Abstract
Introduction
To compare the effect of three different anti-inflammatory regimens consisting of preservative-free dexamethasone (DEX), diclofenac (DICLO) eye drops, and their combination (DEX + DICLO) following trabeculectomy on early postoperative inflammation.
Methods
A prospective randomized controlled trial. Sixty-nine patients undergoing trabeculectomy were randomized to receive either postoperative treatment with topical DEX (n = 23), topical DICLO (n = 23), or a combination of topical DEX and topical DICLO (n = 23) after trabeculectomy. The primary outcome was the anterior chamber flare measurement in the first 3 months postoperatively. Secondary outcomes included intraocular pressure, central corneal thickness, conjunctival injection, and number of cells in the anterior chamber from baseline to 3 months postoperatively.
Results
Anterior chamber flare reached a maximum 1 day after trabeculectomy with an increase of 55% (95% CI 37–73%) for DEX, 64% (95% CI 47–82%) for DICLO, and 57% (95% CI 39–75%) for DEX + DICLO and returned to near pre-operative values 6 weeks after surgery. There were no significant differences in anterior chamber flare [effect size for DICLO: 0.16 (95% CI − 4.3 to 4.6), effect size for DEX + DICLO: 0.09 (95% CI − 4.1 to 4.3)], intraocular pressure, central corneal thickness, conjunctival injection, or number of cells in the anterior chamber between DEX, DICLO, or DEX + DICLO groups.
Conclusion
We found that topical diclofenac was not statistically different from topical dexamethasone in controlling early postoperative inflammation after trabeculectomy, while combining diclofenac and dexamethasone offered no added anti-inflammatory control compared to dexamethasone alone.
Trial Registration
www.clinicaltrials.gov (NCT04054830).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Surgical success of a trabeculectomy depends upon controlling post-operative inflammation to ensure functional drainage of the aqueous from the anterior chamber. |
The purpose of this blinded, randomized study is to investigate which anti-inflammatory prophylaxis provides better control of inflammation following a trabeculectomy by comparing preservative-free dexamethasone (DEX), diclofenac (DICLO) eye drops, and their combination (DEX + DICLO). |
What was learned from the study? |
Topical diclofenac was not statistically different from topical dexamethasone in controlling early postoperative inflammation after trabeculectomy, while combining diclofenac and dexamethasone offered no added anti-inflammatory control compared to dexamethasone alone. |
Our results suggest that topical diclofenac can be considered a usable alternative to dexamethasone in postoperative treatment after trabeculectomy, but studies with longer follow-up time are required. |
Introduction
Glaucoma is the most frequent cause of irreversible blindness globally [1]. It is an optic neuropathy characterized by progressive visual field loss. Elevated intraocular pressure (IOP) is an important risk factor for the development of glaucoma, and is currently the only risk factor that is amenable to therapeutic interventions [1,2,3,4]. The golden standard for lowering intraocular pressure and preserving visual function in medically uncontrolled glaucoma is filtration surgery [5, 6] with trabeculectomy as the most common surgical approach. During this procedure, a channel is created between the anterior chamber of the eye and the subconjunctival space, allowing drainage of aqueous from the anterior chamber directly into the subconjunctival space, thereby reducing IOP [7, 8]. Ensuring the appropriate filtration rate is a delicate balance between hyper- and hypofiltration, where one of the regulating factors is the amount of inflammation ultimately leading to tissue fibrosis [9].
It can be challenging to maintain an ideal long-term IOP postoperatively. The early postoperative period is the most critical phase, and topical anti-inflammatory prophylaxis is used postoperatively for many weeks to prevent imminent failure at this stage [10]. A combination of broad-spectrum antibiotics to prevent infection and topical steroids are often used to reduce postoperative inflammation and fibrosis of the filtering bleb [11, 12]. However, treatment with steroids includes an increased risk of development or progression of cataract, and subsequent cataract surgery may increase the fibrosis of the filtering bleb and ultimately lead to failure of the trabeculectomy [13]. Additionally, steroids may increase the intraocular pressure in some patients during the postoperative treatment (steroid-response) [14], eventually leading to a revision of the trabeculectomy due to a suspicion that the trabeculectomy has failed. Non-steroidal anti-inflammatory drugs (NSAIDs) are attractive alternatives as they have not been associated with cataract formation nor with IOP increase. Previous studies have shown that NSAIDs are superior to topical steroids in controlling inflammation after cataract surgery [15, 16], but their role in trabeculectomy is largely uninvestigated [17, 18].
