Abstract
Introduction
Posterior capsular opacification (PCO) is the most common complication of cataract surgery. In this study, we develop a model to quantitatively predict the probability of Nd:YAG laser capsulotomy for vision-threatening PCO to improve the life quality of postoperative patients.
Methods
A registry analysis of cataract procedures performed between the years 2010 and 2021. Following the screening of 16,802 patients (25,883 eyes), 9768 patients (eyes) were enrolled. The cohort was randomly divided into two groups: training (n = 6838) and validation (n = 2930). To identify relevant risk factors, univariate, multivariate, and Least Absolute Shrinkage and Selection Operator (LASSO) algorithm Cox regression analysis were employed, and a nomogram was created to demonstrate the prediction result.
Results
At 5 years, the overall cumulative incidence of Nd:YAG laser capsulotomy was 12.0% (1169/9768). The following variables were included in the prediction model: sex [hazard ratio (HR) = 1.53, 95% CI 1.32–1.76], age (HR = 0.71, 95% CI 0.56–0.88), intraocular lens (IOL) material (HR = 2.65, 95% CI 2.17–3.24), high myopia (HR = 2.28, 95% CI 1.90–2.75), and fibrinogen (HR = 0.79, 95% CI 0.72–0.88). In the validation cohort, the area under the curve (AUC) of 1-, 3-, and 5-year predictions for Nd:YAG laser capsulotomy were 0.702, 0.691, and 0.688, respectively. For a subgroup of patients with high myopia, the protective effect of hydrophobic IOL disappeared (HR = 0.68, 95% CI 0.51–1.12, P = 0.127).
Conclusion
This model could predict the probability of Nd:YAG laser capsulotomy for vision-threatening PCO after cataract surgery by taking into account factors such as age, gender, IOL material, high myopia, and fibrinogen. Meanwhile, implantation of a hydrophobic IOL in individuals with high myopia did not demonstrate a protective impact against vision-threatening PCO.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Our research revealed a marked reduction in the rates of Nd:YAG laser capsulotomy for vision-threatening posterior capsular opacification (PCO) compared with the results of previous similar studies, and the majority of laser treatment occurred within 3 years after cataract surgery. |
Deficiency of fibrinogen could increase the probability of Nd:YAG laser capsulotomy. |
It reveals no difference in the protective impact of vision-threatening PCO between hydrophobic and hydrophilic intraocular lens (IOLs) in high myopic eyes. |
By referring to factors such as gender, age, IOL material, fibrinogen level, and high myopia, the model could estimate the probability of Nd:YAG laser capsulotomy for vision-threatening PCO. |
Introduction
Cataract has long been the primary cause of blindness worldwide [1, 2]. It has a huge impact on people’s well-being and productivity, drastically lowering their quality of life and resulting in large individual and community economic costs [3]. Surgical intervention is the primary method of treating cataracts in modern clinical practice. Cataract surgery procedures have advanced significantly, with high success rates, and the majority of patients recover with satisfactory visual outcomes and lower problems [4]. However, the most prevalent cause of recurrent vision disruption after cataract surgery is posterior capsular opacification (PCO) [5, 6]. PCO results from the migration and modification of lens epithelial cells (LECs) along the posterior capsule, and leads to diminished visual acuity, impaired contrast sensitivity, and stray light impairment when the visual axis is involved [7, 8]. The prevalence of this complication increases over time and varies from 0.9% to 50% in different studies [9,10,11]. Differences among these stated incidence figures may be due to different study designs, diagnostic criteria, treatment indications for PCO, intraocular lens (IOL) properties, and the duration of follow-up.
The standard treatment for PCO is Nd:YAG laser capsulotomy, and it is utilized as a secondary measure for vision-threatening PCO in prior research [12,13,14,15,16]. With identical symptoms to cataracts, PCO is debilitating and distressing for the postoperative individual. With the invocation of new technologies such as femtosecond laser, cataract treatment will definitely become more personalized in the future [17]. However, we have not yet been able to provide tailored treatment and long-term follow-up recommendations for patients with specific conditions. The risk factors for PCO are often discussed, including perioperative conditions [18, 19], IOL design, surgical technique [20], and so on, but the quantitative prediction of the probability of Nd:YAG laser capsulotomy for vision-threatening PCO has seldom been examined.
