Abstract
Introduction
To investigate changes in the vitreoretinal interface after anti-vascular endothelial growth factor (anti-VEGF) treatment in highly myopic eyes.
Methods
Eyes with myopic choroidal neovascularization (mCNV) treated with intravitreal injection of anti-VEGF in a single-center were retrospectively reviewed. Fundus abnormalities and features of optical computed tomography were studied.
Results
A total of 295 eyes from 254 patients were recruited to the study. Prevalence of myopic macular retinoschisis (MRS) was 25.4%, and the rates of progression and onset of MRS were 75.9% and 16.2%, respectively. Outer retinal schisis (β = 8.586, p = 0.003) and lamellar macular hole (LMH) (β = 5.015, p = 0.043) at baseline were identified risk factors for progression and onset of MRS, whereas male sex (β = 9.000, p = 0.039) and outer retinal schisis at baseline (β = 5.250, p = 0.010) were risk factors for MRS progression. Progression of MRS was first detected in outer retinal layers in 48.3% of eyes. Thirteen eyes required surgical intervention. Spontaneous improvements of MRS were observed in five eyes (6.3%).
Conclusion
Changes in the vitreoretinal interface, such as progression, onset, and improvement of MRS, were observed after anti-VEGF treatment. Outer retinal schisis and LMH were risk factors of progression and onset of MRS after anti-VEGF treatment. Intravitreal injection of ranibizumab and retinal hemorrhage were protective factors for surgical intervention for vision-threatening MRS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
To investigate changes in the vitreoretinal interface after anti-vascular endothelial growth factor (anti-VEGF) treatment in highly myopic eyes. |
What was learned from the study? |
Changes in the vitreoretinal interface, including progression, onset, and improvement of macular retinoschisis (MRS), were observed after anti-VEGF treatment in highly myopic eyes. |
Outer schisis and lamellar holes were risk factors for progression and onset of MRS. |
Intravitreal injection of ranibizumab and retinal hemorrhage were protective factors for progression to vision-threatening MRS that required surgical intervention. |
Introduction
Myopic choroidal neovascularization (mCNV) is a vision-threatening complication in eyes with pathologic myopia [1]. The prevalence of mCNV ranges from 5% to 11% among individuals with high myopia [2,3,4]. In addition to mCNV, intraretinal splitting of the inner and outer retinal layers, recognized as myopic macular retinoschisis (MRS), has been noted in 9% to 34% of highly myopic eyes [5, 6]. The terminology used in discussing this condition has not been consistent, and Panozzo and Mercanti proposed the term “myopic traction maculopathy” (MTM) to describe the changes in the vitreoretinal interface caused by traction forces from the epiretinal membrane or by residual focal vitreoretinal adhesion combined with posterior staphyloma and progressive scleral stretching in myopic eyes [7]. Optical computed tomography (OCT) is essential for the diagnosis of MTM, which manifests as three sometimes overlapping subtypes, i.e., MRS, foveal detachment (FD), and macular holes [8, 9]. The impairments in visual acuity associated with each subtype have been described in previous publications [8, 10]. The natural course of MRS varies, with some eyes with MRS experiencing progressive visual deterioration, while others may have stable visual acuity for years [11, 12]. Surgery is indicated when there is decreased visual acuity, FD, full-thickness macular hole (FTMH), or a schisis shaped like a champagne flute. [12, 13]
The incidence of myopia has increased rapidly in recent decades in many countries, particularly in East Asia [14,15,16] and, consequently, the management of associated comorbidities has become increasingly important. Intravitreal (IV) injection of anti-vascular endothelial growth factor (anti-VEGF) has been shown to effectively improve vision in patients with mCNV [17, 18]. The progression and onset of MRS in mCNV eyes after anti-VEGF treatment have been reported [19,20,21,22], but the risk and protective factors have not yet been studied in a large series. In the present study, we collected cases of eyes with mCNV treated with anti-VEGF, investigated the clinical features, and identified the risk and protective factors for vitreoretinal interface changes after anti-VEGF treatment in highly myopic eyes.
Methods
Medical records were retrospectively reviewed on consecutive patients with high myopia and mCNV who had applied for National Health Insurance coverage of anti-VEGF treatment at the National Taiwan University Hospital between July 2016 and September 2021. The inclusion criteria were: (1) presence of high myopia, defined as a refractive spherical equivalent (SE) of at least — 6.00 diopters (D) or an axial length (AL) of > 26.0 mm; (2) diagnosis of active subfoveal, juxtafoveal, or extrafoveal CNV secondary to pathologic myopia confirmed by complete ocular examination; (3) a minimum follow-up duration of 3 months. Patients who had undergone macular surgery, such as peeling of epiretinal membrane (ERM) and/or the internal limiting membrane (ILM), or photodynamic therapy, were excluded. Patients with other diseases causing retinoschisis or cystoid macular edema, such as retinal vein occlusion, age-related macular degeneration, and uveitis, were also excluded.