In this randomized controlled clinical trial, we sought to determine the effects on early postoperative inflammation after trabeculectomy of different anti-inflammatory prophylactic treatments, including topical preservative-free dexamethasone and preservative-free diclofenac, and a combination of these regimens.
Methods
Study Design
The study was a prospective, randomized controlled clinical trial at the Department of Ophthalmology at Rigshospitalet-Glostrup, Denmark. The study was conducted in accordance with Good Clinical Practice guidelines and adhered to the tenets of the Declaration of Helsinki. Prior to initiation, the study was registered at the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT, 2018-001855-10) and www.clinicaltrials.gov (NCT04054830). Approval was obtained from the Danish Medicines Agency (Journal no.: 2018082465), the Danish Committee on Health Research Ethics (Journal nr.: H-18056701), and The Danish Data Protection Agency (VD-2018-477, I-Suite nr.: 6736). All participants provided written informed consent and received no incentives or compensation for participation in the trial. The Consolidated Standards of Reporting Trials (CONSORT) were followed for all reporting aspects. The sample size was set based on patients undergoing trabeculectomy at our department. Using a sampling of 1.1:1, a power of 0.8, and a type 1 error of 0.05, a minimum of 16 participants was required in each group. We aimed for 24 participants in each group to compensate for possible dropouts.
Interventions
Participants were randomized to one of three interventional groups comparing anti-inflammatory regimens after trabeculectomy. The control group received preservative-free dexamethasone (Menopause 1 mg/ml, Théa) (DEX). The comparison groups received preservative-free diclofenac (Voltaren Ophtha 1 mg/ml; GSK Consumer Healthcare) (DICLO) or a combination of preservative-free dexamethasone (Menopause 1 mg/ml; Thea) and preservative-free diclofenac (Voltage Ophthal. 1 mg/ml; GSK Consumer Healthcare) (DEX + DICLO). A topical antibiotic (Chloramphenicol 5 mg/ml) was prescribed to be used 4 times daily for the first week. Anti-inflammatory treatment was planned to last a minimum of 9 weeks, and the eye drops were used 6 times daily for the first 2 weeks, tapering to 4 drops per day for the next 4 weeks. Thus, a total of 12 drops were used daily in the DEX + DICLO group to start with. Depending on the clinical condition of the eye, the topical anti-inflammatory treatment was reduced by 1 daily drop per week after 6 weeks. If topical treatment had to be extended beyond 15 weeks after filtration surgery, all participants were switched to preservative-free topical dexamethasone.
Study Participants
Participants were recruited among patients referred to the Department of Ophthalmology, Rigshospitalet-Glostrup, for surgery because of medically uncontrolled glaucoma from August 1, 2019, to July 11, 2021. Inclusion criteria were primary open-angle glaucoma, pseudoexfoliation glaucoma, pigment dispersion glaucoma, or ocular hypertension. Participants had to be older than 50 years and women postmenopausal. Lastly, the participants needed to comply with study procedures and provide informed consent to participation. Exclusion criteria were previous intraocular surgery except for cataract surgery more than 6 months prior, medical history of anterior segment dysgenesis, inflammatory/uveitic glaucoma, angle closure glaucoma, neovascular glaucoma, or traumatic glaucoma. In addition, patients who were known to be steroid responders, or who received systemic treatment with steroids or NSAIDs, or had an allergy to any of the contents of the pharmaceuticals used in the study, were excluded.
