Abstract
Introduction
The aim of this study was to compare retinal and choroidal alterations in eyes with severe nonproliferative diabetic retinopathy (NPDR) after panretinal photocoagulation (PRP), using conventional pattern scan laser (PASCAL) and PASCAL with endpoint management (EPM).
Methods
This was a post hoc analysis of a paired randomized clinical trial. Bilateral treatment-naïve eyes of an individual with symmetric severe NPDR were randomly allocated into the threshold PRP group and subthreshold EPM PRP group. Patients had follow-up visits at 1, 3, 6, 9, and 12 months post-treatment. The retinal thickness (RT), choroidal thickness (CT), choroidal area, and choroidal vascularity index (CVI) were compared between the two groups and among different time points within the same group.
Results
Seventy eyes of 35 patients with diabetes mellitus (DM) were finally included for analysis at the 6- and 12-month visits, respectively. At 3 and 6 months post-treatment, the RT in the subthreshold EPM PRP group was significantly thinner than that in the threshold PRP group. CT, stromal area, and luminal area were reduced earlier in the threshold PRP group than in the subthreshold EPM PRP group. CVI was not significantly different within the same group or between groups at most time points.
Conclusion
At 12 months post-treatment, retinal thickening and choroidal disturbance may be slightly less severe and more delayed in eyes receiving PRP using PASCAL with EPM than in those receiving PRP using conventional PASCAL. The EPM algorithm may be a good alternative in PRP when treating severe NPDR.
Trial Registration
ClinicalTrials.gov identifier, NCT01759121.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Endpoint management (EPM) has seldom been used to treat diabetic retinopathy (DR) when using panretinal photocoagulation (PRP). Thus, retinal and choroidal structure and choroidal blood flow alterations after PRP with the EPM algorithm remain unclear. |
To compare retinal and choroidal alterations in eyes with severe nonproliferative diabetic retinopathy (NPDR) after PRP using conventional pattern scan laser (PASCAL) and PASCAL with EPM. |
What was learned from the study? |
At 12 months post-treatment, retinal thickening and choroidal disturbance may be slightly less severe and more delayed in eyes receiving PRP using PASCAL with EPM than in those receiving PRP using conventional PASCAL. |
The EPM algorithm may be a good alternative in PRP when treating severe NPDR. |
Introduction
Diabetic retinopathy (DR), a common complication of diabetes mellitus (DM), remains the major cause of vision loss in the working-age population. In the past 25 years, the number of people suffering from severe vision impairment and blindness caused by DR has doubled worldwide [1, 2]. It is estimated that 190 million people may develop DR by 2030, and a quarter of cases are vision-threatening [3].
Panretinal photocoagulation (PRP) is a traditional and useful DR treatment. According to the Diabetic Retinopathy Preferred Practice Pattern guidelines [4], severe nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) can be treated using PRP. PRP may inevitably impair the peripheral visual field and increase the risk of diabetic macular edema (DME). With the introduction of intraocular antivascular endothelial growth factor (VEGF) treatment, the application of PRP seems to be considered with more caution. However, anti-VEGF treatment requires higher expense, exerts effects for a limited time, and is not effective for weak responders or nonresponders. Anti-VEGF treatment is also not recommended for patients with DR with high stroke risk [5]. Thus, PRP is still essential for DR treatment, and the practice pattern deserves further evaluation.
In the past 10 years, pattern scan laser (PASCAL) photocoagulation has been widely applied in PRP. Compared with conventional photocoagulation, PASCAL utilizes lower energy and shorter pulse duration (10 to 30 ms), which reduces patients’ pain and damage induced by heat diffusion to the tissues adjacent to the retinal pigment epithelium (RPE) [6]. Subvisible and selective strategies in photocoagulation have been constantly developed to minimize tissue damage. On the basis of a calculated model of retinal thermal damage, an algorithm named endpoint management (EPM) has been established to set power and pulse duration for different levels of tissue effect [7]. EPM may help strike a balance between minimization of tissue damage and maximization of treatment effect. Nevertheless, only a handful of studies have used EPM in treating DME and central serous chorioretinopathy [7, 8], and EPM has seldom been used to treat DR when using PRP. Thus, retinal and choroidal structure and choroidal blood flow alterations after PRP with the EPM algorithm remain unclear.