Clinical prediction models use a statistical approach to integrate multiple clinical factors into a graph or scale to forecast prognosis probability and prescribe diagnostic or therapeutic measures [21]. These models are now widely used in clinical and basic medical science research [22].
In this study, we anticipated that clinical features and laboratory data had a significant role in determining the probability of Nd:YAG laser capsulotomy therapies in patients with vision-threatening PCO. Therefore, we construct and validate a clinical prediction model and display the results using a nomogram.
Methods
Patient Selection
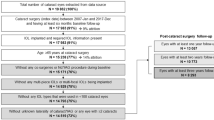
The Department of Ophthalmology, the First Medical Center, Chinese PLA General Hospital undertook this investigation as a single-center retrospective cohort study. From January 2010 to December 2021, a total of 16,802 patients (25,883 eyes) underwent cataract phacoemulsification and in-the-bag IOL implantation surgery. These patients create a dynamic cohort with rolling admissions and follow-ups. The follow-up period was determined by the most recent record at our center. Subjects with the following conditions were excluded: eyes that had undergone combined ophthalmic surgery, including but not limited to vitrectomy and trabeculectomy; eyes with traumatic cataract, complicated cataract, or congenital cataract; eyes with serious ocular illness affecting anterior structures, such as lens subluxation, ocular trauma, corneal scar, and active uveitis; and eyes with a history of retinal vascular disorders including central retinal vein occlusion (CRAO), branch retinal vein occlusion (BRVO), and proliferative diabetic retinopathy (PDR). Patients whose follow-up data were missing were also eliminated from this investigation. If both eyes match the inclusion criteria, the first operated eye will be registered. The enrollment process for this study is shown in Fig. 1. Following the exclusions, 9768 patients were included in our analysis.
This study was carried out following the principles of the Declaration of Helsinki, and the study protocol was approved by the Chinese PLA General Hospital Ethics Committee (no. S2022-415-01). Because all data were anonymized and the study was retrospective, no informed permission was necessary for patient enrollment.
Data Collection
Data were gathered through the use of electronic medical records. Medical information and procedure descriptions were coded along with International Classification of Disease-10 (ICD-10) diagnosis codes. Demographics (gender, age at surgery), medical records (history of hypertension, hyperlipemia, diabetes, and rheumatic diseases, as well as ocular comorbidities, and postoperative record), surgery record (surgeon grade, IOL material, and intraoperative complications), laboratory test results, and Nd:YAG laser capsulotomy event data were all collected. Hydrophilic IOL included Akreos AO60 (Bausch&Lomb), Softec HD (Lenstec), SMART36A (Acritec), and XLSTABI (Zeiss Optic). ZCB00 (Abbott Medical Optics Johnson & Johnson Vision) and ACRYSOF IQ were two hydrophobic IOLs (Alcon Laboratories). The laboratory examination measurements included red blood cell (RBC) count, white blood cell (WBC) count, platelet (PLT) count, hemoglobin, lymphocyte ratio, neutrophil ratio, fasting blood glucose, creatinine, uric acid, total cholesterol (TC), triglyceride, fibrinogen, international normalized ratio (INR), prothrombin time (PT), and thrombin time (TT). High myopia (more than 6D of myopia), dry eye disease (DED), history of infectious keratitis (without surgical treatment, no serious corneal scars remained and improvement of disease for more than 2 years), glaucoma, history of uveitis (improvement of disease for more than 2 years), and age-related macular degeneration (AMD) were among the ocular co-pathologies. Rheumatoid arthritis, Raynaud syndrome, ankylosing spondylitis, sicca syndrome (SS), vasculitis, and systemic lupus erythematosus were all diagnosed in rheumatology. Two ophthalmologists decided on the diagnosis of PCO and the need of Nd:YAG laser capsulotomy based on a two lines decline in corrected distance visual acuity (BCVA) induced by PCO.