All patients underwent a complete ophthalmic examination, which included noncycloplegic refraction assessment using an autorefractometer (KR-800TM; Topcon, Tokyo, Japan), measurement of the best corrected visual acuity (BCVA; in Snellen visual acuity ratios and in logarithms of the minimum angle of resolution [logMAR] when used for statistical analyses), slit-lamp biomicroscopy, indirect ophthalmoscopy, AL measurement using the Lenstar™ (LS 900; Haag-Streit, Mason, OH, USA), color fundus photography, spectral-domain-OCT (SD-OCT; RTVue XR or 100; Optovue, Fremont, CA, USA), and fluorescein angiography (HRA 2; Heidelberg Engineering, Heidelberg, Germany). The horizontal and vertical scans of OCT were used for serial follow-up and comparison. Relevant OCT parameters, such as vitreomacular traction (VMT), vitreomacular adhesion (VMA), the presence of ERM, foveoschisis, extrafoveoschisis, inner retinal schisis, outer retinal schisis, lamellar macular hole (LMH), FD, central foveal thickness (CFT), FTMH, dome-shaped macula, and the location of CNV (subfoveal, juxtafoveal or extrafoveal), were documented for analysis. CFTs were generated automatically by the OCT device. In this study, splitting of the inner and outer retinal layers resulting in intraretinal, hyporeflective cystoid spaces on the OCT is identified as MRS, and splitting within the outer plexiform layer and the outer nuclear layer is defined as outer retinal schisis. Schisis cavities at the level of the inner plexiform, ganglion cell layer, and retinal nerve fiber layer are defined as inner retinal schisis [20, 23, 24]. Progression of MRS is defined as the development of an inner LMH, FD, or FTMH, or an increase of > 100 μm in the height of retinoschisis and an enlargement of the retinoschisis towards the area not having a macular retinoschisis [12]. Onset of MRS is defined as the absence of MRS at baseline but with the detection of signs of MRS during follow-ups. Improvement of MRS is defined as a decrease in the height or extent of the macular retinoschisis without the development of an inner lamellar MH, FD, or FTMH. Myopic maculopathy is classified into five categories: 0, indicating no myopic retinal degenerative lesion; 1, indicating tessellated fundus; 2, indicating diffuse chorioretinal atrophy; 3, indicating patchy chorioretinal atrophy; and 4, indicating macular atrophy [25]. The images were reviewed and analyzed independently by two of the authors (MCT and YTH). In cases of doubt or disagreement, the senior author (TCH) was consulted, and a panel discussion was held to reach a consensus.
This study was approved by the Research Ethics Committee of National Taiwan University Hospital and was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments.
Statistical Analysis
Means and standard deviations (SD) of quantitative variables and frequencies and percentages of categorical variables were calculated. Continuous variables were compared by using the t-test or Mann–Whitney U-test, and categorical variables were analyzed by using the Chi-square test or Fisher’s exact test. Logistic regression was used for investigating factors relevant to progression and onset of MRS. p < 0.05 was considered to be statistically significant. The statistical analysis of the data was carried out by using Statistical Package for Social Sciences (SPSS) version 22 software (SPSS IBM, Armonk, NY, USA). Descriptive statistics was calculated in terms of the mean, SD, median, and range.
Results
A total of 295 eyes with mCNV from 254 patients were included in this study. The mean (± SD) age of the patients was 57.4 ± 13.3 years (range 19–92; median 58 years) at the diagnosis of mCNV. The mean AL was 29.4 ± 6 mm. Prevalence of MRS in mCNV eyes was 25.4%. Of the 295 mCNV eyes, 95 eyes (32.2%) developed progression or onset of MRS at 11.6 ± 14.8 months (range 1–60 months, median 5 months) after the initiation of IV anti-VEGF treatment. The cumulative incidences of progression and onset of MRS were 15.2% at 3 months, 19.3% at 6 months, 23.4% at 1 year, 28.5% at 2 years, 29.2% at 3 years, and 32.2% at 5 years. Eyes with progression or onset of MRS, compared to those without progression or onset of MRS, had a higher ratio of presence of MRS (63.2% vs. 9.5%, p < 0.0001), foveoschisis (36.8% vs. 4.5%, p < 0.0001), extrafoveoschisis (61.1% vs. 10.0%, p < 0.0001), inner retinal schisis (49.5% vs. 9.0%, p < 0.0001), outer retinal schisis (58.9% vs. 6.5%, p < 0.0001), and LMH (16.8% vs. 1.5%, p < 0.0001) at baseline, more MRS in the fellow eye (39.1% vs. 16.1%, p < 0.0001), and greater initial CFT (322.4 ± 89.3 vs. 301.7 ± 77.6 µm, p = 0.047). For multivariate logistic regression analysis adjusted for foveoschisis, extrafoveoschisis, inner and outer retinal schisis, and LMH at baseline, only outer retinal schisis (β = 8.586, p = 0.003) and LMH (β = 5.015, p = 0.043) were identified as variables associated with progression or new onset of MRS (Table 1).
