Abstract
Introduction
Postoperative endophthalmitis is typically caused by the patient's conjunctival bacterial flora. Povidone iodine solution (5%) is used perioperatively to obtain periocular and ocular antisepsis. However, an adjunctive prophylaxis procedure could further help control the conjunctival microbial load. Considering the increase in antibiotic resistance, a progressive shift toward alternative methods would be desirable. Somilux® eye drops (Alfa Intes, lactoferrin-based eye drops) are medical devices containing liposomal lactoferrin (LF). This study evaluates the effects on conjunctival microflora of LF-based eye drops used in the preoperative phase in patients scheduled for cataract surgery.
Methods
LF-based eye drops or a vehicle solution (water solution) were instilled 4 times a day starting 3 days before cataract surgery. Before the therapy (T0) and at the time of surgery (T1), a conjunctival swab was performed in both eyes and processed to detect microbial growth, microbiological isolation, and species identification. The outcome was the quantification and characterization of the local microbial flora before and after using LF-based or vehicle-based eye drops. Safety of the treatments was also evaluated.
Results
88 eyes of 44 patients (mean [± SD] age 75 [± 12.6] years) were enrolled. At baseline, 54 conjunctival swabs showed only saprophytic flora, 27 showed only potential pathogenic flora, and seven showed both of them. LF-based eye drops reduced the proportion of potentially pathogenic bacteria (36% at T0 vs. 9% at T1, p = 0.008) compared with the vehicle (41% at T0 vs. 55% at T1, p = 0.302) without altering the physiological ocular microbial composition. No adverse events have been reported.
Conclusion
Our findings provide a novel contribution to the scientific knowledge on the role of LF in the ophthalmic field, supporting the use of LF-based eye drops as a safe and selective treatment to improve the ocular surface physiological defenses and control the bacterial ocular surface contamination prior to cataract surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In some circumstances (e.g., the preoperative setting), it is fundamental to improve ocular hygiene and control the microbial load on the ocular surface to reduce the risk of infection. |
Prophylaxis with antibiotics has been widely used, but the increased antibiotic resistance pattern of conjunctival bacteria over the years has led to restrictions on their use. |
A shift to alternative methods, such as the use of natural molecules that are able to stimulate the physiological defense mechanisms, would be desirable. |
What was learned from the study? |
LF-based eye drops provide a reduction in the proportion of potentially pathogenic bacteria compared with the vehicle (water solution) while not altering the physiological ocular microbial composition. |
LF-based eye drops are a safe and selective treatment to improve the ocular surface physiological defenses and control bacterial ocular surface contamination prior to cataract surgery. |
Introduction
In physiological conditions, the ocular surface of the eye presents a significant microbial load, called “ocular microbiota” [1]. This is composed of Gram-positive resident microorganisms (staphylococci, streptococci, corynebacteria, and propionibacteria) and Gram-negative bacteria [2]. The role of the microbiota in the ocular surface is similar to that it plays in other body districts, namely interacting with epithelial and immune cells and the coordination of several functions, such as the preservation of the epithelial barrier, the inhibition of apoptosis and inflammation, the competitive exclusion of potential pathogens, and the maintenance of immune response homeostasis [2, 3].
In some circumstances, such as the preoperative setting, it is fundamental to improve ocular hygiene and control the microbial load on the ocular surface in order to reduce the risk of infection (e.g., endophthalmitis). Indeed, postoperative endophthalmitis is typically caused by the patient's conjunctival bacterial flora [4,5,6]. Povidone iodine solution (5%) is commonly used perioperatively to obtain periocular and ocular antisepsis [5, 7]. However, an adjunctive prophylaxis procedure used in the preoperative days could further help control the conjunctival microbial load [8, 9]. For this task, prophylaxis with antibiotics has been widely used in the past. However, the increased antibiotic resistance pattern of conjunctival bacteria over the years has led to restrictions on their use [4]. The effectiveness of different disinfectant agents as prophylactic treatments was subsequently tested, but very rapid microbicidal activity was reported (which can be explained by their chemical composition), attesting to their aggressiveness towards the ocular microflora equilibrium [10, 11]. Consequently, a progressive shift toward alternative and microbiota-friendly methods for ocular prophylaxis, such as the use of natural molecules that are able to stimulate the physiological defense mechanisms, would be desirable.
