrier.
Retinal and subretinal hyporeflective spaces can characterize different OCT findings in retinal diseases, and ophthalmologists should carefully consider their features. In particular, outer retinal tubulations (ORT) have been extensively described in several retinal disorders after retinal damage. ORT are stationary hyporeflective spaces inside the outer nuclear layer with hyperreflective borders [18]. Their stationary nature with a branching network and the localization allow ORT to be distinguished from subretinal pseudocysts (Table 2). Conversely, subretinal cystoid spaces have been described as hyporeflective lesions between the neuroepithelium and the RPE and they do no present hyperreflective borders, which characterize subretinal pseudocysts instead [16] (Table 2).
Although structural characteristics of subretinal pseudocysts have been extensively defined, little is known about their etiopathogenesis. Sacconi et al. [13, 14] speculated that Müller cells could concur in the aetiology of subretinal pseudocysts. As a matter of fact, Müller cells play a key role in electrolytic homeostasis within the retina [19, 20] and it is still debated whether retinal cysts are intracellular or interstitial [21]. In addition, the migration of Müller cells into the subretinal space has been described in different conditions: this event could be due to concurrent ocular conditions and prior to pseudocyst formation [15, 22]. Once migrated, Müller cells have been described beyond the external limiting membrane and their processes have been observed adjacent and within Bruch’s membrane [15]. The high variability of the extension of this glial subretinal scaffold is in agreement with our findings, since pseudocysts have been found to be strictly connected to the neurosensory retina, the RPE, or both. However, in vitro studies of subretinal pseudocysts are still lacking and their disappearance in short-term follow-up could suggest a more precarious scaffold. Since migration of RPE cells has been already documented through in vivo and in vitro studies, we cannot exclude their role in the pathogenesis of subretinal pseudocyst [23,24,25]. However, OCT findings of documented migration of RPE do not refer to subretinal, but rather intraretinal areas, presuming the anatomical connection between RPE and neurosensory retina, as well as RPE clumping prior to migration [23]. As a matter of fact, the features we reported in our series do not meet the aforementioned characteristics of RPE cell migration and make this event unlikely in the considered subset of eyes.
The provisional nature of subretinal pseudocysts could imply a non-organized nature, as suggested by their disappearance at short-term follow-up. The presence of photoreceptor debris in different degenerative retinal diseases has been reported [26, 27]. In addition, photoreceptor debris can present cavitation and these findings could support their role in the pathogenesis of subretinal pseudocysts [26, 28]. On the other hand, the presence of photoreceptor debris presumes photoreceptor degeneration and disruption, and this finding is not consistently present in our series.
Since subretinal pseudocysts present hyperreflective edges and particles inside the cystoid space in the OCT, we cannot conclude whether they come from the presence of organized inert substances, a fibrinous material, or a pooling of blood. However, we should consider that blood or fibrous material usually presents a more hyperreflective appearance on OCT. Thus, we cannot be certain about the exact nature of this novel OCT finding. As discussed above, this transitory cystoid space with hyperreflective edge containing suspended particles implies an organized but provisional scaffold.
In our study, we observed that subretinal pseudocysts generally show a single-visit appearance, which is usually together with the presence of subretinal fluid (SRF). The only case with a consecutive presence of subretinal pseudocyst had indeed an accompanying, persistent SRF on the follow-up visit. It could therefore be argued that rather than solid structural formations that are treatment resistant, subretinal pseudocysts are transient alterations within the photoreceptor outer segments and RPE layer that are dependent on the subretinal space created by SRF and therefore disappear following the resolution of SRF by anti-VEGF treatment. The apparent stability in visual and anatomical outcomes of these lesions in the follow-up could be interpreted in favor of them being transient alterations rather than solid modifications. On the other hand, these lesions could be defined as intermediary findings during the degenerative remodelling of outer retinal layers that are linked to RPE and Müller cell migration or photoreceptor outer segment disruption. However, this hypothesis should be confirmed by a prospective follow-up study arm that is not administered intravitreal injections, which would be subject to both ethical and practical challenges in the current clinical practice.
Despite their nature, subretinal pseudocysts have been associated with photoreceptor loss and signs of incomplete RPE and outer retinal atrophy (iRORA) (Fig. 3). Photoreceptor and RPE stress in several diseases could lead to micro-anatomical changes, resulting in subretinal pseudocyst development: this novel OCT finding could virtually represent a sign of poor retinal anatomical prognosis, since subretinal pseudocyst could be the result of photoreceptor or RPE damage in several retinal diseases and predate retinal atrophy.
