Abstract
Introduction
Myopic atrophic maculopathy is prevalent among patients with pathologic myopia and frequently leads to relentless vision loss. Several grading systems were established to facilitate the understanding of myopic atrophic maculopathy. However, the anatomical details in different stages of myopic maculopathy are so far not clearly elucidated. This study aims to investigate the visual acuity and retinal sublayer features in highly myopic eyes with varying severities of myopic atrophic maculopathy (MAM).
Methods
The clinical records of 111 consecutive patients (158 eyes) with high myopia (refractive error ≤ −6.0 D and axial length ≥ 26.0 mm) were reviewed. Fundus photography, optical coherence tomography (OCT), and best-corrected visual acuity (BCVA) were measured. MAM was graded according to the META-analysis for Pathologic Myopia (META-PM) classification system. Myopic choroidal neovascularization (mCNV) and dome-shaped macula (DSM) were also investigated.
Results
Among the 158 eyes, 18 (11%), 21(13%), 24 (15%), 25 (16%), 23 (15%), and 24 (15%) had tessellated fundus, diffuse chorioretinal atrophy, diffuse chorioretinal atrophy with DSM, patchy atrophy, patchy atrophy with DSM, and MAM with mCNV, respectively. A total of 23 (15%) eyes had macular atrophy without mCNV. Progressive thinning in the Henle’s fiber and outer nuclear layers, myoid and ellipsoid zone (MEZ), outer segment (OS), and interdigitation zone and retinal pigmented epithelium based on the severity of MAM (p-value < 0.001) was found. MEZ and OS were most significantly reduced in thickness (p-value < 0.001). The presence of mCNV demonstrated significant outer retinal layer thinning compared with that of the tessellated fundus (p-value = 0.031). Patchy atrophy with DSM showed statistically poorer BCVA compared with that without (p-value = 0.008).
Conclusion
Visual acuity and outer retinal sublayer characteristics were correlated with the severity of MAM. Outer retinal sublayer analysis by spectrum-domain OCT shed some light on the mechanisms of MAM progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Myopic atrophic maculopathy (MAM) potentially causes legal blindness and subsequent social, economic, and medical burdens. |
The study hypothesized that sublayer thicknesses of outer retina and choroid varies among different stages of myopic atrophic maculopathy. |
What was learned from the study? |
The study found that thickness of outer retinal sublayers and choroid decreases with the severity of MAM; for each sublayer, the trend of thickness reduction is uneven, as MAM progress instead of linear decrease; furthermore, as MAM progress from one stage to more severe ones, the percentage reduction of thickness also varies among different sublayers. |
The findings of this study imply that different stages of myopic atrophic maculopathy may have distinct pathogenic mechanisms. |
Introduction
Pathologic myopia is a highly prevalent disease worldwide and one of the leading causes of legal blindness [1,2,3]. Myopic maculopathy is one of the hallmark traits of pathologic myopia [4], and is a complex disease triggered by several attributing factors [5]. Thus, a reliable, homogeneous grading system is warranted for the classification of pathologic myopia. Several grading systems were devised to facilitate an understanding of myopic maculopathy (MM). Among them, the atrophy-traction-neovascularization (ATN) grading system and the myopic atrophic maculopathy (MAM) grading system of META-analysis for Pathologic Myopia (META-PM) Study Group [4, 5] could be considered the most up to date and popular.
Owing to the advancements in optical coherence tomography (OCT), today the understanding of the outer retina is more elaborate [6]. Differential thinning was reported among the retinal layers in highly myopic eyes compared with that of emmetropic eyes [7]. Generally thinner choroidal thickness was observed in severe MAM [8, 9]. Although the correlation between the fundus morphologies and visual acuity differences among varying atrophic stages of MAM has been studied [8, 10, 11], the exact causal correlation of the thickness difference of each retinal sublayer to different stages of MAM is not clearly understood. The differences in subretinal thickness among the varying atrophic stages of myopia maculopathy may facilitate our understanding of the mechanism underlying MAM progression.
Dome-shaped macula (DSM), myopic choroidal neovascularization (mCNV), and chorioretinal atrophy are important traits associated with MAM. The role of DSM in the best-corrected visual acuity (BCVA) and pathological changes of MAM is, however, inconclusive [12]. Furthermore, the impact of mCNV on the outer retinal layer has not been studied before.