Lactoferrin (LF) is an iron-binding multifunctional glycoprotein found in the blood circulation, mucosal surfaces, and various secretory fluids, such as colostrum, milk, tears, nasal and vaginal secretions, pancreatic juice, and saliva [12, 13]. LF is also produced by neutrophils, which are stored in secondary granules to be secreted at the sites of infection and inflammation [13]. LF has numerous well-known biological roles, including regulating iron absorption, the modulation of immune responses, and antimicrobial, antiviral, antioxidant, anticancer, and anti-inflammatory activities [14,15,16]. Moreover, LF is a critical component in mediating the immune response, particularly for the coordinated interactions between innate immunity and adaptive immunity [17, 18].
After colostrum and milk, the highest concentration of LF is found in the eye (about 2 mg/ml in tears), where it plays a key role in defense mechanisms [15, 19]. Of note, there is some evidence to support the therapeutic activity of LF at the ocular level [20]. In particular, besides its well-known antibacterial effect, novel interest has been growing in its potential application in the field of dry eye and infections [20,21,22]. However, a thorough understanding of the mechanisms of its beneficial effects on the ocular surface requires more in-depth research, and further studies are needed to characterize the role of LF in treating ocular pathological conditions or enhancing the ocular physiological defenses [15, 20].
Somilux® eye drops (Alfa Intes, hereafter termed “LF-based eye drops”) are a recently developed medical device containing liposomal LF, whose use is indicated in different eye conditions, including ocular infection, subconjunctival hemorrhage, ocular hyperemia, and dry eye disease. Incorporating LF into liposomes improves the molecule's stability, as its poor aqueous stability and high nasolacrimal duct drainage may hinder its potential efficacy [23].
The present study aimed to evaluate the effects on conjunctival microflora of LF-based eye drops used in the preoperative phase in patients scheduled for cataract surgery.
Methods
Study Design and Setting
This clinical study was conducted with the collaboration of the Mater Domini University Hospital (Catanzaro, Italy), the University Eye Clinic of Genoa, DINOGMI (Genoa, Italy), and the Veneto Eye Bank Foundation (Venice, Italy). Consecutive patients scheduled for senile cataract surgery were screened for enrollment at the Mater Domini University Hospital—Catanzaro. Exclusion criteria were a history of ocular infection in the affected eye in the last 3 months with or without the use of topical antimicrobial agents, the usage of glaucoma medications or other types of eye drops for the control of inflammatory conditions of the eye (including dry eye), diabetes, and autoimmune diseases.
The study complied with the protocol, good clinical practices, the Declaration of Helsinki, and local legal and regulatory requirements. Informed consent was obtained from all participants. The Ethics Committee of the Calabria Region—Center Area Section approved the study on 17 February 2022.
Study Treatment
LF-based eye drops (Somilux®, Alfa Intes, Casoria, Italy) were instilled 4 times a day starting from 3 days before surgery in the eye to be operated on; a vehicle solution (water solution) was used according to the same posology in the contralateral eye. Before starting the prophylactic therapy (T0), and at the time of surgery before 5% povidone iodine solution application (T1), a conjunctival swab was performed in both the eye to be operated on (the treated eye) and the other eye (the control eye). The conjunctival swab was processed for microbial growth detection and subsequent microbiological isolation and identification of the microbial species. The examining centers (Veneto Eye Bank Foundation, Ophthalmology Center of Catania, and Polilab Srl) were unaware of the treatment used by each patient in both eyes. The normal surgical practice was not altered by the implementation of this clinical study, as the extra activity was limited to two tear film samples with a special non-invasive and non-harmful conjunctival swab.
Study Measures and Procedures
The study’s outcome was the quantitative evaluation and characterization of the local microbial flora before and after using LF-based eye drops or vehicle. The safety of treatment with LF-based eye drops was also evaluated.