Baseline B-scan optical coherence tomography (OCT) showing subretinal pseudocysts (red asterisks, a and b) in a patient with central serous chorioretinopathy (a) and age-related macular degeneration (AMD) (b). On follow-up OCT imaging, subretinal pseudocysts disappeared, with the loss of the external limiting membrane (ELM), the ellipsoid zone (EZ), and the interdigitation zone (EZ) (yellow asterisks, c and d)
The main limitations of our study are its small sample size and retrospective nature. Multimodal imaging criteria with inclusion of OCT-A were achieved by a partial number of patients, which constituted a limiting factor for the evaluation of in vivo intralesional fluid dynamics. The lack of ex vivo histological evidence of subretinal pseudocysts leads to hypothetical assessments of the true nature of these lesions. However, we believe that with the introduction of novel multimodal imaging features, our study would contribute to the enhancement of clinical knowledge concerning subretinal pseudocysts, a recent clinical entity that is encountered during the follow-up of cases that suffer from AMD.
Conclusions
In this study, we attempted to define structural and topographic characteristics of subretinal pseudocysts, a novel finding that could be detected during exudative AMD. The lesions can be present either at the immediate border of a detached neurosensorial retina or within the subretinal space as floating structures. A considerable number of subretinal pseudocysts can show an intralesional low density flow signal which is attributable to the suspended scattering particles in motion effect. Given their transient nature, it is difficult to establish a direct effect of subretinal pseudocysts over visual and anatomical outcomes during the clinical course of AMD; however, further studies with larger sample size and prospective design would be beneficial to perform assessments with higher clinical evidence.
References
Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–36.
Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24.
Hilely A, Au A, Freund KB, et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br J Ophthalmol. 2021;105:1415–20.
Gao SS, Jia Y, Zhang M, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:27–36.
Holz FG, Sadda SVR, Staurenghi G, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:464–78.
Guymer R, Wu Z. Age-related macular degeneration (AMD): More than meets the eye. The role of multimodal imaging in today’s management of AMD—a review. Clin Exp Ophthalmol. 2020;48:983–95.
Sacconi R, Forte P, Tombolini B, et al. OCT predictors of 3-year visual outcome for type 3 macular neovascularization. Ophthalmol Retina. 2022;6:586–94.
Sacconi R, Brambati M, Miere A, et al. Characterisation of macular neovascularisation in geographic atrophy. Br J Ophthalmol. 2022;106:1282–7.
Borrelli E, Bandello F, Souied EH, et al. Neovascular age-related macular degeneration: advancement in retinal imaging builds a bridge between histopathology and clinical findings. Graefes Arch Clin Exp Ophthalmol. 2022. https://doi.org/10.1007/s00417-022-05577-x.
Sacconi R, Fragiotta S, Sarraf D, et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog Retin Eye Res. 2022. https://doi.org/10.1016/j.preteyeres.2022.101113.
Cohen SY, Dubois L, Nghiem-Buff S, et al. Retinal pseudocysts in age-related geographic atrophy. Am J Ophthalmol. 2010;150(2):211–7.
Querques G, Coscas F, Forte R, Massamba N, Sterkers M, Souied EH. Cystoid macular degeneration in exudative age-related macular degeneration. Am J Ophthalmol. 2011;152:100–107.e2.
Sacconi R, Lutty GA, Mullins RF, Borrelli E, Bandello F, Querques G. Subretinal pseudocysts: a novel OCT finding in diabetic macular edema. Am J Ophthalmol Case Rep. 2019;16:4–6.
Sacconi R, Mullins RF, Lutty GA, Borrelli E, Bandello F, Querques G. Subretinal pseudocyst: a novel optical coherence tomography finding in age-related macular degeneration. Eur J Ophthalmol. 2020;30:24–6.
Edwards MM, McLeod DS, Bhutto IA, Grebe R, Duffy M, Lutty GA. Subretinal glial membranes in eyes with geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58:1352–67.
Hayashi-Mercado R, Pérez-Montaño C, Reyes-Sánchez J, Ramírez-Estudillo A. Findings of uncertain significance by optical coherence tomography (OCT) as prognostic factors in neovascular age-related macular degeneration (nAMD) treated with ranibizumab. Int J Retina Vitreous. 2022;8:1–7.
Kashani AH, Green KM, Kwon J, et al. Suspended scattering particles in motion: a novel feature of OCT angiography in exudative maculopathies. Ophthalmol Retina. 2018;2(7):694–770.
Damasceno NA, Damasceno EF, Silva FQ, Singh RP. Outer retinal tubulation and neovascular age-related macular degeneration: a review of the pathogenesis and clinical implications. Ophthalmic Surg Lasers Imaging Retina. 2018;49(11):870–6.