In this study, we aimed to study the thickness difference of the outer retinal layers and its functional impact on different grades of atrophic myopia maculopathy. In addition, the functional and anatomical effects of two pathologic changes in MAM, namely DSM and mCNV, were evaluated. We hypothesized that the thickness of each outer retinal sublayer is composed of a varying percentage of total retinal thickness among different stages of MAM and that it might directly correlate with vision functions.
Methods
This was a retrospective, cross-sectional chart review study in a single tertiary center. All the consecutive cases diagnosed as high myopia in the High Myopia Clinic of National Taiwan University Hospital from December 2018 to December 2021 with axial length > 26.0 mm were included in this study. Cases with clinically significant cataract, myopic tractional maculopathy, history of trauma, uveitis, significant vitreous opacity, optic neuropathy, diabetic retinopathy, clinically significant hypertensive retinopathy, or cerebral blindness were excluded. Moreover, macular degenerations of other etiologies, such as age-related macular degeneration, diabetic maculopathy, macular dystrophy, and so on, were all excluded. Related medical records of all the cases were collected, including the BCVA and basic demographics. Imaging modalities, including color fundus, OCT, fluorescence angiography, and indocyanine angiography, were obtained. This study was approved by the Ethics Committee and Institutional Review Board of the National Taiwan University Hospital (reference nos. 202112074RINA). This study was performed in accordance with the Declaration of Helsinki of 1964 and its later amendments. Due to the retrospective nature of the study, the waiver of informed consent was approved by the Institutional Review Board listed above. No identifying information is included in the manuscript. The consecutive patient cases collected from the outpatient clinic were equally accessible and considered adequate to prevent selection bias in terms of sex and gender.
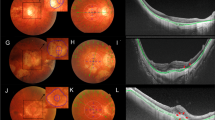
Standard, macula-centered, 45-degree color fundus photograph was obtained using a Canon CR-2 AF Digital retinal camera (Canon, Inc., Tokyo, Japan). OCT images were derived from Optovue RTVue XR OCT (Optovue, Inc., Freemont, CA). During OCT examination, standard, horizontal cut, 6-mm images in the macula, which passed through the fovea, were obtained. According to the META-PM classification system [4, 5], all the cases were graded and categorized according to their MAM stage, i.e., tessellated fundus (M1), diffuse chorioretinal atrophy (M2), patchy chorioretinal atrophy (M3), and macular chorioretinal atrophy (M4). Furthermore, cases having dome-shaped macular configuration or mCNV were separately categorized into another three groups, i.e., diffuse chorioretinal atrophy with DSM, patchy chorioretinal atrophy with DSM, and any stages of MAM with mCNV. The grading of MAM was conducted by two qualified retinal specialists reviewing the standard color fundus photographs and finalized by a third grader if interpretation was inconsistent. The five layers of the outer retina are the outer plexiform layer (OPL), outer nuclear layer (ONL), myoid and ellipsoid zone (MEZ), outer segment of the photoreceptor (OS), and interdigitation zone and retinal pigmented epithelium (IZ and RPE). With the standard OCT image of each case, the thickness of each of these five layers, as well as choroid thickness, was measured at the central fovea, 500 µm, and 1000 µm from the central fovea. At the fovea, Henle’s fiber layer (OPL) and ONL were measured as a single layer due to an inability to differentiate (Fig. 1). For cases having a certain degree of retinal atrophy, plus signs, or scarring tissue within an area of interest, the remnant outer retinal sublayers were carefully outlined and measured by two qualified retinal specialists. If opinions differed, the third retinal specialist was involved, and discussions were held until a consensus was reached.
The illustration of measuring methods, in which a horizontal macula OCT image that transects fovea was obtained. Each sublayer of the outer retina was identified (i). Five vertical lines (white) that were perpendicular to the RPE and Buch’s membrane were drawn at the fovea center, 500 μm from fovea, and 1000 μm from the fovea, respectively. Thickness of the outer retinal sublayers and choroid along each vertical line was measured (ii). OPL outer plexiform layer, HFL + ONL Henle’s fiber layer and outer nuclear layer, MEZ myoid and ellipsoid zone, OS outer segment of the photoreceptor, IZ + RPE interdigitation zone and retinal pigmented epithelium
BCVA was tested using the Snellen chart and converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Statistical analysis was conducted using IBM SPSS for Windows, version 19. The demographics of the different grading groups were compared using the analysis of variance (ANOVA) test. The BCVA, thickness of the outer retinal sublayers, and the choroid of each MAM stage were also compared using ANOVA. Intergroup differences were subsequently analyzed using Tamhane’s T2 post hoc test. Pairwise comparisons between groups with or without DSM or mCNV were evaluated using the independent t-test.