After collection, the conjunctival swab was mixed with Amies transport medium for 15 s and incubated at 32 °C for 30 min. After incubation, the swab was re-mixed for 15 s, and 500 µL of Amies medium were seeded into an HB&L® culture bottle (Alifax Srl, Italy) under sterile conditions. The culture bottles were then placed in an HB&L® instrument (Alifax Srl, Italy) and incubated for 24 h. The bacterial flora was quantified by evaluating the growth curve based on the initial values of nephelometric readings (CFU/mL) and the corresponding growth curve provided by the HB&L® instrument.
In the case of a positive swab for microbial growth, the sample was inoculated into 1 mL brain heart infusion broth (Biomérieux SA), incubated at 30 °C for 15 min, and then spread on selective media. Tryptic soy agar or Sabouraud dextrose agar plates were used for non-exigent bacteria or fungi, respectively. In contrast, Baird-Parker agar and cetrimide agar plates were used to isolate Staphylococcus spp. and Pseudomonas spp., respectively. Chocolate agar and 5% sheep blood agar plates were used to encourage the growth of exigent bacteria. Plates were incubated at 37 °C for 24–48 h for microorganisms and at 22–25 °C for 5 days for fungi.
Isolated bacteria were identified by MicroScan Specialty ID Panels (Beckman Coulter®) and classified as saprophytic or potentially pathogenic according to Koneman's Color Atlas and Textbook of Diagnostic Microbiology (4th edition) and the Dizionario di batteriologia umana normale e patologica (Dictionary of Normal and Pathological Human Bacteriology) (Table 1).
In vitro susceptibility testing for commercially available topical ophthalmic antibiotics (netilmicin, tobramycin, ofloxacin, levofloxacin, moxifloxacin, chloramphenicol, and azithromycin) plus oxacillin and cefuroxime was performed using the Kirby–Bauer disk diffusion method, following the National Committee for Clinical Laboratory Standard Institute protocols.
Statistical Analysis
Means, medians, and standard deviations (SDs) were calculated for continuous variables, while absolute values and frequencies (percentages) were calculated for categorical variables. The comparison of continuous values between groups was performed using the Mann–Whitney U test, while the comparison of continuous values within the group was performed using the Wilcoxon signed-ranks test. The comparison of proportions within the group was performed using McNemar’s test, and the relationship between treatments and the presence (or absence) of potentially pathogenic bacteria was assessed using the the odds ratio (OR). p < 0.05 was considered statistically significant. A post-hoc power analysis based on McNemar’s test was calculated considering OR = 0.1 (eyes with potentially pathogenic bacteria, treated vs. vehicle), α = 0.05, and the proportion of discordant pairs = 0.3.
Results
Overall, 88 eyes of 44 patients (25 males, 19 females, mean [± SD] age 75 [± 12.6] years) were enrolled in the study. At baseline, 54 conjunctival swabs (61.4% of the total) showed only saprophytic flora, 27 (30.7%) only potential pathogenic flora, and 7 (8.0%) both saprophytic and potentially pathogenic bacteria (mixed flora). The distribution of the detected bacteria in the whole sample is reported in Table 2. The in vitro susceptibility test highlighted the presence of resistance profiles among isolated bacteria toward some commonly used antibiotics. In particular, the reported methicillin resistance was useful for identifying MRSA (detected in 8.0% of conjunctival swabs at the baseline) and MRSE (detected in 6.8% of conjunctival swabs at the baseline) species (Table 2).
Microbial Load
At the baseline visit, the microbial load was equal to 40 × 106 UFC/mL (IQR range: 50 × 106) in both treated and control eyes. At T1, the microbial load was not statistically different from that at T0 in both groups. In detail, the microbial load was equal to 15 × 106 UFC/mL (IQR range: 50 × 106) in the treated eyes (p = 0.279) and to 17.5 × 106 UFC/mL (IQR range: 40 × 106) in the control eyes (p = 0.253). The list of bacteria detected at T1 according to the treatment is reported in Table 2.