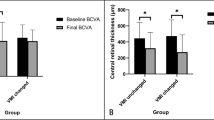
Of the 295 eyes, 13 showed progression, with 15.4% of these progressing to FTMH (1 case is shown in Fig. 1), 15.4% to FD (1 case is shown in Fig. 2), 30.8% to macular hole retinal detachment (MHRD), 7.7% to LMH, and 30.8% to other conditions at risk of vision threatening, such as foveoschisis shaped like a champagne flute [12, 13, 26] (1 case is shown in Fig. 3). Consequently, these eyes received surgical intervention at 12.7 ± 9.1 months (range 2–34 months, median 10 months) after initial injection. (Table 2). One eye (Fig. 3) received IV perfluoropropane (C3F8) [27, 28] and the other 12 eyes received pars plana vitrectomy combined with the inverted ILM flap technique [29, 30] (5 eyes; 1 case is shown in Fig. 1), the center non-peeling ILM surgery technique [31,32,33,34] (3 eyes), or simple ILM peeling (4 eyes; 1 case is shown in Fig. 2). Of the 13 eyes with progression, 11 (84.6%) had MRS at baseline. Risk factors for vision-threatening MRS requiring surgical intervention in eyes with progression and onset of MRS were evaluated by using logistic regression models (Table 3). AL, AL of fellow eye, initial CFT, myopic maculopathy category at baseline, and presence of foveoschisis, inner retinal schisis, ERM, and LMH at baseline were identified to be risk factors, while intravitreal ranibizumab (IVR) and presence of retinal hemorrhage at baseline were protective factors. Eyes requiring surgical intervention compared to those with no need of surgical intervention had poorer BCVA at 1 month after injection (logMAR 0.757 ± 0.363 vs. logMAR 0.510 ± 0.410, p = 0.026), at 6 months (logMAR 0.799 ± 0.402 vs. logMAR 0.521 ± 0.451, p = 0.011), at 1 year (logMAR 0.882 ± 0.528 vs. logMAR 0.530 ± 0.413, p = 0.012), and at the last follow-up visit (logMAR 1.038 ± 0.767 vs. logMAR 0.667 ± 0.624, p = 0.030), and had greater CFT at 1 month (370.7 ± 147.5 vs. 274.9 ± 57.9 µm, p = 0.009), at 3 months (353.4 ± 129.9 vs. 268.8 ± 56.6 µm, p = 0.003), and at 6 months (415.8 ± 196.5 vs. 274.5 ± 52.1 µm, p = 0.001). In addition, the former had higher ratios of categories 3 and 4 myopic maculopathy at the last follow-up visit. We also found that there was no association of age, gender, initial BCVA, location of CNV, the number of total injections, the number of intravitreal bevacizumab (IVB), IVR, and intravitreal aflibercept (IVA) injections, as well as presence of lacquer cracks, punctate inner choroidopathy (PIC), staphyloma, MRS, outer retinal schisis, VMT, VMA, and dome-shaped macula at baseline (p > 0.05 for all) (Table 3). In eyes with MRS progression requiring surgery, the progression was found in the following ratios: 53.8% in the outer retinal layers, 23.1% presence of LMH, 7.7% in both the outer and inner retinal layers, 7.7% in inner retinal layers, and 7.7% presence of FTMH.
Progression of myopic macular retinoschisis (MRS) in the right eye of a 56-year-old woman with myopic choroidal neovascularization (mCNV). a, b Before anti-vascular endothelial growth factor (anti-VEGF) treatment, hyperfluoresence, and leakage of mCNV on late phase of the fluorescein angiography (a) and category 2 myopic maculopathy on color photo (b) are shown. c 7 years after operation, macular atrophy (category 4 myopic maculopathy) was noted on color photo. d, e Before treatment, myopic macular retinoschisis (MRS) with lamellar macular hole (LMH) and juxtafoveal mCNV (arrow) are shown on optical computed tomography (OCT). f 2 years after intravitreal bevacizumab (IVB) treatment, the size of mCNV became smaller (arrow), and best-corrected visual acuity (BCVA) was 20/40. g 32 months after IVB treatment, full-thickness macular hole (FTMH) and new mCNV (arrow) were noted on OCT, and BCVA was 20/200. h 2 years after pars plana vitrectomy and the inverted internal limiting membrane (ILM) flap technique, the FTMH sealed. i 7 years after surgical intervention, the FTMH remained sealed but there were progressive chorioretinal atrophy. BCVA was 20/400
Progression of MRS in the right eye of a 53-year-old woman with mCNV. a Before anti-VEGF treatment, subfoveal mCNV (arrow) and outer retinal schisis were shown on OCT. BCVA was 20/100. b 2 months after 2 injections of IVB, mCNV became smaller (arrow), BCVA improved to 20/40, and MRS remained stationary. c 6 months after injections, MRS progression to foveal detachment (FD) (asterisk) was observed, and BCVA decreased to 20/67. d 6 months after pars plana vitrectomy and ILM peeling, FD and MRS improved. e 1 year after operation, resolution of FD and improvement of MRS were noted on OCT. f 2 years after operation, attached retina without MRS was observed and BCVA was 20/50
Progression of MRS in the right eye of a 44-year-old woman with mCNV. a Before treatment, subfoveal mCNV (arrow), inner and outer retinal schisis, and posterior hyaloid membrane (arrowheads) were observed on OCT. BCVA was 20/50. b 3 months after 2 injections of IVR, mCNV became smaller (arrow) and inner and outer retinal schisis slightly increased. c 6 months after IVR, progression of outer retinal schisis to foveoschisis shaped like a champagne flute was observed on OCT. BCVA decreased to 20/100. d, e 2 weeks (d) and 1 year (e) after intravitreal injection of C3F8 0.3 ml, MRS improved. BCVA returned to 20/29 at 1 year
Table 4 shows the characteristics of eyes with or without MRS at baseline. Compared to eyes without MRS at baseline, eyes with MRS at baseline were associated with older age, fewer total injections of anti-VEGF, fewer injections of ranibizumab, longer AL, longer AL of the fellow eye, higher proportion of staphyloma, ERM (62.0% vs. 26.9%, p < 0.0001), LMH, MRS in the fellow eye at baseline, and greater CFT at baseline, at 1 month, at 3 months, at 6 months, at 1 year, and at the last follow-up visit.