Therefore, the purpose of this study was to comprehensively compare retinal and choroidal alterations in severe NPDR eyes after PRP using conventional PASCAL and PASCAL with EPM, and to investigate the relationship between retinal and choroidal changes after treatment with these two laser patterns. It may provide stronger evidence for using this algorithm for treating DR.
Methods
Study Design and Participants
This was a post hoc analysis of a paired randomized clinical trial (RCT) assessing retinal and choroidal alterations as the primary outcome in the present study. The detailed methods for this trial have been described in our previously published work [9]. Briefly, bilateral eyes with symmetric severe NPDR of the same individual were randomly allocated into the threshold PRP group and subthreshold EPM PRP group. It was a double-masked, parallel controlled RCT. Patients had follow-up visits at 1, 3, 6, 9, and 12 months post-treatment. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Zhongshan Ophthalmic Center, Sun Yat-sen University (2012KYNL056). The study was registered on ClinicalTrials.gov (https://www.clinicaltrials.gov), and the registration number was NCT01759121.
Eighty-four eyes of 42 patients with DM who were recruited from Zhongshan Ophthalmic Center, Sun Yat-sen University between March 2016 and January 2020 were included. Written informed consent was acquired from all participants. The inclusion criteria for this study included the following: (1) patients with type 2 DM aged ≥ 18 years old, (2) patients diagnosed with symmetric severe NPDR (with or without DME) in bilateral eyes, (3) the included eyes were treatment-naïve, (4) BCVA [Early Treatment Diabetic Retinopathy Study (ETDRS)] ≥ 50, and (5) ability and willingness to provide informed consent. The exclusion criteria were as follows: (1) HbA1c ≥ 10%, (2) uncontrollable high blood pressure (HBP) (≥ 180/110 mmHg), malignant tumors and other uncontrolled systemic diseases, (3) refractive error > −6 diopters or axial length > 26 mm, (4) media opacities affecting imaging, (5) a history of laser treatment, anti-VEGF intravitreal injection, or intraocular surgeries, (6) glaucoma, uveitis, and other eye diseases affecting visual acuity, and (7) patients who were not able to receive examinations or treatments.
PASCAL PRP Protocol
All PRP treatments were performed by the same operator (JCJ). Before treatment, topical anesthesia was applied to all treated eyes. Both PRP patterns (threshold PRP and subthreshold EPM PRP, Supplementary Fig. 1) were performed through a Mainster wide-field lens (Ocular Instruments Inc., Bellevue, WA USA) using a PASCAL photocoagulator (Optimedica, Santa Clara, CA, USA). Titration was performed using a single laser spot with a 30 ms impulse duration and a 200 μm spot size. The power was initially set at 200 mW and was gradually increased by 25 mW per time until a gray-white burn was obtained. In the threshold PRP group, a 4 × 4 square grid was then applied in two sessions with an interval of 1 week between treatments. In the subthreshold EPM PRP group, the operator switched the laser pattern to EPM mode after titration and performed the subsequent laser treatment using the same square grid and interval in two sessions. Finally, the laser spots at four corners of the square grid maintained the titration power, while the energy was reduced to 50% in the remaining spots, which were barely visible.
Laser treatment could be complemented during the follow-up visits. Rescue threshold conventional PRP with a single spot was performed when PDR developed. Anti-VEGF and intraocular surgeries were performed when vitreous hemorrhage (VH), neovascular glaucoma, or retinal detachment occurred.