Characterization of the Ocular Microbiota
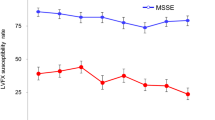
A statistically significant reduction in the proportion of potentially pathogenic bacteria was reported after the treatment with LF-based eye drops compared with the baseline evaluation (36% at T0 vs. 9% at T1, p = 0.008). Conversely, this difference was not statistically significant after the treatment with the vehicle (41% at T0 vs. 55% at T1, p = 0.302) (Fig. 1). The post-hoc power analysis based on McNemar’s test indicated a power of 87%.
Combining the presence or absence of potentially pathogenic strains at T0 and T1 (Table 3), it was observed that eyes treated with LF-based eye drops had a significantly higher chance of reducing the presence of potentially pathogenic strains (OR: 4.0 [1.3–12.4]; p = 0.015), a significantly lower risk of increasing the presence of potentially pathogenic strains (OR: 0.2 [0.06–0.98], p = 0.046), a significantly higher chance of maintaining saprophytic flora (OR: 5.2 [1.2–21.9]; p = 0.024), and a significantly higher chance of turning back to saprophytic flora (OR: 39.0 [4.0–378.2]; p = 0.002) than eyes treated with the vehicle (Fig. 2).
Odds ratio for change in bacterial flora over time. In the treatment group, eyes had a better chance of reducing the presence of potentially pathogenic strains (improve; odds ratio, OR: 4.0 [1.3–12.4]), a lower risk of increasing the presence of potentially pathogenic strains (not getting worse; OR: 0.2 [0.06–0.98]), and a better chance of keeping saprophytic flora (OR: 5.2 [1.2–21.9]) and turning back to saprophytic flora (OR: 39.0 [4.0–378.2])
LF-based eye drops had the ability to modulate the potentially pathogenic strains and favor the saprophytic flora affected by both Gram-positive (25% at T0 vs. 7% at T1, p = 0.039) and Gram-negative (14% at T0 vs. 0% at T1; p = 0.039) bacteria (Table 4).
Safety
All patients used the study treatment and the vehicle as per our instructions. No adverse events were reported during the study period for either group.
Discussion
Even though ocular surgery is performed with sterile instruments and under aseptic conditions, postoperative endophthalmitis represents an unavoidable risk [24]. It is widely known that the patient's ocular flora is the main source of the microbes responsible for most cases of intraocular infection [4,5,6]. Therefore, reducing the microbes on the ocular surface and eliminating potential pathogenic flora in the preoperative phase are useful strategies to decrease the risk of endophthalmitis [4]. For this purpose, prophylaxis with various classes of topical antibiotics has been widely used in recent decades [25]. However, several studies have evaluated the antibiotic resistance pattern of conjunctival bacterial flora in patients undergoing cataract surgery. These studies suggest that an increase in bacterial resistance has been registered due to the inappropriate and widespread use of antibiotics and argue for the need for alternative prophylaxis methods [4, 26,27,28].
In recent years, a progressive shift toward different types of disinfectants that are able to reduce, without selectivity, the microbial load of the conjunctiva has been taking place [29,30,31].
In the present study, using LF-based eye drops for 4 days significantly reduced the proportion of potentially pathogenic bacteria compared with the vehicle without altering the physiological ocular microbial composition. In addition, our findings suggest a higher chance of reducing the presence of potentially pathogenic bacterial strains, a lower risk of increasing the presence of potentially pathogenic bacterial strains, and a higher chance of maintaining or turning back to saprophytic flora during the treatment with LF-based eye drops than with the vehicle. According to this activity, unlike conventional disinfectants, the LF-based eye drops did not produce profound changes in the saprophytic flora (e.g., some coagulase-negative Staphylococci spp.) that is known to have a physiological role in the regulation of the immune homeostasis of the ocular surface system. At the same time, in our cohort, the susceptibility test reported the presence of MRSA and MRSE species in 8.0% and 6.8% of conjunctival swabs at the baseline, respectively, which were not retrieved after the LF-based treatment. These pathogens have been frequently associated with ocular infections; thus, controlling them represents an important therapeutic objective, as these species are often associated with high resistance to other antibiotic classes [32, 33].