Correlation between the average thickness of outer retina sublayers and visual acuity, as well as central outer retinal sublayer thickness and BCVA, were analyzed using the Pearson correlation test.
Adjustments for multiple testing were managed with post hoc analysis of ANOVA. As for pairwise comparisons between groups with and without DSM, and groups with and without CNV, the adjustment was regarded redundant due to different populations. Adjustment of retinal and choroidal thickness according to axial length or refraction has been proven to be unnecessary [13, 14]. Further, multivariable regression analysis was performed for analyzing confounding factors.
The results were considered statistically significant if p < 0.05.
Results
Importantly, 158 eyes from 111 patients were included in this study. The patients were categorized accordingly. The number of cases per condition, average age, sex ratio, and average BCVA of each group are listed in Table 1, and Supplementary Material Table S1. Significant intergroup differences were observed in mean age (p-value = 0.024) and average BCVA (p-value < 0.001). Multivariable analysis was thus conducted for confounding factor analysis, and the results are listed in Supplementary Material Table S2. Sex and age were not significant confounding factors.
Analysis of the average BCVA, thickness of the outer retinal sublayers, and choroid of MAM, as well as subgroup comparison, are listed in Table 2. Significantly poorer BCVA (p-value = 0.040), thinner OS (p-value = 0.032), and thinner choroid (p-value < 0.001) were observed in the M2 stage (compared with the M1 stage). No significance was found in the BCVA, thickness of the outer retinal sublayers, and thickness of the choroid layer between the M2 stage and M3 stage. Significant decrease in the BCVA and thinning in every outer retinal sublayer (p-value < 0.001) were observed between the M3 stage and M4 stage, except for the choroid layer (p-value = 0.767).
Comparisons between groups of MAM with and without DSM are presented in Table 3. Significant thinning in the choroid thickness (p-value = 0.018) was observed in the M2 stage with DSM compared with that without. Further, significant decrease in the average BCVA (p-value = 0.008) was found in the group of M3 with DSM compared with that without. In addition to these, there was no difference in the outer retinal layer thickness between the groups with or without DSM.
The comparisons between groups of MAM with or without mCNV are listed in Table 4. Significant decrease in the average BCVA (p-value = 0.031) and thickness of the OS layer (p-value = 0.005) were observed in the group with mCNV compared with that without. No significant difference was found in other outer retinal or choroidal sublayers between the two groups.
Table 5 presents the correlation analysis between the average thickness of the choroidal and outer retinal layers and visual acuity. For all MAM stages, from M1 to M4, excluding the presence of DSM and mCNV, the thickness of the choroid and all the outer retinal layers demonstrated significant negative correlation with the BCVA (p-value < 0.001). Among them, the OS and MEZ thickness had the highest Pearson correlation in absolute value (0.71) with BCVA. Sublayer thickness at central fovea (umbo) showed similar correlation profiles.
Discussion
Correlation of Outer Retinal Sublayers with Different Grading of MAM
Our results provide an insight into the anatomical changes in varying severities of MAM by investigating the correlation between visual acuity and the sublayer thickness of the outer retina and choroid.
For myopic eyes, the choroid and retinal layers, including the HFL and ONL, MEZ, and IZ and RPE, were found to be thinner than those of the emmetropic eyes [11]. In fact, macular thickness increased in the very early stage of myopia [15], which is attributed to vitreomacular traction. The retinal layer, as well as the choroid thinning owing to axial elongation, were considered important pathophysiological parameters in the myopic macular change [16]. Subsequent tangential retinal stretch leads to decreased photoreceptor density [17, 18], photoreceptor shrinkage, and photoreceptor cell apoptosis [19] and resultant retinal thinning. Although prominent thinning in inner retinal layers of myopic eyes was demonstrated [20], changes focusing on the outer retinal layers have not been well addressed.
In the early stage of MAM, choroid thinning and reduced perfusion in the myopic eyes were considered two of the important mechanisms responsible for causing retinal degeneration in myopic eyes [21]. Hayashi et al. found that the majority of patients progressed to the severe atrophic myopic stage without axial elongation [22]. In our study, only the OS and choroid showed significant thinning diffuse atrophy compared with that of tessellation. Photoreceptors are high oxygen-demand type cells [23]. In a hypobaric hypoxia mouse model, prominent shortening of the OS of the rod cells was observed [24]; thus, its especially high susceptibility to choroid perfusion decrease is reasonable. Our study revealed that a compromise in choroid perfusion and resultant OS thinning may be an important trait in the early stage of MAM; however, further studies are needed to prove the hypothesis.