During follow-up, the onset of MRS developed in 35 of the 216 eyes (16.2%) of high myopic eyes without MRS at baseline at 17.9 ± 17.2 months (range 1–60 months; median 12 months) after initiation of anti-VEGF treatment. New MRS developed more likely in the inner retinal layers (31.4%, 11/35), followed by in the outer retinal layers (25.7%, 9/35), in both the outer and inner retinal layers (20.0%, 7/35), presence of LMH (17.1%. 6/35), and presence of FTMH (5.7%, 2/35). Onset of MRS was not associated with age, gender, laterality, the total number of injections of anti-VEGF, the numbers of injections of bevacizumab, ranibizumab or aflibercept, the numbers of injections at 3 months, 6 months, 1 year, and yearly until 7 years, AL, initial BCVA, initial CFT, dome-shaped macula, BCVA at 3 months, 6 months, 1 year and yearly until 5 years, and BCVA at the last follow-up visit. However, eyes with onset of MRS had significantly longer duration of follow-up (62.3 ± 33.0 vs. 45.8 ± 34.1 months, p = 0.009) and better BCVA at 1 month (logMAR 0.467 ± 0.312 vs. logMAR 0.643 ± 0.536, p = 0.018). Of the 35 eyes (5.7%) with onset of MRS, two had MRS progression to a FTMH or MHRD and received surgical intervention at 12 and 14 months, respectively.
Of the 79 eyes with MRS at baseline, 60 eyes (75.9%) had MRS progression at 7.2 ± 10.1 months (range 1–48 months, median 3 months) after IV anti-VEGF. MRS progression was associated with male sex (odds ratio [OR] 9.000, p = 0.039), and outer retinal schisis at baseline (OR 5.250, p = 0.010). MRS progression was found in the following ratios: 48.3% in the outer retinal layers, 31.7% in both the outer and inner retinal layers, 13.3% presence of LMH, and 6.7% in the inner retinal layers. Of the 79 eyes, 11 required surgical intervention at 11.1 ± 8.6 months; the surgical indications were FTMH (9.1%), MHRD (27.3%), FD (18.2%), LMH (9.1%), and others (36.4%). Five of the 79 eyes (6.3%) had improvement of MRS at 9.6 ± 7.1 months (range 2–19 months, median 12 months) after IV anti-VEGF (Fig. 4). The height or extent of MRS on OCT decreased in four of the five eyes, while complete resolution was achieved in the other one eye. MRS improvement was first detected in the outer retinal layers (60.0%) and in both the outer and inner retinal layers (40.0%). ILM disruption was observed in 3 of the 5 eyes.
Improvement of MRS in the right eye of a 47-year-old woman. a, b Color photos before treatment (a) and 2 years after anti-VEGF treatment (b) showed macular atrophy (category 4 myopic maculopathy). c Before treatment, outer retinal schisis and suspected disruption of ILM (arrowhead) were shown on OCT. BCVA was 20/67. d 1 year after intravitreal bevacizumab and aflibercept, MRS remained stable. e 19 months after IV anti-VEGF treatment, improvement of MRS and suspected disruption of ILM (arrowhead) were observed on OCT. BCVA became 20/25. f 2 years after treatment, near total resolution of MRS and suspected disruption of ILM (arrowhead) were noted on OCT. BCVA was 20/22
In this study, BCVA improved significantly from logMAR 0.785 ± 0.492 to logMAR 0.608 ± 0.505 at 1 month (p < 0.0001), to logMAR 0.568 ± 0.498 at 3 months (p < 0.0001), to logMAR 0.587 ± 0.502 at 6 months (p < 0.0001), to logMAR 0.617 ± 502 at 1 year (p < 0.0001), to logMAR 0.641 ± 0.549 at 2 years (p < 0.0001), and to logMAR 0.693 ± 0.622 at 3 years (p = 0.016) after anti-VEGF treatment.