Statistical Analysis
The analyses were carried out using R software (version 4.2.1), with the primary packages utilized in the study being the Caret package (version 6.0-93) for data cleaning and the rms package (version 6.3-0) for predictive model construction.
Clinical characteristics of the eyes included in the study were provided as counts and percentages for categorical variables, and mean (standard deviation) or median [interquartile range (IQR)] for numerical variables. The Kolmogorov–Smirnov test or the Shapiro–Wilk test were utilized to evaluate whether each attribute had a normal distribution.
The total data were randomly divided into a training cohort (70%) and a validation cohort (30%). The Wilcoxon–Mann–Whitney test or Fisher’s exact test was performed to verify the balance of the distribution of variables between the training and validation cohorts. To explore potential risk variables for Nd:YAG laser capsulotomy, univariate and multivariate Cox regression analyses were performed. Statistical significance was defined as a two-tailed P < 0.05 (*). Risk factors discovered from univariate analysis (P < 0.20) were included in the multivariate analysis. The Least Absolute Shrinkage and Selection Operator (LASSO) Cox regression algorithm were used to select the most useful predictive features from the training cohort data. Cross-validation was used to determine the LASSO–Cox regression minimal tuning parameter (l). The predictive model for Nd:YAG laser capsulotomy, which includes risk indicators, was displayed as a nomogram and then verified using an internal validation cohort. A calibration plot was used to test predictive accuracy by measuring the correlation between anticipated probabilities and observed proportions using bootstrapping with 1000 resamples. The plots included the P value for the Hosmer–Lemeshow tests goodness-of-fit. The model’s overall discriminative capacity was evaluated using the area under the curve (AUC). Decision curve analysis (DCA) was used to assess the model’s utility and net benefit of decision-making. Lastly, a subgroup analysis was conducted for potential risk factors.
Results
Baseline Variables
This study includes 9768 eyes of equal number to the individuals who underwent conventional phacoemulsification combined with IOL implantation. The average age at surgery was 73.0 (65.0, 80.0) years, with 56.7% of the patient being female. The average follow-up time after surgery was 26 months. The overall cumulative incidence of Nd:YAG laser capsulotomy was 2.0% (191/9768) at 1 year, 9.5% (925/9768) at 3 years, and 12.0% (1169/9768) at 5 years. The baseline clinical features of the training and validation cohorts are presented in Table 1, and the statistical test showed balance in the two randomly divided cohorts.
Risk Factor Selection and the Prediction Model Development
In the training cohort, univariate log-rank analysis was performed to analyze the potential risk factors for Nd:YAG laser capsulotomy, and then multivariate Cox regression analysis was used after including 21 variables (P < 0.20, according to the findings of univariate analysis). The results of the univariate log-rank analysis and multivariate Cox regression analyses are disclosed in Table 2. It revealed that the following factors were independent of Nd:YAG laser capsulotomy: sex (female, HR = 1.48, 95% CI 1.24–1.78), age (60–80 years, HR = 0.79, 95% CI 0.65–0.97; > = 80 years, HR = 0.75, 95% CI 0.59–0.95), IOL material (hydrophilic IOL, HR = 2.68, 95% CI 2.19–3.27), high myopia (HR = 2.23, 95% CI 1.85–2.70), DED (HR = 1.28, 95% CI 1.09–1.50), rheumatic disease (HR = 1.27, 95% CI 1.04–1.55), and fibrinogen (HR = 0.83, 95% CI 0.75–0.92).
To decrease the influence of collinearity among variables and to simplify the structure of the prediction model, the LASSO Cox regression algorithm was performed to select the most important variables. Thirty clinical factors were used in the LASSO Cox regression for 1000 bootstrap iterations, and finally, five features were selected for the model with a minimum lambda value of 0.0023 (Fig. 2). This model finally included five factors: sex (female, HR = 1.53, 95% CI 1.32–1.76), age (60–80 years, HR = 0.75, 95% CI 0.62–0.91; > = 80 years, HR = 0.71, 95% CI 0.56–0.88), IOL material (hydrophilic IOL, HR = 2.65, 95% CI 2.17–3.24), high myopia (HR = 2.28, 95% CI 1.90–2.75), and fibrinogen (HR = 0.79, 95% CI 0.72–0.88). The results of the final multivariate Cox model are displayed in Table 3. We then used a nomogram (Fig. 3) to depict the prediction result for 1-, 3-, and 5-year treatment probability of Nd:YAG laser capsulotomy based on the analytical results.