In the intermediate stage of MAM, the presence of lacquer crack marks the initiation of degeneration from diffuse atrophy, in which retinal atrophy developed alongside the lacquer cracks into patchy atrophy [22]. Although it is easy to morphologically differentiate diffuse atrophy from patchy atrophy [4], our study found no significant difference in BCVA and the thickness of each outer retinal layer and the choroid. Our study results thus imply that the atrophic pathways in these stages did not significantly cause the vertical shrinkage of the individual outer retinal layers. Instead, it is the topographical extension of the atrophy area that dictates the progression from diffuse chorioretinal atrophy to patchy chorioretinal atrophy.
In the late stage of MAM, severe retinal thinning can be anticipated [5]. Our study shows that significant outer retinal thinning is present in all sublayers as patchy atrophy progresses into macular atrophy. Among them, the MEZ and OS layers exhibited the highest thickness reduction in percentage (Fig. 2). This finding implies that the outer portion of photoreceptors is more susceptible to atrophy in end-stage MAM, which is highly energy demanding.
Illustration of average thickness (i) and composition percentage (B) of the outer retinal layers in patchy atrophy and macular atrophy. Reduction in the percentage of each sublayer is presented in (ii). OPL outer plexiform layer, HFL + ONL Henle’s fiber layer and outer nuclear layer, MEZ myoid and ellipsoid zone, OS outer segment of the photoreceptor, IZ + RPE interdigitation zone and retinal pigmented epithelium
Choroidal Thickness
Choroidal thinning has long been considered a key feature of myopic change [5, 25], and its relentless thinning as MAM progresses is a common occurrence [5, 26]. However, our study shows that the trends vary in different stages of MAM.
In early stages of MAM, our study shows significant choroidal thinning in diffuse atrophy group compared with that of the tessellated fundus as mentioned above. This implies that choroidal thinning and perfusion compromise may be an early sign (even before development of retinal atrophy); the exception being the OS layer. Early choroidal thinning in a highly myopic eye had been observed even before the MAM [9]. Choroidal thinning is considered a sign of choroidal perfusion compromise [27], and choroidal vessel occlusion also results in choroidal thickness shrinkage and further compromises outer retinal perfusion in myopic eyes [28]. Consequently, our study shows that the choroid layer thinning and possible choroid perfusion compromise develop early in MAM.
However, choroidal thinning did not continue during intermediate stages. Our results show no significant difference in the choroidal thickness between diffuse and patchy atrophy. This led to the hypothesis that the underlying atrophic pathophysiology does not further compromise choroid perfusion and structure in these stages. In addition, choroid reduction does not play a key role in topographical atrophy of the outer retina as diffuse atrophy progresses into patchy atrophy. Progression of MAM independent of choroidal thinning has also been reported in a previous study [29]. Instead, it may be the disruption of Bruch’s membrane and subsequent lacquer cracks that plays a critical role in these stages.
In the end stages of MAM, our study shows no significant difference in choroid thickness between macular atrophy and patchy atrophy. Zhao et al. also found no significant difference in the choroid thickness between the macular atrophy and patchy atrophy groups [8]. On the basis of that discovery, they proposed that patchy and macular atrophy could be two distinct subtypes of end-stage MAM, instead of a consecutive relationship as proposed by Ohno-Matsui and Ruiz-Medrano et al. [4, 10] Furthermore, in a retrospective observational cases series [30], Fang et. al. found that patients with patchy atrophy rarely progressed into macular atrophy. Our findings support the idea that patchy atrophy and macular atrophy may be two distinct subtypes of end-stage MAM (Fig. 3). Alternatively, it may also be possible that the atrophic process driving patchy atrophy toward macular atrophy somehow spares the choroid. Further investigation into pathohistological mechanisms in end-stage MAM is needed to solve this puzzle.