Discussion
In this retrospective study, vitreoretinal interface changes, including progression and onset of MRS after IV anti-VEGF treatment for eyes with mCNV, were evaluated. Factors contributing to progression of MRS have been reported to be diverse and to include AL, macular chorioretinal atrophy, VMT, and intrinsic noncompliance of the ILM [6, 12, 23, 35, 36]. Whether IV anti-VEGF treatment for mCNV increases the risk of MRS incidence or progression remains controversial. Shimada et al. reported that 5.4% of 74 eyes that had IVB for the treatment of mCNV developed macular retinal detachment following treatment, and four eyes had foveoschisis around the CNV [12]. Huang et al. found progression of MRS in seven of the 11 patients with preexisting ERM and MRS, and no progression of MRS in the six contralateral eyes which had MRS and ERM but were not treated with IV anti-VEGF [21]. Lai et al. reported that two of the 37 eyes had progression of MRS and another one eye had FTMH after IVB or IVR [22]. Ceklic et al. observed new MRS in eight of 474 eyes (1.7%) in the outer retinal layers, and new MRS developed in seven eyes treated with IVR but only in one untreated fellow eye. None of the eight eyes had progression of MRS to a FTMH or FD during the follow-up period of 12 months [20]. Zhou et al. reported ten eyes had MRS progression and two eyes had new-onset MRS in 122 mCNV eyes treated with Conbercept [19].
In our study, we observed 94 of 295 eyes (31.9%) had progression or onset of MRS. Of 216 eyes, 35 (16.2%) without MRS at baseline developed MRS, and 60 of 79 eyes (75.9%) with MRS at baseline had progression of MRS. The rates of progression and incidence of MRS were higher than those previously reported. The reasons for our results might be that we did not include fellow eyes, which usually did not have pathological changes, and that with a better understanding of MRS, we could differentiate MRS from macular edema and recognize it earlier with OCT. In addition, both the average AL and the average duration of follow-up were relatively longer and the average age was older in our study compared to other studies. Comparison of the higher rate of progression of MRS in our study to the 11.6–69.0% reported in studies of natural course of MRS [12, 13] implies that IV anti-VEGF might play a role in changing the vitreoretinal interface. The injection procedure may cause vitreous disturbance and induce posterior vitreous detachment (PVD) [37]. The rapid influx of the drug may lead to vitreous perturbation [19]. The anti-VEGF drugs themselves may enter the space between the vitreous and the retina and mechanically influence the adhesion or traction of the vitreous on the retina. The drug fluid mixed with the vitreous body potentially change the density of vitreous body. Furthermore, shrinkage of the fibrovascular tissue induced by anti-VEGF agents may result in tractional forces and enhance the separation of different retinal layers, which may result in the incidence or progression of MRS. The risk factors for progression and new onset of MRS were outer retinal schisis and LMH at baseline in the multivariate logistic regression model, while the risk factors for progression of MRS were outer schisis and male sex using univariate logistic regression analysis. Outer retinal schisis represents disruption of attachment of outer processes of glial Müller cells from photoreceptors, leading to an unstable structure and an increase in the risk of MRS progression. The result is consistent with that of outer schisis, classified in stage 2 in the staging system for myopic traction maculopathy recently proposed by Parolini et al. [38], and might further progress into macular detachment (stage 3). LMH was found to be another risk factor for progression and onset of MRS in our study, and this echoes the report that LMH with MRS is more susceptible to anatomical progression [39]. In our study, male sex was identified as another risk factor for MRS progression in eyes with mCNV, which is a female-predominant disease [40]. Although no study has yet addressed the gender predilection for MRS progression, several studies have shown that male sex was associated with VMA [41,42,43,44], and Palacio et al. found that males had significantly larger area of VMA. The reasons for this may be related to a lower concentration of sodium hyaluronate in women, leading to earlier PVD [45]. It should be noted that MRS progression was first detected in the outer retinal layers while onset of MRS was more likely to occur in the inner retinal layers, and this again confirms the staging system that stage 1 indicates inner or inner-outer schisis and stage 2 indicates predominantly outer schisis [38].
The mechanisms of MRS improvement are also diverse. Polito et al. reported that a complete PVD occurred before the resolution of macular RD and suggested that the PVD contributed to resolution of MRS [46]. Shimada et al. suggested that rupture of ILM reduces traction and leads to resolution of MRS and that disruption of the ILM may occur when ILM is detached from the underlying retina and is stretched extensively [12]. Lai et al. reported spontaneous resolution of MRS in 8 eyes without evidence of PVD and ILM disruption [47]. In our study, 5 eyes (6.3%) with MRS at baseline had improvements of MRS, and ILM disruption was detected in 3 of the 5 eyes. This is, to the best of our knowledge, the first report of MRS improvement after IV anti-VEGF treatment for mCNV. Likewise, the higher rate of MRS improvement in our study compared to 3.9% reported in the study of natural course of MRS [12] indicates that IV anti-VEGF did play a role in changing the vitreoretinal interface. We suspect that the profibrotic effect of anti-VEGF may either increase tractional force causing the extremely-stretched ILM to break or consolidate the connections among different retinal layers. In either ways, the traction on the retina is released or decreased, leading to improvement of MRS [12].