Nagelhus EA, Horio Y, Inanobe A, et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54.
Nagelhus EA, Veruki ML, Torp R, et al. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–19.
Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016;36:1823–42.
Lewis GP, Chapin EA, Luna G, Linberg KA, Fisher SK. The fate of Müller’s glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol Vis. 2010;16:1361.
Ho J, Witkin AJ, Liu J, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118:687–93.
Qiu S, Jiang Z, Huang Z, et al. Migration of retinal pigment epithelium cells is regulated by protein kinase Cα in vitro. Invest Ophthalmol Vis Sci. 2013;54:7082–90.
Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-α. Invest Ophthalmol Vis Sci. 2000;41(13):4324–32.
Cherepanoff S, Killingsworth MC, Zhu M, et al. Ultrastructural and clinical evidence of subretinal debris accumulation in type 2 macular telangiectasia. Br J Ophthalmol. 2012;96(11):1404–9.
Valter K, Maslim J, Bowers F, Stone J. Photoreceptor dystrophy in the RCS rat: roles of oxygen, debris, and bFGF. Invest Ophthalmol Vis Sci. 1998;39:2427–42.
Hisatomi T, Sakamoto T, Sonoda KH, et al. Clearance of apoptotic photoreceptors: elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin αvβ3. Am J Pathol. 2003;162:1869–79.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Giuseppe Querques, Francesco Bandello, Riccardo Sacconi, Alberto Quarta; Methodology: Matteo Menean, Cem Kesim, Riccardo Sacconi, Francesco Bandello, Giuseppe Querques; Formal analysis and investigation: Matteo Menean, Riccardo Sacconi, Stela Vujosevic, Cem Kesim, Alberto Quarta, Nicolò Ribarich, Leonardo Bottazzi, Assaf Hilely, Vittorio Capuano, Eric H Souied, David Sarraf, Francesco Bandello, Giuseppe Querques, Writing—original: Matteo Menean, Cem Kesim, Riccardo Sacconi, Francesco Bandello, Giuseppe Querques Writing—review and editing: Matteo Menean, Riccardo Sacconi, Giuseppe Querques Supervision: Riccardo Sacconi, Francesco Bandello, Giuseppe Querques.
Disclosures
Riccardo Sacconi has the following disclosures: Allergan Inc (consultant), Bayer Shering-Pharma (consultant), Medivis (consultant), Novartis (consultant), Zeiss (consultant). Eric H Souied has the following disclosures: Allergan (consultant), Novartis (consultant), Bayer (consultant), Roche (consultant). David Sarraf has the following disclosures: Amgen (consultant), Bayer (consultant), Genentech (consultant), Heidelberg (consultant), Novartis (consultant), Optovue (consultant), Regeneron (consultant), Topcon (consultant). Stela Vujosevic has the following disclosures: Abbvie-Allergan (consultant), Apellis (consultant), Bayer (consultant), Novartis (consultant), Roche (consultant). Giuseppe Querques has the following disclosures: Alimera Sciences (consultant), Allergan Inc (consultant), Amgen (consultant), Bayer Shering-Pharma (consultant), Heidelberg (consultant), KBH (consultant), LEH Pharma (consultant), Lumithera (consultant), Novartis (consultant), Sandoz (consultant), Sifi (consultant), Sooft-Fidea (consultant), Zeiss (consultant). Francesco Bandello has the following disclosures: Alcon (consultant), Alimera Sciences (consultant), Allergan Inc (consultant), Farmila-Thea (consultant), Bayer Shering-Pharma (consultant), Bausch and Lomb Genentech (consultant), Hoffmann-La-Roche (consultant), Novagali Pharma (consultant), Novartis (consultant), Sanofi-Aventis (consultant), Thrombogenics (consultant), Zeiss (consultant). Matteo Menean, Assaf Hilely, Nicolò Ribarich, Leonardo Bottazzi, Cem Kesim, Alberto Quarta and Vittorio Capuano have nothing to disclose.
Compliance with Ethics Guidelines
The ethics committee of IRCCS Ospedale San Raffaele was notified about this retrospective study. According to Italian law, retrospective studies require the ethics committee to be notified, but do not require its approval. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study and publication of the information.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Menean, M., Sacconi, R., Vujosevic, S. et al. Subretinal Pseudocysts: A Comprehensive Analysis of this Novel OCT Finding. Ophthalmol Ther 12, 2035–2048 (2023). https://doi.org/10.1007/s40123-023-00727-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00727-8