The illustration of sublayer thickness changes of the outer retinal layer based on the severities of MAM. (i) Tessellated fundus, (ii) diffuse chorioretinal atrophy, (iii) patchy chorioretinal atrophy, and (iv) macular chorioretinal atrophy. The relative thickness of each sublayer is strictly proportional to the average thickness of corresponding sublayer in this study (Table 2). Special attention should be paid to the fact that the thickness difference for the same sublayers between two different MAM stages does not imply statistical significance. For the statistical analysis and results, please refer to Table 2. Modern understanding of the progression of MAM hypothesized that MAM progressed sequentially from (i) to (iv). As tessellated fundus progressed into diffuse chorioretinal atrophy, significant thinning was only observed in OS and choroid layers. Further, progression from diffuse chorioretinal atrophy to patchy chorioretinal atrophy (ii to iii) was not associated with significant thinning in any sublayer, which implied that horizontal (geographical) atrophy dictated this period. Macular chorioretinal atrophy revealed significant thinning in almost all outer retinal sublayers. However, macular chorioretinal atrophy showed no significant difference in choroid thickness with respect to patchy chorioretinal atrophy. This finding is not consistent to the conventional hypothesis that macular chorioretinal atrophy develops from patchy chorioretinal atrophy (iii to iv). Our finding suggested that macular chorioretinal atrophy may develop from stages of tessellated fundus or diffuse chorioretinal atrophy. OPL outer plexiform layer, HFL + ONL Henle’s fiber layer and outer nuclear layer, MEZ myoid and ellipsoid zone, OS outer segment of the photoreceptor, IZ + RPE interdigitation zone and retinal pigmented epithelium
Myopic Choroidal Neovascularization
In any stage of MAM, mCNV can develop, and is usually followed by enlarging atrophic area and vision deterioration [5]. Thinner choroid was found in MAM with mCNV compared with that without [31]. In our study, MAM with mCNV shows significant OS layer thinning and corresponding BCVA deterioration, while the other outer retinal layers and choroid remained unchanged. Several possible mechanisms have been attributed to choroidal thinning in MAM. The hemodynamic theory proposes that compromised perfusion results in a thinner choroid and subsequent development of mCNV [32]. On the contrary, a histological study conducted by Shefali et al. did not reveal any change in the thickness of choriocapillaries, indicating that the presence of mCNV is not well correlated with choroidal thinning [33]. This study proposed that an increase in the fibrotic component and the subsequent centipedal contracture thicken the surrounding choroid. Owing to multiple contributing factors, we postulate that the change in choroidal thickness in MAM with mCNV can be variable.
While the choroidal thickness change in MAM with mCNV is not representative of a particular result, the BCVA deterioration is definite [5]. According to the studies mentioned above, we hypothesized that compromised choroid perfusion still developed in the absence of thickness change. With the outer segment being the most susceptible layer in an oxygen-deficit environment [20], it is reasonable that OS reduces more significantly than other outer retinal layers in the presence of compromised choroidal perfusion.
Aside from mCNV, lacquer cracks and Fuchs spots are two “plus signs” defined by Ohno-Matsui et al. [4] Lacquer crack is caused by disruption in Bruch’s membrane, while Fuchs spot is reactive hyperpigmentation of RPE of nearby mCNV. Both of these plus signs disintegrate the normal retinochoroidal histology, and thus may theoretically disrupt retinal function and even lead to outer retinal atrophy. However, further study is needed to investigate their associations with the thickness of outer retinal sublayers.
Dome-Shaped Macula
DSM has no effect on BCVA deterioration [34], but increases the risk of subfoveal serous detachment and parafoveal retinoschisis [35]. The bulging configuration around the fovea presumably increased the mechanical stress on the RPE layer, resulting in subsequent anatomical and functional deterioration [36]. However, in our study, significant BCVA decrease was found in the patchy atrophy group with DSM compared with that without; while no significant difference in the BCVA was found between the diffuse atrophy group with or without DSM. Our finding suggests that the presence of DSM mostly does not affect BCVA, outer retina, and choroid structures. However, if accompanied by a more severe stage of MAM, such as patchy atrophy, vision may deteriorate.
Correlation between Visual Acuity and Thickness of the Outer Retina and Choroid
Thickness reduction in MEZ has been found to have the highest correlation to vision deterioration in myopic eyes [11]. In addition, thinner choroid was associated with poor visual acuity in highly myopic eyes [21].
In our study, thickness of OS, followed by thickness of MEZ, was the most sensitive visual acuity indicator (Table 5). Furthermore, our study also showed that the correlation profiles of visual acuity between point thickness at umbo or average thickness in central fovea are similar (Table 5). Although outer retinal thickness at central fovea (umbo) is highly associated with visual acuity [37], increase in topographic atrophy in the macula also has a significant effect on BCVA deterioration [11]. As MAM is a process involving a large portion of macula, analysis between the relationship of area-average thickness and BCVA is regarded as being reasonable.