The baseline prevalence of MRS was 26.8% in this study, which is also higher than the 6–18% reported in studies including fellow eyes without mCNV [20, 48,49,50], but is similar to the prevalence (23.8%) in a study of mCNV [19]. Our data also confirm that MRS was more prevalent in older patients with high myopia [5, 20] and that eyes with MRS at baseline had more staphyloma and greater AL [6, 7]. Patients with MRS at baseline were reported to have received more injections than the other patients without MRS (MRS [n = 15 eyes]: 5.8 ± 2.1 vs. non-MRS [n = 207 eyes]: 4.0 ± 2.9; p = 0.0001) [20]. However, in our study, eyes with MRS at baseline received fewer total injections (p = 0.037), and fewer IVR (p = 0.038) than eyes without MRS at baseline. We attributed the treatment protocol to be the major contributor to this discrepancy. In the RADIANCE trial, two injections of ranibizumab were given in the first month and treatments were continued if there was a loss in visual acuity, intraretinal or subretinal fluid, or active mCNV. In early stages, MRS might be mistaken as intraretinal cysts or fluids, and injections were continually given. In addition, loss in visual acuity might be caused by progression of MRS. However, injections were repeatedly given until stable visual acuity was reached. In addition, the number of cases in our study was higher and we might have much experience of detecting MRS.
In this study, BCVA improved significantly at 1 month, 3 months, 6 months, and yearly until 3 years after treatment. This finding in turn suggests a beneficial effect of anti-VEGF treatment in eyes with mCNV despite the possibility of MRS progression or incidence.
Interestingly, IVR and presence of retinal hemorrhage at baseline were found to be protective factors of surgical intervention for vision-threatening MRS. Although no previous studies on the difference in the risk of MRS among different anti-VEGF agents are available, we suspected that the different fibrotic effects of anti-VEGF agents are associated with different risks of progression to vision-threatening MRS. Gillies et al. reported no significant differences in the rate of development or growth of macular atrophy over 24 months between ranibizumab and aflibercept in patients with neovascular age-related macular degeneration (nAMD) treated using an treat-and-extend regimen [51]. However, some studies have shown that the CNV in ranibizumab-treated nAMD patients demonstrated less fibrosis on OCT [52] and that ranibizumab prevented epidural fibrosis in a postlaminectomy rat model [53]. The result that IVR was a protective factor of vision-threatening MRS requiring surgical intervention in our study supports the notion that ranibizumab might have less fibrotic effect. Further studies with long-term treatment using a single anti-VEGF agent are needed to accurately identify the effect of different agents on the development of vision-threatening MRS. On the other hand, blood components have long been used to improve the hole closure rate for macular hole [54,55,56,57]. Blood contains many growth factors that promote wound healing [58] and cell migration [59]. Therefore, retinal hemorrhage may play a role in cell migration and healing in MRS and may help consolidate the retinal structure. Eyes with onset of MRS had better BCVA at 1 month after IV anti-VEGF treatment, and we suspect that these eyes responded more positively to anti-VEGF agents. Thus, the profibrotic effects of anti-VEGF may be more obvious in these eyes. The stronger the profibrotic effects induced by anti-VEGF agents, the greater the effect of vitreoretinal interface changes developing.
Our study is limited by the retrospective nature of the study and the various treatment regimens of anti-VEGF. The lack of control group is another limitation. The optimal control group (eyes with mCNV without anti-VEGF treatment) was unavailable. Instead, we compared the data in our study to data from other studies on natural course of MRS, which is not an ideal solution. Nevertheless, this is the largest real-world study to evaluate vitreoretinal interface changes after anti-VEGF treatment for mCNV. A number of eyes (16.2%) without MRS at baseline developed new MRS, and 75.9% of eyes with MRS at baseline had progression of MRS. Furthermore, improvement of MRS was first reported after anti-VEGF treatment. Risk factors of progression and onset of MRS were outer retinal schisis and LMH while risk factors for MRS progression were outer schisis and male sex. IVR and presence of retinal hemorrhage were protective factors for vision-threatening MRS requiring surgical intervention. An understanding of the vitreoretinal interface changes after anti-VEGF treatment for eyes with mCNV may help in guiding follow-up schedule, surgical planning, and management.
References
Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018;63:92–106.
Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–611.
Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(9–25):e12.
Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12:127–33.
Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–6.
Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135:338–42.
Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122:1455–60.
Ikuno Y, Sayanagi K, Soga K, Oshima Y, Ohji M, Tano Y. Foveal anatomical status and surgical results in vitrectomy for myopic foveoschisis. Jpn J Ophthalmol. 2008;52:269–76.
Wang SW, Hung KC, Tsai CY, Chen MS, Ho TC. Myopic traction maculopathy biomarkers on optical coherence tomography angiography—an overlooked mechanism of visual acuity correction in myopic eyes. Eye (Lond). 2019;33:1305–13.