Internal Validation of the Nd:YAG Laser Capsulotomy Prediction Model
The performance of the prediction model was evaluated by discrimination and calibration. In the training cohort, the AUC of 1-, 3-, and 5-year predictions of the Nd:YAG laser capsulotomy were 0.650, 0.683, and 0.678, respectively (Fig. 4A). In the validation cohort, the AUC of 1-, 3-, and 5-year were 0.702, 0.691, and 0.688, respectively (Fig. 4B). In both cohorts, the 1-, 3-, and 5-year calibration plots for predicting the probability of Nd:YAG laser capsulotomy showed good agreement (P > 0.05) (Fig. 5). The DCA shows that the prediction model provides a net benefit within the reasonable threshold range of 5–30% (Fig. 6).
ROC curves of the training and validation cohorts. A In the training cohort, the AUC of 1-, 3-, and 5-year predictions of the Nd:YAG laser capsulotomy were 0.650, 0.683, 0.678, respectively. B In the validation cohort, the AUC of 1-, 3-, and 5-year predictions of the Nd:YAG laser capsulotomy were 0.702, 0.691, 0.688, respectively
Subgroup Analysis of the Influence of IOL material
We used a forest plot to demonstrate the impacts of the IOL material among subgroups (Fig. 7). For the probability of Nd:YAG laser capsulotomy, the protective effect of hydrophobic material IOL implantation is not affected by age, gender, the diagnoses of DED, glaucoma, diabetes, hyperlipemia, hypertension, or RD (P < 0.01). However, for subgroups with ocular comorbidities such as with history of keratoiditis (HR = 0.31, 95% CI 0.09–1.03, P = 0.057), AMD (HR = 0.81, 95% CI 0.28–2.34, P = 0.700), history of uveitis (HR = 0.50, 95% CI 0.15–1.67, P = 0.259), and high myopia (HR = 0.68, 95% CI 0.51–1.12, P = 0.127), this protective effect disappeared. The P-value of interaction between IOL material and high myopia was 0.021.
Discussion
The goal of our study was to develop a prediction model for future Nd:YAG laser capsulotomy treatment in patients with vision-threatening PCO. With the advancements in PCO prevention, such as the use of novel IOLs and femtosecond laser-assisted primary posterior capsulotomy, the management of PCO is generally evolving. This predictive information can help doctors make informed decisions about patient management and preventive interventions in the early postoperative period, lowering the probability of vision-threatening PCO in the long-term postoperative period.
The incidence of Nd:YAG laser capsulotomy was similar to that described in recent years by Lindholm et al. [13] (13.2% at 5-year, 2019) and Hecht et al. [23] (10.2% at 4-year, 2020), but significantly lower than that reported much earlier by Baratzc et al. [24] (33.0% at 5-year, 2001) and Ando et al. [25] (32.7% at 5-year, 2003). This reduction can be ascribed to advancements in surgical techniques and IOL design and materials. Considering the trend in vision-threatening PCO development, we discovered an increased likelihood of Nd:YAG laser capsulotomy within 3 years after surgery. To the best of our knowledge, early PCO development is caused by flaws in the capsule fusion process. This may result in a delayed or partial creation of the capsular bend at the posterior IOL edge. Thus, LECs can migrate to the gap between the IOL optic and the posterior capsule [26]. Within 3–5 years after surgery, just a small minority of patients develop new cases of vision-threatening PCO. The existing migratory LECs beneath the IOL edge provide a tight barrier, inhibiting LECs migration toward the central visual axis [27,28,29].