No adverse events were reported during the study, in agreement with the safety results reported in preclinical studies [23].
The observed protective effect of LF can be explained by the peculiar activity of this molecule in preventing inflammation and acting against microbial infections [17]. LF directly affects pathogens through iron sequestration and its ability to bind lipopolysaccharide and block interactions with receptors used for entry into host cells [34]. Furthermore, it has been reported that LF plays a critical mediating role in the interactions between innate and adaptive immunity [17, 18]. Indeed, LF is a pleiotropic molecule that can directly contribute to the influence of presenting cells for the development of T-helper cell polarization [17, 35, 36]. The discovery of LF receptors on a wide variety of immune cells and their demonstrated capability to bind LF further support the potential of this molecule to modulate and influence both innate and adaptive immune system responses [37, 38].
The protective effects of LF have been described in different human districts thanks to its presence in various fluids. Human colostrum, a key player in the bacterial colonization of the neonatal gut, contains the highest LF concentrations [39]. Several studies have shown the usefulness of LF supplementation in children, particularly preterm infants, in reducing the incidence of late-onset sepsis and necrotizing enterocolitis by strengthening the intestinal epithelial barrier, promoting antagonism toward enteropathogens, and balancing anti- and pro-inflammatory responses [40,41,42]. LF also showed anti-inflammatory, immunomodulatory, and antimicrobial activities relevant to maintaining gut microbiota–host mutualism [43, 44].
In addition, it has been reported that salivary LF can counteract oral pathogen growth, including that of Streptococcus spp., Candida albicans, and other anaerobic periodontopathic bacteria (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia) residing in the biofilm of supragingival and subgingival plaque, respectively [45, 46]. Interestingly, LF also inhibits the biofilm formation of these bacteria at physiological concentrations [16].
Protective activities of LF have also been reported during vaginal dysbiosis, characterized by low amounts of vaginal lactobacilli and increased levels of endogenous anaerobic bacteria [47]. In this condition, increased levels of LF have been reported and related to an immune-modulator action of LF, similar to the role normally played by healthy microbiota in vaginal mucosa [47, 48].
These mechanisms could explain the control activity of LF against potentially pathogenic microorganisms observed in our study, thus providing preliminary evidence of the natural protective and immunomodulatory activity of LF in the ocular district as well.
Obtained results support the use of LF-based eye drops as a safe and specific treatment to prepare the ocular surface for surgery through the improvement of the ocular physiological defenses and the control of bacterial contamination. Moreover, our findings suggest that the LF-based eye drops can be useful for restoring the homeostasis of the ocular surface during the postoperative course, or in the case of recurrent/chronic infections (e.g., blepharitis, blepharoconjunctivitis). In addition, the LF-based eye drops can be used after standard medical therapy for the management of postoperative ocular conditions characterized by inflammation and/or an altered microbial profile (e.g., cataract surgery). This hypothesis is supported by previous studies on cell culture or preclinical assays in which topical eye drops of LF-loaded biodegradable nanoparticles were effective in treating various conditions characterized by ocular inflammatory processes [23]. However, any difference in the reconstitution of the bacterial flora in the postoperative phase should be assessed in the future after treatment with LF-based eye drops to further characterize its activity.
The present study suffers from some limitations that deserve mentioning and are related to the relatively small sample size and the non-randomized design. In addition, the clinical relevance of this treatment to the endophthalmitis rate, as well as to other parameters related to an altered ocular microbial profile, was not addressed and will represent the subject of future large studies. However, our findings suggest that LF-based eye drops can be useful to stimulate the ocular physiological defenses and modulate the microbial equilibrium toward the saprophytic component. This action is carried out without altering the biodiversity of the ocular microbiome, whose equilibrium is fundamental to immunomodulatory function and the maintenance of relationships with other microbes and organs, and LF-based eye drops thus represent a natural, safe, and selective prophylactic treatment [3].