Taniuchi S, Hirakata A, Itoh Y, Hirota K, Inoue M. Vitrectomy with or without internal limiting membrane peeling for each stage of myopic traction maculopathy. Retina. 2013;33:2018–25.
Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133:794–800.
Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156(948–957): e941.
Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143:455–62.
Tsai TH, Liu YL, Ma IH, et al. Evolution of the prevalence of myopia among Taiwanese schoolchildren: a review of survey data from 1983 through 2017. Ophthalmology. 2021;128:290–301.
Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
Wong YL, Saw SM. Epidemiology of pathologic myopia in Asia and worldwide. Asia Pac J Ophthalmol (Phila). 2016;5:394–402.
El Matri L, Chebil A, Kort F. Current and emerging treatment options for myopic choroidal neovascularization. Clin Ophthalmol. 2015;9:733–44.
Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121:682–92.
Zhou Y, Yang S, Yuan Y, et al. Progression and new onset of macular retinoschisis in myopic choroidal neovascularization eyes after Conbercept therapy: a post-hoc analysis. Eye (Lond). 2020;34:523–9.
Ceklic L, Munk MR, Wolf-Schnurrbusch U, Gekkieva M, Wolf S. Visual acuity outcomes of ranibizumab treatment in pathologic myopic eyes with macular retinoschisis and choroidal neovascularization. Retina. 2017;37:687–93.
Huang J, Chen T, Lu Y, Long L, Dai H. Retinoschisis and intravitreal ranibizumab treatment for myopic choroidal neovascularization. Chin Med J (Engl). 2014;127:2053–7.
Lai TY, Luk FO, Lee GK, Lam DS. Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond). 2012;26:1004–11.
Fujimoto M, Hangai M, Suda K, Yoshimura N. Features associated with foveal retinal detachment in myopic macular retinoschisis. Am J Ophthalmol. 2010;150:863–70.
Sayanagi K, Ikuno Y, Tano Y. Tractional internal limiting membrane detachment in highly myopic eyes. Am J Ophthalmol. 2006;142:850–2.
Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(877–883): e877.
Wang SW, Hsia Y, Huang CJ, Hung KC, Chen MS, Ho TC. Biomarkers in the pathogenesis of epiretinal membrane and myopic traction maculopathy: effects of internal limiting membrane incompliance and posterior staphyloma. Photodiagnosis Photodyn Ther. 2021;33: 102208.
Chen FT, Yeh PT, Lin CP, Chen MS, Yang CH, Yang CM. Intravitreal gas injection for macular hole with localized retinal detachment in highly myopic patients. Acta Ophthalmol. 2011;89:172–8.
Ma IH, Hsieh YT, Yeh PT, Yang CH, Yang CM. Long-term results and risk factors influencing outcome of gas tamponade for myopic foveoschisis with foveal detachment. Eye (Lond). 2020;34:392–9.
Lin JP, Yang CM. Combined fovea-sparing internal limiting membrane peeling with internal limiting membrane flap technique for progressive myopic traction maculopathy. Graefes Arch Clin Exp Ophthalmol. 2022;260:489–96.
Tsui MC, Yang CM. Early and late macular changes after the inverted internal limiting membrane flap technique for a full-thickness macular hole. Retina. 2021;41:20–8.
Ho TC, Ho AY, Chen MS. Reconstructing foveola by foveolar internal limiting membrane non-peeling and tissue repositioning for lamellar hole-related epiretinal proliferation. Sci Rep. 2019;9:16030.
Ho TC, Chen MS, Huang JS, Shih YF, Ho H, Huang YH. Foveola nonpeeling technique in internal limiting membrane peeling of myopic foveoschisis surgery. Retina. 2012;32:631–4.
Ho TC, Yang CM, Huang JS, Yang CH, Chen MS. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch Clin Exp Ophthalmol. 2014;252:1553–60.
Ho TC, Yang CM, Huang JS, et al. Long-term outcome of foveolar internal limiting membrane nonpeeling for myopic traction maculopathy. Retina. 2014;34:1833–40.
VanderBeek BL, Johnson MW. The diversity of traction mechanisms in myopic traction maculopathy. Am J Ophthalmol. 2012;153:93–102.
Kuhn F. Internal limiting membrane removal for macular detachment in highly myopic eyes. Am J Ophthalmol. 2003;135:547–9.
Veloso CE, Kanadani TM, Pereira FB, Nehemy MB. Vitreomacular interface after anti-vascular endothelial growth factor injections in neovascular age-related macular degeneration. Ophthalmology. 2015;122:1569–72.
Parolini B, Palmieri M, Finzi A, et al. The new myopic traction maculopathy staging system. Eur J Ophthalmol. 2021;31:1299–312.
Hsia Y, Lee CY, Ho TC, Yang CH, Yang CM. The development and evolution of lamellar macular hole in highly myopic eyes. Eye (Lond). 2022. https://doi.org/10.1038/s41433-022-02086-3.