We then created a nomogram for individualized prediction of Nd:YAG laser capsulotomy in patients with a probability of developing vision-threatening PCO. The model performance, as reflected by the AUC of 5-year prediction, was 0.678 in the training cohort and 0.688 in the validation cohort. The goodness-of-fit test results demonstrate a good calibration of the model (P > 0.05). By reference to several preoperative clinical factors, we could specify the possibility of Nd:YAG laser capsulotomy treatment for individual patients. Apart from fibrinogen, the prediction model incorporates five items: sex, age, IOL material, fibrinogen, and high myopia, all of which have previously been described as risk factors for PCO. This identification would not add to the burden of cataract patients, but it would allow for earlier management in those at high risk. Although these parameters cannot be directly regulated, understanding their impact on PCO risk can help guide the selection of IOL and the decision of intraoperative management such as femtosecond laser-assisted primary posterior capsulotomy.
In recent clinical practice, both hydrophobic and hydrophilic acrylic IOLs are widely used. Various research has summarized that IOLs made of hydrophobic substances were overall superior to hydrophilic acrylic IOL in decreasing the PCO score and Nd:YAG capsulotomy rate [11, 30, 31]. This might be because the hydrophobic acrylic material has an adhesive surface to the collagen membrane, resulting in quick and tight opposition of the lens in capsular bag and raised adhesion through fibronectin [19, 29]. In addition, it has been demonstrated that the hydrophilic surface qualities create an optimum matrix for LECs growth and migration toward the center of the visual axis [32]. Utilizing scanning electron microscopy, the posterior edge profiles of the hydrophobic IOL revealed a sharper square edge than the hydrophilic IOL, which is critical for the prevention of PCO [33].
Consistent with these prior findings, high myopia is also revealed to be a risk factor for vision-threatening PCO in our research. This may, at least in part, be because high myopic eyes have not only a longer axial length but also a larger capsular bag [34]. The specific biometric characteristics may elevate the risk of PCO formation. Pupil dilation in myopic eyes may cause an inadvertently large capsulorhexis [23, 35], which then results in partial anterior capsule overlap, elevating the risk of PCO [36,37,38]. Large capsular bags could cause weak posterior capsule adhesion to the IOL in high myopic eyes, alongside several types of capsular bending [39]. The classical no space no cell theory highlights the “barrier effect” of the IOL contact on the posterior capsule [40]. Furthermore, cataract surgery in high myopic eyes is generally linked to lower-diopter IOL implantation, which has thinner optics and less posterior convexity [34]. The weaker biomechanical effects of thinner optics could result in delayed bend types and a higher rate of incomplete capsular contractures. Aside from the above analyses, higher levels of TGF-β present in the aqueous humor of pathologically myopic eyes have been proposed to affect the development of PCO [41]. The most obvious outcome from our subgroup analysis is that there is no difference in the protective effect of vision-threatening PCO between hydrophobic and hydrophilic IOLs in high myopic eyes. To our knowledge, the present study is the first to focus on the influence of IOL material to PCO development in high myopic eyes.
A surprising variable observed to be significantly linked to the probability of Nd:YAG laser capsulotomy in our model was fibrinogen. Fibrinogen is a glycoprotein synthesized in hepatocytes and is normally present in human blood plasma [42]. Fibrinogen and fibrin function primarily in multiple biological processes besides hemostasis and thrombosis, namely wound healing, inflammation, infection, cell migration, cell–cell interactions, angiogenesis, tumor growth, and metastasis [43,44,45]. The quantitative disorders of fibrinogen are mainly caused by chain-encoding gene mutations or liver disease [46, 47]. Cataract surgery, as a disruption of ocular integrity, instigates a wound-healing response [48]. J A RemijnIn et al. [49] examined that the absence of fibrinogen results in large but loosely packed thrombi and thrombotic complications. Similarly, in mice studies, fibrinogen deficiency could change the pattern of epithelial cell migration and increase epithelial hyperplasia [45]. These factors may explain the association between fibrinogen and Nd:YAG laser capsulotomy. Alternatively, fibrinogen deficiency may influence the mechanical stability of the newly formed tissue, provoking a persistent local inflammatory response, which functions crucially in lens-associated fibrotic post-cataract surgery. Owing to the intraoperative disturbance, the breaching blood-aqueous barrier permits several blood proteins to enter the ocular environment. These blood factors might aid the promoting effect of epithelial cell proliferation in residual LECs. Concerning fibrinogen, although related treatments are not standard in ophthalmic care, our outcomes imply a potential avenue for further research and clinical investigation. The molecular and cellular reasons for the correlation between fibrinogen and vision-threatening PCO are yet to be understood. We suggest a comprehensive review based on more reliable study designs.