Conclusions
LF is an ingredient in many supplements and medicines, but a thorough understanding of the mechanisms for its beneficial effects requires further in-depth research, especially in the ocular tissues. Our findings provide a novel contribution to the knowledge on the role of LF in the ophthalmic field, supporting the use of LF-based eye drops as a safe and specific treatment to improve the ocular surface physiological defenses and control bacterial ocular surface contamination before cataract surgery. More in-depth studies focusing on LF-induced changes in the ocular microbiome may further confirm these observations and extend the application of this protocol from the prevention of postsurgical eye infections to the management of postsurgical ocular conditions characterized by an altered microbial community profile.
References
Li JJ, Yi S, Wei L. Ocular microbiota and intraocular inflammation. Front Immunol. 2020;11:609765. https://doi.org/10.3389/fimmu.2020.609765.
Aragona P, Baudouin C, Benitez Del Castillo JM, et al. The ocular microbiome and microbiota affect ocular surface pathophysiology and disorders. Surv Ophthalmol. 2021;66(6):907–25. https://doi.org/10.1016/j.survophthal.2021.03.010.
Gomes JÁP, Frizon L, Demeda VF. Ocular surface microbiome in health and disease. Asia Pac J Ophthalmol (Phila). 2020;9(6):505–11. https://doi.org/10.1097/APO.0000000000000330.
Simina DS, Larisa I, Otilia C, et al. The ocular surface bacterial contamination and its management in the prophylaxis of post cataract surgery endophthalmitis. Rom J Ophthalmol. 2021;65(1):2–9. https://doi.org/10.22336/rjo.2021.2.
Shimada H, Nakashizuka H. Cataract surgery by intraoperative surface irrigation with 0.25% povidone-iodine. J Clin Med. 2021;10(16):3611. https://doi.org/10.3390/jcm10163611.
American Academy of Ophthalmology. Cataract in the adult eye; preferred practice pattern. San Francisco, CA: American Academy of Ophthalmology; 2011. http://www.aao.org/asset.axd?idZ8d66318f-ff50-408e-9bb1-73d277cf14ce. Accessed 7 Oct 2022.
Shimada H, Nakashizuka H, Grzybowski A. Prevention and treatment of postoperative endophthalmitis using povidone-iodine. Curr Pharm Des. 2017;23(4):574–85. https://doi.org/10.2174/1381612822666161205105404.
Halachmi-Eyal O, Lang Y, Keness Y, et al. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg. 2009;35(12):2109–14. https://doi.org/10.1016/j.jcrs.2009.06.038.
Grzybowski A, Schwartz SG, Matsuura K, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns around the world. Curr Pharm Des. 2017;23(4):565–73. https://doi.org/10.2174/1381612822666161216122230.
Barkana Y, Almer Z, Segal O, et al. Reduction of conjunctival bacterial flora by povidone-iodine, ofloxacin and chlorhexidine in an outpatient setting. Acta Ophthalmol Scand. 2005;83(3):360–3. https://doi.org/10.1111/j.1600-0420.2005.00414.x.
Tognetto D, Pastore MR, Guerin GM, et al. Bactericidal activity of three different antiseptic ophthalmic preparations as surgical prophylaxis. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):289–93. https://doi.org/10.1007/s00417-021-05361-3.
Albar AH, Almehdar HA, Uversky VN, et al. Structural heterogeneity and multifunctionality of lactoferrin. Curr Protein Pept Sci. 2014;15(8):778–97. https://doi.org/10.2174/1389203715666140919124530.
Rascón-Cruz Q, Espinoza-Sánchez EA, Siqueiros-Cendón TS, et al. Lactoferrin: a glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules. 2021;26(1):205. https://doi.org/10.3390/molecules26010205.
Hao L, Shan Q, Wei J, et al. Lactoferrin: major physiological functions and applications. Curr Protein Pept Sci. 2019;20(2):139–44. https://doi.org/10.2174/1389203719666180514150921.