Hsu CR, Lai TT, Hsieh YT, Ho TC, Yang CM, Yang CH. Baseline predictors for good visual gains after anti-vascular endothelial growth factor therapy for myopic choroidal neovascularization. Sci Rep. 2022;12:6800.
Gattoussi S, Cougnard-Gregoire A, Delyfer MN, et al. Vitreomacular adhesion and its association with age-related macular degeneration in a population-based setting: the alienor study. Invest Ophthalmol Vis Sci. 2017;58:2180–6.
Shao L, Xu L, You QS, et al. Prevalence and associations of incomplete posterior vitreous detachment in adult Chinese: the Beijing Eye Study. PLoS ONE. 2013;8: e58498.
Quinn NB, Steel DH, Chakravarthy U, et al. Assessment of the vitreomacular interface using high-resolution OCT in a population-based cohort study of older adults. Ophthalmol Retina. 2020;4:801–13.
Palacio AC, Gupta A, Nesmith BL, Jadav PR, Schaal Y, Schaal S. Vitreomacular adhesion evolution with age in healthy human eyes. Retina. 2017;37:118–23.
Larsson L, Osterlin S. Posterior vitreous detachment. A combined clinical and physiochemical study. Graefes Arch Clin Exp Ophthalmol. 1985;223:92–5.
Polito A, Lanzetta P, Del Borrello M, Bandello F. Spontaneous resolution of a shallow detachment of the macula in a highly myopic eye. Am J Ophthalmol. 2003;135:546–7.
Lai TT, Ho TC, Yang CM. Spontaneous resolution of foveal detachment in traction maculopathy in high myopia unrelated to posterior vitreous detachment. BMC Ophthalmol. 2016;16:18.
Henaine-Berra A, Zand-Hadas IM, Fromow-Guerra J, Garcia-Aguirre G. Prevalence of macular anatomic abnormalities in high myopia. Ophthalmic Surg Lasers Imaging Retina. 2013;44:140–4.
Kamal-Salah R, Morillo-Sanchez MJ, Rius-Diaz F, Garcia-Campos JM. Relationship between paravascular abnormalities and foveoschisis in highly myopic patients. Eye (Lond). 2015;29:280–5.
Fang X, Weng Y, Xu S, et al. Optical coherence tomographic characteristics and surgical outcome of eyes with myopic foveoschisis. Eye (Lond). 2009;23:1336–42.
Gillies MC, Hunyor AP, Arnold JJ, et al. Macular atrophy in neovascular age-related macular degeneration: a randomized clinical trial comparing ranibizumab and aflibercept (RIVAL study). Ophthalmology. 2020;127:198–210.
Kaiser PK, Blodi BA, Shapiro H, Acharya NR, Group MS. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–75.
Yilmaz A, Karatay M, Yildirim T, et al. Prevention of epidural fibrosis using ranibizumab in a postlaminectomy rat model. Turk Neurosurg. 2017;27:119–23.
Korobelnik JF, Hannouche D, Belayachi N, Branger M, Guez JE, Hoang-Xuan T. Autologous platelet concentrate as an adjunct in macular hole healing: a pilot study. Ophthalmology. 1996;103:590–4.
Liggett PE, Skolik DS, Horio B, Saito Y, Alfaro V, Mieler W. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology. 1995;102:1071–6.
Ryan EA, Lee S, Chern S. Use of intravitreal autologous blood to identify posterior cortical vitreous in macular hole surgery. Arch Ophthalmol. 1995;113:822–3.
Lai CC, Chen YP, Wang NK, et al. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122:1889–98.
Ksander GA, Sawamura SJ, Ogawa Y, Sundsmo J, McPherson JM. The effect of platelet releasate on wound healing in animal models. J Am Acad Dermatol. 1990;22:781–91.
Campochiaro PA, Jerdan JA, Glaser BM. Serum contains chemoattractants for human retinal pigment epithelial cells. Arch Ophthalmol. 1984;102:1830–3.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Mei-Chi Tsui wrote the main manuscript text and prepared Figs. 1, 2, 3, and 4. Yi-Ting Hsieh, Tso-Ting Lai, Yun Hsia, Shih-Wen Wang, I-Hsin Ma, Kuo-Chi Hung, Chang-Pin Lin, Chang-Hao Yang, Chung-May Yang, and Tzyy-Chang Ho reviewed the manuscript.
Prior Publication
Neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere.
Disclosures
All authors (Mei-Chi Tsui, Yi-Ting Hsieh, Tso-Ting Lai, Yun Hsia, Shih-Wen Wang, I-Hsin Ma, Kuo-Chi Hung, Chang-Pin Lin, Chang-Hao Yang, Chung-May Yang, and Tzyy-Chang Ho) have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Research Ethics Committee of National Taiwan University Hospital and was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tsui, MC., Hsieh, YT., Lai, TT. et al. Vitreoretinal Interface Changes After Anti-vascular Endothelial Growth Factor Treatment in Highly Myopic Eyes: A Real-World Study. Ophthalmol Ther 12, 1693–1710 (2023). https://doi.org/10.1007/s40123-023-00701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00701-4