Our analysis had several strengths. First, this study formed a large postoperative cohort with a sufficient length of follow-up time. Second, based on the superior cohort, we comprehensively integrated multiple clinical factors into a prediction model and employed LASSO Cox regression to lower the influence of multicollinearity among them. For the first time, we determined fibrinogen as an independent risk factor and developed a model to estimate the probability of Nd:YAG laser capsulotomy for vision-threatening PCO. The simple model showed decent discrimination and calibration, and may be beneficial for clinical application.
Nonetheless, some limitations of our study should be noted. First, owing to the retrospective study design, we were unable to obtain all the factors that may affect the results, such as postoperative inflammation, energies used, the substances employed during the surgery, and so on. We could enroll only homogeneous patients to minimize the effect of these factors. Second, the single-center nature of the study may limit the generalizability of our results in guiding future clinical decisions. To address this limitation, we recommend performing multicenter studies in future research. Third, the inclusion of additional clinical variables could improve the model's discriminative ability. The application of novel method such as deep learning, pattern recognition, and natural language processing, is required for developing more precise predictive models in subsequent investigations.
Conclusion
The model is capable of predicting the probability of Nd:YAG laser capsulotomy for vision-threatening PCO after cataract surgery by considering variables such as age, sex, IOL material, high myopia, and fibrinogen levels. In addition, the subgroup analysis demonstrated no difference in the protective effect against vision-threatening PCO between hydrophobic and hydrophilic IOLs in patients with high myopia.
References
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34.
GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:144–60.
Hong T, Mitchell P, Burlutsky G, Gopinath B, Liew G, Wang JJ. Visual impairment and depressive symptoms in an older Australian cohort: longitudinal findings from the Blue Mountains Eye Study. Br J Ophthalmol. 2015;99:1017–21.
Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. The Lancet. 2017;390:600–12.
Nibourg LM, Gelens E, Kuijer R, Hooymans JMM, van Kooten TG, Koopmans SA. Prevention of posterior capsular opacification. Exp Eye Res. 2015;136:100–15.
Wormstone IM, Eldred JA. Experimental models for posterior capsule opacification research. Exp Eye Res. 2016;142:2–12.
Maedel S, Evans JR, Harrer-Seely A, Findl O. Intraocular lens optic edge design for the prevention of posterior capsule opacification after cataract surgery. Cochrane Database Syst Rev. 2021;8:CD012516.
van Bree MCJ, van den Berg TJTP, Zijlmans BLM. Posterior capsule opacification severity, assessed with straylight measurement, as main indicator of early visual function deterioration. Ophthalmology. 2013;120:20–33.
Apple DJ, Peng Q, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, et al. Eradication of posterior capsule opacification: documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108:505–18.
Spalton DJ. Posterior capsular opacification after cataract surgery. Eye (Lond). 1999;13(Pt 3b):489–92.
Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine (Baltimore). 2017;96: e8301.
Bai H, Li H, Zheng S, Sun L, Wu X. Nd:YAG capsulotomy rates with two multifocal intraocular lenses. Int J Gen Med. 2021;14:8975–80.
Lindholm J-M, Laine I, Tuuminen R. Five-year cumulative incidence and risk factors of Nd:YAG capsulotomy in 10 044 hydrophobic acrylic 1-piece and 3-piece intraocular lenses. Am J Ophthalmol. 2019;200:218–23.
Hecht I, Karesvuo P, Achiron A, Elbaz U, Laine I, Tuuminen R. Anti-inflammatory medication after cataract surgery and posterior capsular opacification. Am J Ophthalmol. 2020;215:104–11.