Our study had certain limitations. Firstly, this is a retrospective cross-sectional study similar to several relevant studies in this field [38, 39]. It is extremely hard to observe MAM longitudinally because it takes decades for MAM to progress [30, 40] However, by consecutive collection of cases over many years, and by statistical adjustment of confounders, many biases could be neutralized. Furthermore, this study provides invaluable information that sheds light on the functional and anatomical evolution of myopic atrophy maculopathy, paving the way for future longitudinal studies. Secondly, this study excluded tractional MAM, which is also an important pathology of MAM. However, by excluding the tractional effect, which could jeopardize the sublayer thickness measurement, we were able to precisely evaluate the difference in thickness of the outer retinal layers and the choroid among the different atrophic stages, and investigate individual outer retinal layer thickness of MAM with DSM or mCNV. The findings of the outer retinal and choroid thickness difference along the different atrophic stages may shed light onto the underlying pathogenic mechanism of MAM. The third limitation is that this study did not analyze the effects of the other two “plus signs”; lacquer cracks and Fuchs spots. Indeed, like mCNV, these two “plus signs” may also have a negative effect on functional and anatomical traits of outer retina. Howevver, due to the heterogeneity and complexity of the “plus signs,” it may be more appropriate to analyze them in another separate and focused study.
Conclusions
Our study found that as MAM progresses, the outer retinal layers, including HF and ONL, MEZ, OS, and IZ and RPE, undergo progressive thinning. The thinning process among the outer retinal layers and choroid along the atrophic pathway is uneven. Between the tessellation fundus and diffuse atrophy groups, only the OS and choroid are significantly thinner in the diffuse atrophy group. Further, no significant difference in all the outer retinal layers and choroid between the patchy and diffuse atrophy groups was observed. In macular atrophy, all the outer retinal layers are thinner than those observed in patchy atrophy; the exception being choroid. Among them, the MEZ and OS exhibited the most significant reduction in thickness, accounting for the thinnest layers in macular atrophy. The results suggest that patchy and macular atrophy are two types of end-stage MAM (instead of representing a chronological relationship). mCNV also showed significant thinning in OS and BCVA deterioration. The presence of DSM is associated with thinner choroid in M2 stage, while the presence of DSM is associated with poorer vision in M3 stage. OS thickness has the highest negative correlation with BCVA in all the atrophic stages of pathologic myopia, followed by the MEZ thickness. The present study suggested that the thickness of each outer retinal sublayer makes up the varying percentage of total retinal thickness among different stages of MAM, and that directly correlates with vision functions. Different findings between different stages of myopic atrophic pathways suggested that each atrophic stage could be driven by different pathogenic mechanisms.
References
Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157:9-25.e12. https://doi.org/10.1016/j.ajo.2013.08.010.
Wong Y-L, Saw S-M. Epidemiology of pathologic myopia in Asia and worldwide. Asia-Pac J Ophthalmol. 2016;5:394–402. https://doi.org/10.1097/apo.0000000000000234.
Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102:855–62. https://doi.org/10.1136/bjophthalmol-2017-311266.
Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877-883 e877. https://doi.org/10.1016/j.ajo.2015.01.022.
Ruiz-Medrano J, Montero JA, Flores-Moreno I, et al. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115. https://doi.org/10.1016/j.preteyeres.2018.10.005.
Zhou Y, Song M, Zhou M, et al. Choroidal and retinal thickness of highly myopic eyes with early stage of myopic chorioretinopathy: tessellation. J Ophthalmol. 2018;2018:2181602. https://doi.org/10.1155/2018/2181602.
Liu X, Shen M, Yuan Y, et al. Macular thickness profiles of intraretinal layers in myopia evaluated by ultrahigh-resolution optical coherence tomography. Am J Ophthalmol. 2015;160:53–61e52. https://doi.org/10.1016/j.ajo.2015.03.012.
Zhao X, Ding X, Lyu C, et al. Morphological characteristics and visual acuity of highly myopic eyes with different severities of myopic maculopathy. Retina. 2020;40:461–7. https://doi.org/10.1097/IAE.0000000000002418.
Barteselli G, Lee SN, El-Emam S, et al. Macular choroidal volume variations in highly myopic eyes with myopic traction maculopathy and choroidal neovascularization. Retina. 2014;34:880–9. https://doi.org/10.1097/IAE.0000000000000015.