In our study, DME referred to clinically significant macular edema, which was defined by the ETDRS [10]. The diagnosis of DME was mainly based on fundus photography and optical coherence tomography (OCT) images. Eyes with DME at baseline received focal or grid laser. A repeat macular laser was applied in nonresponsive or worsening DME eyes with a BCVA change ≤ 10. Anti-VEGF would be provided in DME eyes with a BCVA change > 10 during the follow-up visits and these eyes were excluded from this study.
Patient Follow-up and Assessment
Demographics (age, sex) and systemic clinical characteristics [height, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), HbA1c, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)] were recorded at baseline.
BCVA, intraocular pressure (IOP) (Canon TX-20 noncontact tonometer, Canon Inc., Tokyo, Japan), dilated pupil fundus examination, ETDRS 7-field fundus photography (Carl Zeiss Meditec, Jena, Germany), and OCT (Spectralis HRA + OCT, Heidelberg Engineering, Germany) were performed at baseline and at 1, 3, 6, 9, and 12 months post-treatment. Fundus fluorescein angiography (FFA) (Spectralis HRA + OCT, Heidelberg Engineering, Germany) was performed at baseline and at 6 and 12 months post-treatment.
DR Progression
The ratio of DME at baseline and at 6 and 12 months post-treatment were determined. In addition, the presence of PDR and VH was also recorded at 6 and 12 months post-treatment.
Retinal and Choroidal Parameter Measurements
Enhanced depth imaging spectral-domain OCT (EDI SD-OCT) B-scan images in the horizontal and vertical directions were collected. Two experienced examiners (LZJ and LT) independently measured the retinal and choroidal parameters. Examiners repeated the measurements when the two examiners’ results differed by > 15%.
The retinal and choroidal thicknesses were manually measured at the fovea, 750 μm from the fovea, and 1500 μm from the fovea in four sectors (superior, temporal, inferior, and nasal). The retinal thickness was defined as the distance between the inner limiting membrane and the outermost edge of the RPE, and the choroidal thickness was defined as the distance between the outermost edge of the RPE and the innermost edge of the scleral border. Similar to previous studies [11, 12], ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA) was used to binarize the images and to quantify the choroidal area and choroidal vascularity index (CVI). The measured area was selected to be 1500 μm and 3000 μm in width in the macular area using ImageJ ROI manager. Three choroidal lumens > 100 μm in diameter were randomly chosen, and the average reflectivity of those areas was obtained. The average reflectivity was set as the minimum value to minimize noise in the images. Afterward, the images were converted to 8 bits and adjusted by Auto Local Threshold using the Niblack algorithm. The total choroidal area, luminal area, stromal area, and CVI were automatically calculated after adding the distance between the pixels. The light areas were defined as the stromal areas, and the dark areas were defined as the luminal areas. Details are shown in Fig. 1.
Enhanced depth imaging spectral-domain optical coherence tomography (EDI SD-OCT) images and the corresponding binarized images. A, B Left: en face images where the green lines indicate the scan direction. A Middle: horizontal OCT B-scan image, B middle: vertical OCT B-scan image. The retinal and choroidal thickness were measured at the fovea (orange lines), 750 μm from the fovea (red lines), and 1500 μm from the fovea (yellow lines), respectively. A Right: horizontal OCT B-scan binarized image, B right: vertical OCT B-scan binarized image. The choroidal area was measured within the 1500-μm area and within the 3000-μm area, and the blue lines indicate the border of the choroid
The measurements at four sectors/different scan directions were averaged to create the examiners’ measurements. Measurements by two experienced examiners were then averaged as the ultimate data.
Statistical Analyses
The statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). The mean and standard deviation are presented for quantitative variables, and the number and percentage are presented for categorical variables. Paired samples t tests were performed to compare normally distributed unrepeated variables and to compare the follow-up data at different timepoints and the corresponding baseline data within the same group. Categorical variables were analyzed with the chi-squared test. A generalized estimating equation (GEE) was used to compare the dynamic longitudinal variables between the two groups at all time points, adjusting the intereye correlation and correlation among the repeated measurements. The correlation between retinal thickness changes (compared with baseline data) and choroidal thickness/choroidal area/CVI changes (compared with baseline data) was investigated in two groups at 6 and 12 months post-treatment using Pearson correlation analysis. The agreement between the examiners for measurements of the retinal and choroidal parameters were analyzed using the intraclass correlation coefficient (ICC). Statistical significance was set at p < 0.05.