Kim JW, Eom Y, Yoon EG, Choi Y, Song JS, Jeong JW, et al. Comparison of Nd:YAG laser capsulotomy rates between refractive segmented multifocal and multifocal toric intraocular lenses. Am J Ophthalmol. 2021;222:359–67.
Belda JI, Dabán JP, Elvira JC, O’Boyle D, Puig X, Pérez-Vives C, et al. Nd:YAG capsulotomy incidence associated with five different single-piece monofocal intraocular lenses: a 3-year Spanish real-world evidence study of 8293 eyes. Eye (Lond). 2022;36:2205–10.
Schojai M, Schultz T, Haeussler-Sinangin Y, Boecker J, Dick HB. Safety of femtosecond laser-assisted primary posterior capsulotomy immediately after cataract surgery. J Cataract Refract Surg. 2017;43:1171–6.
Ma B, Yang L, Jing R, Liu J, Quan Y, Hui Q, et al. Effects of Interleukin-6 on posterior capsular opacification. Exp Eye Res. 2018;172:94–103.
Shihan MH, Kanwar M, Wang Y, Jackson EE, Faranda AP, Duncan MK. Fibronectin has multifunctional roles in posterior capsular opacification (PCO). Matrix Biol. 2020;90:79–108.
Kim S-Y, Kim J-H, Choi J-S, Joo C-K. Comparison of posterior capsule opacification in rabbits receiving either mitomycin-C or distilled water for sealed-capsule irrigation during cataract surgery. Clin Exp Ophthalmol. 2007;35:755–8.
Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol. 2016;17:339–50.
Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiat. 2020;77:534–40.
Hecht I, Dubinsky-Pertzov B, Karesvuo P, Achiron A, Tuuminen R. Association between intraocular lens diopter and posterior capsular opacification. Clin Exp Ophthalmol. 2020;48:889–94.
Baratz KH, Cook BE, Hodge DO. Probability of Nd:YAG laser capsulotomy after cataract surgery in Olmsted County. Minnesota American Journal of Ophthalmology. 2001;131:161–6.
Ando H, Ando N, Oshika T. Cumulative probability of neodymium: YAG laser posterior capsulotomy after phacoemulsification. J Cataract Refract Surg. 2003;29:2148–54.
Sugita M, Kato S, Sugita G, Oshika T. Migration of lens epithelial cells through haptic root of single-piece acrylic-foldable intraocular lens. Am J Ophthalmol. 2004;137:377–9.
Nixon DR, Apple DJ. Evaluation of lens epithelial cell migration in vivo at the haptic-optic junction of a one-piece hydrophobic acrylic intraocular lens. Am J Ophthalmol. 2006;142:557–62.
Nixon DR, Woodcock MG. Pattern of posterior capsule opacification models 2 years postoperatively with 2 single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2010;36:929–34.
Praveen MR, Shah GD, Vasavada AR, Dave KH. The effect of single-piece hydrophobic acrylic intraocular lenses on the development of posterior capsule opacification. Am J Ophthalmol. 2015;160:470-478.e1.
Wu Q, Wu L, Wang C-Y. Hydrophobic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: a Meta-analysis. Int J Ophthalmol. 2022;15:997–1004.
Leydolt C, Davidovic S, Sacu S, Menapace R, Neumayer T, Prinz A, et al. Long-term effect of 1-piece and 3-piece hydrophobic acrylic intraocular lens on posterior capsule opacification: a randomized trial. Ophthalmology. 2007;114:1663–9.
Dorey MW, Brownstein S, Hill VE, Mathew B, Botton G, Kertes PJ, et al. Proposed pathogenesis for the delayed postoperative opacification of the hydroview hydrogel intraocular lens. Am J Ophthalmol. 2003;135:591–8.
Iwase T, Nishi Y, Oveson BC, Jo Y-J. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. J Cataract Refract Surg. 2011;37:1060–8.
Vass C, Menapace R, Schmetterer K, Findl O, Rainer G, Steineck I. Prediction of pseudophakic capsular bag diameter based on biometric variables. J Cataract Refract Surg. 1999;25:1376–81.