Kowalczyk P, Kaczyńska K, Kleczkowska P, et al. The lactoferrin phenomenon—a miracle molecule. Molecules. 2022;27(9):2941. https://doi.org/10.3390/molecules27092941.
Rosa L, Cutone A, Lepanto MS, et al. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci. 2017;18(9):1985. https://doi.org/10.3390/ijms18091985.
Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15(17):1956–73. https://doi.org/10.2174/138161209788453202.
de la Rosa G, Yang D, Tewary P, et al. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180(10):6868–76. https://doi.org/10.4049/jimmunol.180.10.6868.
Kijlstra A, Jeurissen SH, Koning KM. Lactoferrin levels in normal human tears. Br J Ophthalmol. 1983;67(3):199–202. https://doi.org/10.1136/bjo.67.3.199.
Vagge A, Senni C, Bernabei F, et al. Therapeutic effects of lactoferrin in ocular diseases: from dry eye disease to infections. Int J Mol Sci. 2020;21(18):6668. https://doi.org/10.3390/ijms21186668.
Dogru M, Matsumoto Y, Yamamoto Y, et al. Lactoferrin in Sjögren’s syndrome. Ophthalmology. 2007;114(12):2366–7. https://doi.org/10.1016/j.ophtha.2007.06.027.
Devendra J, Singh S. Effect of oral lactoferrin on cataract surgery induced dry eye: a randomised controlled trial. J Clin Diagn Res. 2015;9(10):NC06–9. https://doi.org/10.7860/JCDR/2015/15797.6670.
López-Machado A, Díaz-Garrido N, Cano A, et al. Development of lactoferrin-loaded liposomes for the management of dry eye disease and ocular inflammation. Pharmaceutics. 2021;13(10):1698. https://doi.org/10.3390/pharmaceutics13101698.
Sun J, Guo Z, Li H, et al. Acute infectious endophthalmitis after cataract surgery: epidemiological characteristics, risk factors and incidence trends, 2008–2019. Infect Drug Resist. 2021;14:1231–8. https://doi.org/10.2147/IDR.S304675.
Gower EW, Lindsley K, Tulenko SE, et al. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2017;2(2):CD006364. https://doi.org/10.1002/14651858.CD006364.pub3.
Keshav BR, Basu S. Normal conjunctival flora and their antibiotic sensitivity in Omanis undergoing cataract surgery. Oman J Ophthalmol. 2012;5(1):16–8.
Fernández-Rubio E, Urcelay JL, Cuesta-Rodriguez T. The antibiotic resistance pattern of conjunctival bacteria: a key for designing a cataract surgery prophylaxis. Eye (Lond). 2009;23(6):1321–8.
Arantes TEF, Cavalcanti RF, Diniz MFA, et al. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Arq Bras Oftalmol. 2006;69(1):33–6.
Gili NJ, Noren T, Törnquist E, et al. Preoperative preparation of eye with chlorhexidine solution significantly reduces bacterial load prior to 23-gauge vitrectomy in Swedish health care. BMC Ophthalmol. 2018;18(1):167. https://doi.org/10.1186/s12886-018-0844-9.
Vagge A, Ferro Desideri L, Carnevali A, et al. Efficacy of a new commercial ocular spray containing oftasecur citrus extract for reducing microbial load in the conjunctiva of patients receiving intravitreal injections. Ophthalmol Ther. 2021;10(4):1025–32. https://doi.org/10.1007/s40123-021-00384-9.
Barkana Y, Dorairaj S, Tham R, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis (Ophthalmology 2014;121:2081–90). Ophthalmology. 2015;122(7):e40–1. https://doi.org/10.1016/j.ophtha.2014.11.030.
Deguchi H, Kitazawa K, Kayukawa K, et al. The trend of resistance to antibiotics for ocular infection of Staphylococcus aureus, coagulase-negative staphylococci, and Corynebacterium compared with 10-years previous: a retrospective observational study. PLoS ONE. 2018;13(9):e0203705. https://doi.org/10.1371/journal.pone.0203705.