Results
Patient Demographics and Systemic Profiles
Forty-two patients were included in this study, and 35 patients with bilateral eyes were finally included for analysis at the 6- and 12-month visits. The flow diagram of this study is shown in Fig. 2. The mean age was 57.76 ± 9.84 years old, and the male to female ratio was 19:23. Other baseline details are shown in Table 1. The levels of HbA1c, LDL-C, and HDL-C at 6 and 12 months post-treatment were not significantly different from those at baseline (Supplementary Fig. 2). However, there was a steadily increasing trend in the levels of HbA1c and LDL-C.
Baseline Ocular Profiles
There was no significant difference between the two groups in any baseline ocular parameters, including eye (right/left), BCVA, IOP, ratio of DME, central macular thickness (CMT), subfoveal choroidal thickness (SFCT), retinal thickness (RT) at 1.5 mm and 3 mm, choroidal thickness (CT) at 1.5 mm and 3 mm, and choroidal area and CVI in the parafoveal zone (ring diameter 1.5 mm), the perifoveal zone (ring diameter 1.5 to 3 mm), and the para- and perifoveal zone (ring diameter 3 mm). Details are shown in Table 2.
BCVA
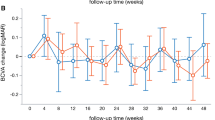
At 6 months post-treatment, BCVA in the subthreshold EPM PRP group was significantly better than that in the threshold PRP group, while there was no significant difference in BCVA at other time points between groups (Fig. 3A). In these two groups, BCVA decreased at all follow-up time points compared with that at baseline (Fig. 3A). The detailed data are shown in Supplementary Table 2.
BCVA and retinal and choroidal parameters at different time points during the follow-up period. A BCVA (ETDRS), B CMT, C SFCT, D RT at 1.5 mm, E RT at 3 mm, F CT at 1.5 mm, G CT at 3 mm, H choroidal area and CVI within 1.5 mm in the threshold PRP group, I choroidal area and CVI within 3 mm in the threshold PRP group, J choroidal area and CVI from 1.5 mm to 3 mm in the threshold PRP group, K choroidal area and CVI within 1.5 mm in the subthreshold EPM PRP group, L choroidal area and CVI within 3 mm in the subthreshold EPM PRP group, M choroidal area and CVI from 1.5 mm to 3 mm in the subthreshold EPM PRP group. BCVA best corrected visual acuity,ETDRS Early Treatment Diabetic Retinopathy Study, CMT central macular thickness, SFCT subfoveal choroidal thickness, RT retinal thickness, CT choroidal thickness, CVI choroidal vascularity index, PRP panretinal photocoagulation, EPM endpoint management. *p < 0.05 using paired samples t test; #p < 0.05 using generalized estimating equation
Laser Treatment and DR Progression
In the initial treatment, the actual PRP power used in the subthreshold EPM PRP group was significantly lower than that in the threshold PRP group, while no significant difference was observed in the number of PRP shots and macular laser use (yes/no). No significant difference was observed in the ratio of DME eyes at 6 and 12 months post-treatment. Moreover, additional PRP or macular laser times and the ratio of PDR or VH were not significantly different at 6 or 12 months post-treatment. Details are shown in Table 3.
Retinal and Choroidal Parameter Profiles
The ICC exceeded 0.9 in all retinal and choroidal parameters, which suggested a high reproducibility between the two examiners (Supplementary Table 1).