Ruiz-Medrano J, Flores-Moreno I, Ohno-Matsui K, et al. Correlation between atrophy-traction-neovascularization grade for myopic maculopathy and clinical severity. Retina. 2021;41:1867–73. https://doi.org/10.1097/IAE.0000000000003129.
Ye J, Shen M, Huang S, et al. Visual acuity in pathological myopia is correlated with the photoreceptor myoid and ellipsoid zone thickness and affected by choroid thickness. Invest Ophthalmol Vis Sci. 2019;60:1714–23. https://doi.org/10.1167/iovs.18-26086.
Jain M, Gopal L, Padhi TR. Dome-shaped maculopathy: a review. Eye (Lond). 2021;35:2458–67. https://doi.org/10.1038/s41433-021-01518-w.
Salmon AE, Sajdak BS, Atry F, et al. Axial scaling is independent of ocular magnification in OCT images. Invest Ophthalmol Vis Sci. 2018;59:3037–40. https://doi.org/10.1167/iovs.17-23549.
Sampson DM, Gong P, An D, et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:3065–72. https://doi.org/10.1167/iovs.17-21551.
Lam DS, Leung KS, Mohamed S, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007;48:376–82. https://doi.org/10.1167/iovs.06-0426.
Curtin BJ. Pathologic myopia. Acta Ophthalmol Suppl. 1988;185:105–6. https://doi.org/10.1111/j.1755-3768.1988.tb02680.x.
Jaworski A, Gentle A, Zele AJ, et al. Altered visual sensitivity in axial high myopia: a local postreceptoral phenomenon? Invest Ophthalmol Vis Sci. 2006;47:3695–702. https://doi.org/10.1167/iovs.05-1569.
Chui TY, Song H, Burns SA. Individual variations in human cone photoreceptor packing density: variations with refractive error. Invest Ophthalmol Vis Sci. 2008;49:4679–87. https://doi.org/10.1167/iovs.08-2135.
Xu GZ, Li WW, Tso MO. Apoptosis in human retinal degenerations. Trans Am Ophthalmol Soc. 1996;94:411–30 (discussion 430–411).
Abbott CJ, Grünert U, Pianta MJ, et al. Retinal thinning in tree shrews with induced high myopia: optical coherence tomography and histological assessment. Vis Res. 2011;51:376–85. https://doi.org/10.1016/j.visres.2010.12.005.
Nishida Y, Fujiwara T, Imamura Y, et al. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012;32:1229–36. https://doi.org/10.1097/IAE.0b013e318242b990.
Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117(1595–1611):1611.e1591-1594. https://doi.org/10.1016/j.ophtha.2009.11.003.
Kooragayala K, Gotoh N, Cogliati T, et al. Quantification of oxygen consumption in retina ex vivo demonstrates limited reserve capacity of photoreceptor mitochondria. Invest Ophthalmol Vis Sci. 2015;56:8428–36. https://doi.org/10.1167/iovs.15-17901.
Ebner LJA, Samardzija M, Storti F, et al. Transcriptomic analysis of the mouse retina after acute and chronic normobaric and hypobaric hypoxia. Sci Rep. 2021;11:16666. https://doi.org/10.1038/s41598-021-96150-9.
Curtin BJ. Physiologic vs pathologic myopia: genetics vs environment. Ophthalmology. 1979;86:681–91. https://doi.org/10.1016/s0161-6420(79)35466-5.
Wong CW, Phua V, Lee SY, et al. Is choroidal or scleral thickness related to myopic macular degeneration? Invest Ophthalmol Vis Sci. 2017;58:907–13. https://doi.org/10.1167/iovs.16-20742.
Wu H, Zhang G, Shen M, et al. Assessment of choroidal vascularity and choriocapillaris blood perfusion in anisomyopic adults by SS-OCT/OCTA. Invest Ophthalmol Vis Sci. 2021;62:8. https://doi.org/10.1167/iovs.62.1.8.
Okabe S, Matsuo N, Okamoto S, et al. Electron microscopic studies on retinochoroidal atrophy in the human eye. Acta Med Okayama. 1982;36:11–21. https://doi.org/10.18926/amo/30704.
Zaben A, Zapata M, Garcia-Arumi J. Retinal sensitivity and choroidal thickness in high myopia. Retina. 2015;35:398–406. https://doi.org/10.1097/iae.0000000000000367.
Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;125:863–77. https://doi.org/10.1016/j.ophtha.2017.12.005.
Cheung CM, Loh BK, Li X, et al. Choroidal thickness and risk characteristics of eyes with myopic choroidal neovascularization. Acta Ophthalmol. 2013;91:e580-581. https://doi.org/10.1111/aos.12117.