Fukuda M, Ohashi H, Matsumoto C, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus ocular surface infection efficacy of chloramphenicol eye drops. Cornea. 2002;21(7 Suppl):S86–9. https://doi.org/10.1097/01.ico.0000263125.99262.42.
Redwan EM, Uversky VN, El-Fakharany EM, et al. Potential lactoferrin activity against pathogenic viruses. C R Biol. 2014;337(10):581–95. https://doi.org/10.1016/j.crvi.2014.08.003.
Puddu P, Valenti P, Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91(1):11–8. https://doi.org/10.1016/j.biochi.2008.05.005.
Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62(22):2540–8. https://doi.org/10.1007/s00018-005-5369-8.
Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62:2560–75.
González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301.e1-8. https://doi.org/10.1016/j.ijantimicag.2008.07.020.
Toscano M, De Grandi R, Grossi E, Drago L. Role of the human breast milk-associated microbiota on the newborns’ immune system: a mini review. Front Microbiol. 2017;25(8):2100. https://doi.org/10.3389/fmicb.2017.02100.
Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–8. https://doi.org/10.1001/jama.2009.1403.
Ochoa TJ, Zegarra J, Cam L, et al. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr Infect Dis J. 2015;34(6):571–6. https://doi.org/10.1097/INF.0000000000000593.
Meyer MP, Alexander T. Reduction in necrotizing enterocolitis and improved outcomes in preterm infants following routine supplementation with Lactobacillus GG in combination with bovine lactoferrin. J Neonatal Perinatal Med. 2017;10(3):249–55. https://doi.org/10.3233/NPM-16130.
Mastromarino P, Capobianco D, Campagna G, et al. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals. 2014;27(5):1077–86. https://doi.org/10.1007/s10534-014-9762-3.
Giansanti F, Panella G, Leboffe L, et al. Lactoferrin from milk: nutraceutical and pharmacological properties. Pharmaceuticals (Basel). 2016;9(4):61. https://doi.org/10.3390/ph9040061.
Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–5. https://doi.org/10.1038/417552a.
Wakabayashi H, Yamauchi K, Kobayashi T, Yaeshima T, Iwatsuki K, Yoshie H. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob Agents Chemother. 2009;53(8):3308–16. https://doi.org/10.1128/AAC.01688-08.
Valenti P, Rosa L, Capobianco D, et al. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front Immunol. 2018;9:376. https://doi.org/10.3389/fimmu.2018.00376.
Spear GT, Kendrick SR, Chen HY, et al. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ß and lactoferrin. PLoS ONE. 2011;6(5):e19560. https://doi.org/10.1371/journal.pone.0019560.
Acknowledgements
The authors would like to thank Dr. Anna Rita Blanco (Medical Liaison, Alfa Intes) for scientific support during the study.
Funding
No funding or sponsorship was received for this study or the publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing and Editorial Assistance
Editorial, graphical, and statistical assistance were provided by Simonetta Papa, PhD, Valentina Mirisola, PhD, Massimiliano Pianta, Valentina Attanasio, and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by Alfa Intes.
Author Contributions
Study design: GG, VS, AV, NPL, CDN, CET; data collection and interpretation: all; manuscript writing: GG, AV; manuscript editing: all; approval to submit: all.
Disclosures
Giuseppe Giannaccare, Sofia Comis, Virginia Jannuzzi, Davide Camposampiero, Diego Ponzin, Sergio Cambria, Marcello Santocono, Nicola Pallozzi Lavorante, Chiara Del Noce, Vincenzo Scorcia, Carlo E. Traverso, and Aldo Vagge have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted within the protocol approved by the Ethics Committee of the Calabria Region (Sezione Area Centro) on February 17, 2022. It adhered to the principles of the Declaration of Helsinki. All the participants signed an informed consent form.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Giannaccare, G., Comis, S., Jannuzzi, V. et al. Effect of Liposomal-Lactoferrin-Based Eye Drops on the Conjunctival Microflora of Patients Undergoing Cataract Surgery. Ophthalmol Ther 12, 1315–1326 (2023). https://doi.org/10.1007/s40123-023-00673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00673-5