At 3 and 6 months post-treatment, CMT and RT at 1.5 mm and 3 mm in the subthreshold EPM PRP group were significantly thinner than those in the threshold PRP group. However, at 9 and 12 months post-treatment, no significant difference was noticed between groups in CMT or RT at 1.5 mm and 3 mm. Compared with baseline, CMT and RT at 1.5 mm and 3 mm became significantly increased at 1 month post-treatment in both groups. (Fig. 3B, D, E).
For SFCT and CT at 1.5 mm and 3 mm, no significant difference was observed between groups. Compared with baseline, CT became significantly thinner at 1 month post-treatment in the threshold PRP group, but became significantly thinner at 6 months post-treatment in the subthreshold EPM PRP group (Fig. 3C, F, G). In the parafoveal zone (Fig. 3H, K), the stromal area was significantly reduced at 3 months post-treatment in the threshold PRP group and at 6 months post-treatment in the subthreshold EPM PRP group; the luminal area was significantly reduced at 1 month post-treatment in the threshold PRP group, but was significantly reduced at 6 months post-treatment in the subthreshold EPM PRP group. Similar changes were noticed in the stromal area and the luminal area between groups and within the same group in the perifoveal zone (Fig. 3J, M) and the para- and perifoveal zone (Fig. 3I, L). In the perifoveal zone, the stromal area was significantly reduced in the threshold PRP group at 9 and 12 months post-treatment compared with that in the subthreshold EPM PRP group (Fig. 3J, M). Moreover, the reduction of the stromal area and luminal area began earlier in the parafoveal zone than in the perifoveal zone within the same group [threshold PRP group: (stromal area: parafoveal zone 3 months post-treatment, perifoveal zone 12 months post-treatment; luminal area: parafoveal zone 1 month post-treatment, perifoveal zone 6 months post-treatment), subthreshold EPM PRP group: (stromal area: parafoveal zone 6 months post-treatment, perifoveal zone none; luminal area: parafoveal zone 6 months post-treatment, perifoveal zone 9 months post-treatment)] (threshold PRP group: Fig. 3H, J; subthreshold EPM PRP group: Fig. 3K, M).
At 9 months post-treatment, the CVI of the perifoveal zone in the subthreshold EPM PRP group was significantly reduced compared with that in the threshold PRP group (Fig. 3J, M). However, there was no significant difference in CVI at 1, 3, 6, or 12 months post-treatment between groups (Fig. 3H–M).
The above data are shown in Supplementary Table 2.
Relationship Between Retinal and Choroidal Parameters
There was no significant difference in the correlation between retinal thickness changes and choroidal thickness/choroidal area/CVI changes within the same group in either group at 6 and 12 months post-treatment.
Discussion
Eyes in the subthreshold EPM PRP group seemed to have a better BCVA and a thinner RT than those in the threshold PRP group at 6 months post-treatment, while no significant differences were observed between groups at 9 or 12 months post-treatment. Retinal inflammation and edema triggered by thermal effects secondary to PRP are major reasons for increased RT in the early post-treatment phase [13]. It is possible that the lower power used in the subthreshold EPM PRP group induced less retinal thickening within approximately 6 months. A shorter pulse duration is used in PASCAL treatment compared with conventional laser treatment, while a stronger power will be required with PASCAL [6]. The application of the EPM algorithm calculated and lowered the laser power to some degree, minimizing the thermal effect in the early post-laser stage. In addition, a lower power in the threshold PRP group may also indicate less damage to the retinal vessels, therefore causing less retinal atrophy after more than 6 months. Although some differences may exist in RT changes between the two groups, the proportions of DME, VH, and PDR in the two groups were similar; hence, the introduction of the EPM algorithm in PRP seemed to be as effective as the conventional PASCAL method.