Ohno-Matsui K, Ikuno Y, Lai TYY, et al. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018;63:92–106. https://doi.org/10.1016/j.preteyeres.2017.10.005.
Jonas SB, Panda-Jonas S, Jonas JB, et al. Histology of neovascular myopic macular degeneration. Sci Rep. 2021;11:21908. https://doi.org/10.1038/s41598-021-01500-2.
Fang D, Zhang Z, Wei Y, et al. The morphological relationship between dome-shaped macula and myopic retinoschisis: a cross-sectional study of 409 highly myopic eyes. Invest Ophthalmol Vis Sci. 2020;61:19. https://doi.org/10.1167/iovs.61.3.19.
Zhao X, Ding X, Lyu C, et al. Observational study of clinical characteristics of dome-shaped macula in Chinese Han with high myopia at Zhongshan Ophthalmic Centre. BMJ Open. 2018;8:e021887. https://doi.org/10.1136/bmjopen-2018-021887.
Ohsugi H, Ikuno Y, Oshima K, et al. Morphologic characteristics of macular complications of a dome-shaped macula determined by swept-source optical coherence tomography. Am J Ophthalmol. 2014;158:162-170 e161. https://doi.org/10.1016/j.ajo.2014.02.054.
Nanegrungsunk O, Gu SZ, Bressler SB, et al. Correlation of change in central subfield thickness and change in visual acuity in neovascular AMD: post hoc analysis of VIEW 1 and 2. Am J Ophthalmol. 2022;238:97–102. https://doi.org/10.1016/j.ajo.2021.11.020.
Farinha CL, Baltar AS, Nunes SG, et al. Progression of myopic maculopathy after treatment of choroidal neovascularization. Ophthalmologica. 2014;231:211–20. https://doi.org/10.1159/000357290.
Fang Y, Du R, Nagaoka N, et al. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology. 2019;126:1018–32. https://doi.org/10.1016/j.ophtha.2019.01.012.
Li Z, Liu R, Xiao O, et al. Progression of myopic maculopathy in highly myopic Chinese eyes. Invest Ophthalmol Vis Sci. 2019;60:1096–104. https://doi.org/10.1167/iovs.18-25800.
Acknowledgements
The authors sincerely thank all study participants for their invaluable data.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
Free resources of statistics were used from the website of the statistics education center. Yu-Cheng Lee, who is affiliated to the College of Medicine at National Taiwan University, helped us with the statistics. No funding was involved in the statistical analysis process.
Author Contributions
Cheng-Yung Lee, Yun Hsia, Mei-Chi Tsui, Shih-Wen Wang, Chien-Jung Huang, I-Hsin Ma, Kuo-Chi Hung, Muh-Shy Chen, and Tzyy-Chang Ho contributed to the study conception and design. Material preparation, data collection and analysis were performed by Cheng-Yung Lee, Yun Hsia, and I-Hsin Ma. The first draft of the manuscript was written by Cheng-Yung Lee. Cheng-Yung Lee, Yun Hsia, Mei-Chi Tsui, Shih-Wen Wang, Chien-Jung Huang, I-Hsin Ma, Kuo-Chi Hung, Muh-Shy Chen, and Tzyy-Chang Ho commented on the previous versions of the manuscript. And finally, Cheng-Yung Lee, Yun Hsia, Mei-Chi Tsui, Shih-Wen Wang, Chien-Jung Huang, I-Hsin Ma, Kuo-Chi Hung, Muh-Shy Chen, and Tzyy-Chang Ho read and approved the final manuscript.
Disclosures
Cheng-Yung Lee, Yun Hsia, Mei-Chi Tsui, Shih-Wen Wang, Chien-Jung Huang, I-Hsin Ma, Kuo-Chi Hung, Muh-Shy Chen, and Tzyy-Chang Ho declare that they have no competing interests.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee and Institutional Review Board of the National Taiwan University Hospital (reference numbers: 202112074RINA). This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Due to the retrospective nature of the study, the waiver of informed consent was approved by the Institutional Review Board listed above. No identifying information is included in the manuscript.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed in the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, CY., Hsia, Y., Tsui, MC. et al. Correlation of Visual Acuity and Outer Retinal Thickness in Myopic Atrophic Maculopathy: A Retrospective Review. Ophthalmol Ther 12, 1989–2003 (2023). https://doi.org/10.1007/s40123-023-00710-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00710-3