Xi W, Yang M, Wan J, Wang Y, Qiao Y, Huang X, et al. Effect of pupil dilation on biometry measurements and intraocular lens power in eyes with high myopia. Front Med (Lausanne). 2022;9: 963599.
Gu X, Chen X, Jin G, Wang L, Zhang E, Wang W, et al. Early-onset posterior capsule opacification: incidence, severity, and risk factors. Ophthalmol Ther. 2022;11:113–23.
Wren SME, Spalton DJ, Jose R, Boyce J, Heatley CJ. Factors that influence the development of posterior capsule opacification with a polyacrylic intraocular lens. Am J Ophthalmol. 2005;139:691–5.
Smith SR, Daynes T, Hinckley M, Wallin TR, Olson RJ. The effect of lens edge design versus anterior capsule overlap on posterior capsule opacification. Am J Ophthalmol. 2004;138:521–6.
Zhao Y, Li J, Lu W, Chang P, Lu P, Yu F, et al. Capsular adhesion to intraocular lens in highly myopic eyes evaluated in vivo using ultralong-scan-depth optical coherence tomography. Am J Ophthalmol. 2013;155:484-491.e1.
Peng Q, Visessook N, Apple DJ, Pandey SK, Werner L, Escobar-Gomez M, et al. Surgical prevention of posterior capsule opacification. Part 3: intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg. 2000;26:198–213.
Yan W, Zhang Y, Cao J, Yan H. TGF-β2 levels in the aqueous humor are elevated in the second eye of high myopia within two weeks after sequential cataract surgery. Sci Rep. 2022;12:17974.
Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–99.
Neerman-Arbez M, Casini A. Clinical consequences and molecular bases of low fibrinogen levels. Int J Mol Sci. 2018;19:192.
Neerman-Arbez M, de Moerloose P. Hereditary fibrinogen abnormalities. 8th ed. New York: McGraw-Hil; 2010.
Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691–8.
Neerman-Arbez M, Honsberger A, Antonarakis SE, Morris MA. Deletion of the fibrinogen [correction of fibrogen] alpha-chain gene (FGA) causes congenital afibrogenemia. J Clin Invest. 1999;103:215–8.
Casini A, Sokollik C, Lukowski SW, Lurz E, Rieubland C, de Moerloose P, et al. Hypofibrinogenemia and liver disease: a new case of Aguadilla fibrinogen and review of the literature. Haemophilia. 2015;21:820–7.
Kohnen T. Preventing posterior capsule opacification: what have we learned? J Cataract Refract Surg. 2011;37:623–4.
Remijn JA, Wu YP, Ijsseldijk MJ, Zwaginga JJ, Sixma JJ, de Groot PG. Absence of fibrinogen in afibrinogenemia results in large but loosely packed thrombi under flow conditions. Thromb Haemost. 2001;85:736–42.
Acknowledgements
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 82070937, and No. 82101097). The study sponsor is also funding the journal’s Rapid Service Fee.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Zhaohui Li and Zi Ye; Methodology: Tianju Ma, Wenqian Chen; Formal analysis and investigation: Xuanlong Li, Jinglan Li, Di Sun; Writing—original draft preparation: Xuanlong Li; Writing—review and editing: Xuanlong Li, Zi Ye; Funding acquisition: Zhaohui Li and Zi Ye. All authors approved the final manuscript prior to submission and are accountable for all aspects in ensuring the accuracy and integrity of work across all phases of the project.
Disclosures
All named authors confirm that they have no competing interests to declare.
Compliance with Ethics Guidelines
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Chinese PLA General Hospital (no. S2022-415–01). Informed consent was not required for patient enrollment because all data were anonymized and the study was retrospective in nature.
Data Availability
Because we did not acquire consent from the patients in this study to share data publicly, health data of individuals cannot be available online for the public. However, the datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, X., Li, J., Sun, D. et al. Development and Validation of a Prediction Model for Nd:YAG Laser Capsulotomy: A Retrospective Cohort Study of 9768 eyes. Ophthalmol Ther 12, 1893–1912 (2023). https://doi.org/10.1007/s40123-023-00723-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00723-y