BCVA in both groups gradually decreased and remained stable at the 12-month follow-up. Corresponding to the BCVA alterations, CMT had a generally increased tendency in both groups. These results may suggest that increased CMT mainly contributes to worsening BCVA. Regarding the causes of increased CMT, PRP may not be the only factor. Systemic conditions are possibly responsible for DR development. In our study, the steady increase in levels of HbA1c and LDL-C implied relatively unsatisfactory glycemic control and a disturbance of lipid metabolism, which may worsen DR, as stated in previous studies [14, 15].
In our study, the constant thinning of CT was consistent with most previous studies, although laser patterns for PRP may vary in these studies. The possible reasons for CT reduction may be complicated [16]. Thermal damage causes redistribution and autoregulation of choroidal reperfusion. In addition, decreased levels of VEGF secondary to RPE impairment have a negative effect on choroidal vessel permeability and dilation. These factors may consequently result in CT reduction. Nevertheless, no significant change in CT at the 12-month follow-up was observed in Huang’s study [13]. The discrepancy may be due to the criteria for patient inclusion. Unlike other studies, DME eyes were not included in Huang’s study. According to some studies, SFCT was found to be thicker in eyes with DME than in eyes without DME. As DME resolves in the treatment process, SFCT may decrease. Therefore, a thinner baseline SFCT may partially account for the variation in CT changes in Huang’s study. Zhu and colleagues [17] reported a significantly increased macular CT at 1 and 3 months post-treatment. CT of the photocoagulated area after PRP was also measured. They argued that a redistribution of choroidal blood flow forward to the fovea, caused by reduced blood flow in the peripheral retina, may be the determinant. Transiently increased SFCT lasting for 1 or 2 weeks has been reported in some studies [16, 18], and the reason may be choroidal effusion and vasodilation. However, increased SFCT may seldom be present at more than 1 month post-treatment, as in Zhu’s study. The mechanism deserves further exploration.
No significant difference was observed in CT between groups, but interestingly, the thinning of CT occurred earlier in the threshold PRP group (1 month post-treatment) than in the subthreshold EPM PRP group (6 months post-treatment). Lavinsky’s study [19] revealed that 50% of the EPM titration power in rabbit eyes primarily caused damage limited to the RPE, and restoration of RPE continuity and synapse reconstruction between bipolar cells and photoreceptors were observed in a few weeks. Unfortunately, a higher percentage of titration power may cause irreversible damage to the RPE, photoreceptors, and other adjacent structures. Thus, we hypothesize that the prompt restoration of the retinal structures in the subthreshold EPM PRP group reduces the diffuse effect interfering with the choroid. In addition, restoration of the RPE may help to maintain a VEGF balance and choroidal reperfusion by dilating the vessels.
Corresponding to decreased CT, gradually reduced stromal area and luminal area were observed, which was consistent with Mikoshiba’s study [20]. Similar to the CT changes, the reduction of these areas also occurred earlier in the threshold PRP group than in the subthreshold EPM PRP group, which may provide extra evidence for the choroidal shrinkage process post-laser. For the intergroup comparison, a significantly reduced stromal area in the threshold PRP group was observed only in the perifoveal zone at 9 and 12 months post-treatment compared with that in the subthreshold EPM PRP group. The facts may further prove that the choroidal effects of an EPM algorithm may be similar or even less than those of conventional PASCAL in PRP.
Moreover, the reduction in stromal area and luminal area began earlier in the parafoveal zone than in the perifoveal zone in the two groups. Nonvascular smooth muscle cells were reported to accumulate, especially behind the fovea [21]. The contraction of these cells may oppose the thickening choroid caused by lacunae expansion, which possibly further reduces the choroidal area. We hypothesize that this might account for the discrepancy between the parafoveal and perifoveal zones in these areas.
Macular choroidal circulation has been assessed in different studies, and the results have varied. Okamoto’s [22] and Mikoshiba’s [20] studies revealed reduced subfoveal choroidal blood flow (CBF) at 1 and 3 months post-laser using laser speckle flowgraphy. They attributed the changes to the decreased level of VEGF and insufficient CBF compensation for peripheral choriocapillaris destruction, respectively. On the other hand, in Takahashi’s study [23], an increased subfoveal CBF was detected at 1 month post-laser using doppler flowmetry. They also emphasized that choriocapillary vasodilation in the macular region is the main reason. In our study, the macular choroidal circulation was not significantly different within the same group or between groups at most timepoints. The variation in these results may be partially due to different laser patterns and types. For instance, PASCAL may cause less damage to the choriocapillaris than the conventional laser, and EPM further minimizes this damage, thereby causing fewer choroidal disturbances. However, some of these disturbances might be subtle and cannot be detected by current applications. Advanced methods are warranted to provide a more accurate and comprehensive assessment of choroidal changes post-laser. In addition, the measurement methods for choroidal circulation, choroidal autoregulated ability, and disease severity may also contribute to the variation [21, 24].
The lack of a relationship between RT changes and choroidal changes in both groups in our study was in agreement with the results of another study [20]. A previous study [12] showed that naïve DME eyes had thicker CT and higher choroidal areas than naïve non-DME eyes. Even in normal eyes [25], CBF was positively correlated with SFCT. According to Okamoto’s research [11], in the non-PRP group, CBF was significantly reduced, and a positive correlation between SFCT and CBF was observed. However, in the PRP group, such a difference was not observed. Taken together, RT and choroidal parameters may be affected differently by PRP. RT may change more than the choroidal parameters. A plausible explanation for the asymmetric relationship between RT and choroidal changes might be the stronger autoregulated ability of the choroid. In addition to DME, DR stage progression [26], systemic changes [27, 28], and other factors may also alter the choroidal parameters and cause varied results.
The strengths of this study were as follows: (1) it was the first prospective study to perform PRP in DR treatment using PASCAL with EPM [9], (2) the changes in RT, CT, choroidal areas ,and choroidal circulation in the macular area after PASCAL with EPM were presented for the first time, and (3) choroidal parameters were measured both at 1.5 mm and at 3 mm, which included a broader area compared with previous studies [12, 29]. Nevertheless, there were some limitations in this study. First, the measured areas were limited to the macular area and not to the entire retina and choroid. Second, retinal microvasculature was not assessed. Moreover, the data were manually measured, and advanced techniques are warranted to effectively obtain accurate and repeatable results.
In conclusion, at 12 months post-treatment, retinal thickening and choroidal disturbance may be slightly less severe and more delayed in eyes receiving PRP using PASCAL with EPM than in those receiving PRP using conventional PASCAL. The EPM algorithm may be a good alternative in PRP when treating severe NPDR.
References
Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–34.
Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337–47.
Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–31.
Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern. Ophthalmology. 2020;127(1):P66-p145.
Porta M, Striglia E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med. 2020;15(2):199–210.
Alasil T, Waheed NK. Pan retinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Curr Opin Ophthalmol. 2014;25(3):164–70.
Lavinsky D, Palanker D. Nondamaging photothermal therapy for the retina: initial clinical experience with chronic central serous retinopathy. Retina. 2015;35(2):213–22.
Hamada M, Ohkoshi K, Inagaki K, et al. Subthreshold photocoagulation using endpoint management in the PASCAL® system for diffuse diabetic macular edema. J Ophthalmol. 2018;2018:7465794.
Lai K, Zhao H, Zhou L, et al. Subthreshold pan-retinal photocoagulation using endpoint management algorithm for severe nonproliferative diabetic retinopathy: a paired controlled pilot prospective study. Ophthalmic Res. 2021;64(4):648–55.
Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103(12):1796–806.
Okamoto M, Yamashita M, Ogata N. Effects of intravitreal injection of ranibizumab on choroidal structure and blood flow in eyes with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):885–92.
Kase S, Endo H, Takahashi M, et al. Alteration of choroidal vascular structure in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):971–7.
Huang T, Li X, Xie J, et al. Long-term retinal neurovascular and choroidal changes after panretinal photocoagulation in diabetic retinopathy. Front Med (Lausanne). 2021;8: 752538.
Eid S, Sas KM, Abcouwer SF, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539–49.
Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–44.
Zhang Z, Meng X, Wu Z, et al. Changes in choroidal thickness after panretinal photocoagulation for diabetic retinopathy: a 12-week longitudinal study. Invest Ophthalmol Vis Sci. 2015;56(4):2631–8.
Zhu Y, Zhang T, Wang K, et al. Changes in choroidal thickness after panretinal photocoagulation in patients with type 2 diabetes. Retina. 2015;35(4):695–703.
Cho GE, Cho HY, Kim YT. Change in subfoveal choroidal thickness after argon laser panretinal photocoagulation. Int J Ophthalmol. 2013;6(4):505–9.
Lavinsky D, Sramek C, Wang J, et al. Subvisible retinal laser therapy: titration algorithm and tissue response. Retina. 2014;34(1):87–97.
Mikoshiba Y, Iwase T, Ueno Y, et al. A randomized clinical trial evaluating choroidal blood flow and morphology after conventional and pattern scan laser panretinal photocoagulation. Sci Rep. 2018;8(1):14128.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–68.
Okamoto M, Matsuura T, Ogata N. effects of panretinal photocoagulation on choroidal thickness and choroidal blood flow in patients with severe nonproliferative diabetic retinopathy. Retina. 2016;36(4):805–11.
Takahashi A, Nagaoka T, Sato E, et al. Effect of panretinal photocoagulation on choroidal circulation in the foveal region in patients with severe diabetic retinopathy. Br J Ophthalmol. 2008;92(10):1369–73.
Zhao T, Chen Y, Liu D, et al. Optical coherence tomography angiography assessment of macular choriocapillaris and choroid following panretinal photocoagulation in a diverse population with advanced diabetic retinopathy. Asia Pac J Ophthalmol (Phila). 2020;10(2):203–7.
Iwase T, Yamamoto K, Kobayashi M, et al. What ocular and systemic variables affect choroidal circulation in healthy eyes. Medicine (Baltimore). 2016;95(43): e5102.
Kim JT, Lee DH, Joe SG, et al. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(5):3378–84.
Wong IY, Wong RL, Zhao P, et al. Choroidal thickness in relation to hypercholesterolemia on enhanced depth imaging optical coherence tomography. Retina. 2013;33(2):423–8.
Xu J, Xu L, Du KF, et al. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology. 2013;120(10):2023–8.
Wang H, Tao Y. Choroidal structural changes correlate with severity of diabetic retinopathy in diabetes mellitus. BMC Ophthalmol. 2019;19(1):186.
Acknowledgements
Funding
The research was funded by Science and Technology Projects of Guangzhou, China (202201020624). Funding for journal’s Rapid Service fee was provided by Chenjin Jin.
Author Contributions
Zijing Li, Tu Lu, Lijun Zhou, Chuangxin Huang, Hongkun Zhao, Jiandong Liang, Cong Li, Qifeng Cong, Yuqing Lan, and Chenjin Jin contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Zijing Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Zijing Li, Tu Lu, Lijun Zhou, Chuangxin Huang, Hongkun Zhao, Jiandong Liang, Cong Li, Qifeng Cong, Yuqing Lan, and Chenjin Jin declare that they have no competing interests.
Compliance with Ethics Guidelines
It was approved by the institutional review board of Zhongshan Ophthalmic Center, Sun Yat-sen University (2012KYNL056). Written informed consent was provided by all participates. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Data Availability
The author will provide the original data and material at the request of readers.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Z., Lu, T., Zhou, L. et al. Retinal and Choroidal Alterations in Diabetic Retinopathy Treatment using Subthreshold Panretinal Photocoagulation with Endpoint Management Algorithm: A Secondary Analysis of a Randomized Clinical Trial. Ophthalmol Ther 12, 1867–1880 (2023). https://doi.org/10.1007/s40123-023-00713-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00713-0