Randomization and Blinding
Participants were randomized by a computerized algorithm 1:1:1 ratio to each of the three interventional groups using the randomization instrument in Research Electronic Data Capture (REDCap) hosted at Capital Region, Denmark [19, 20]. Before study initiation, a block-randomized list was created using https://www.sealedenvelope.com/simple-randomiser/v1/lists by an independent researcher and uploaded to REDCap. The list length was 123, with block sizes 6 and 9 in random order. Only one eye could be included for each participant. A computerized algorithm would decide which eye to include in the study if both eyes were eligible. Participants could not be blinded to whether they received monotherapy or both types of topical medication. The primary outcome assessors were blinded to the randomization status. All statistical calculations were performed in a blinded manner.
Surgical Technique
All surgeries were performed by experienced glaucoma surgeons, defined as having performed more than 200 trabeculectomies. Preoperatively, the eye was instilled with oxybuprocaine 0.4%, pilocarpine 2%, and iopidine 1%, and disinfected with povidone iodine 1% and chlorhexidine ethanol 0.5%, and covered in sterile drapings. One operation was performed in general anesthesia, and all other surgical procedures were performed using peribulbar anesthesia with an equal mixture of bupivacaine 5 mg/ml and lidocaine 20 mg/ml, or topical anesthesia with oxybuprocaine 0.4% and subconjunctivally instilled lidocaine 10 mg/ml, according to the surgeon´s preference. The surgeon chose the optimal location for the filtration area and placed a corneal traction suture. A conjunctival incision was made to expose the sclera and a limbus-based scleral flap, 3 × 4 mm and 2/3 of scleral thickness. Antimetabolite MMC 0.2 mg/ml was applied with soaked sponges under the conjunctiva for 3 min. Two sutures were placed in the corners of the scleral flap. Then, a sclerostomy was performed, and a block of cornea and sclera at the level of the trabecular meshwork was removed, followed by a peripheral iridectomy. The flap was sutured to restrict outflow, and the conjunctiva was closed, with watertight 10–0 ethilon being used for both procedures. The surgeon concluded by applying 1 mL cefuroxime 2.5 mg/ml into the anterior chamber and injecting 0.5 mL of 4 mg/ml dexamethasone 180° from the trabeculectomy subconjunctivally.
Follow-Up Examinations
Participants were examined preoperatively and at 1 day, 1, 2, 3, 4, and 6 weeks, and 3 months postoperatively. The examinations included manifest refraction, visual acuity using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, conjunctival injection objectively graded from 0 to 5 with a score ≥ 3 defined as significant injection, slit-lamp biomicroscopy, ophthalmoscopy, grading of the number of cells in the anterior chamber using Standardization of Uveitis Nomenclature (SUN) [21], and IOP was measured by Goldmann applanation tonometry. Two measurements were taken and averaged to determine the mean IOP if the two values were within 2 mmHg. A third measurement was taken if the first two deviated by more than 2 mmHg, in which case the median value was used. Anterior chamber flare was measured by a flare photometer (FM-600; KOWA) on undilated pupils using an average of five reliable measurements. Central corneal thickness (CCT) was measured by anterior segment optical coherence tomography (Tomey CASIA-II). Additional anti-inflammatory treatment was initiated if the participant presented with signs of uncontrolled inflammation. Eye-related adverse events were grouped into 9 groups (hypotony, elevated IOP, bleb leak, slit lamp-/surgical intervention, corneal edema, dryness, corneal abrasion, corneal dellen, and hyphema). Hypotony was noted as an adverse reaction if treatment with atropine was initiated. Elevated IOP was managed with pressure lowering eyedrops, needling, or revision. The participants underwent Seidel testing on every visit, regardless of IOP or bleb appearance. If the leak was managed with a scleral lens, it was defined as slit-lamp intervention, and it was classified as surgical intervention if it required re-suturing.
Outcomes
The primary outcome measure was the anterior chamber flare change from baseline to 3 months postoperatively. Secondary outcome measures were IOP, CCT, conjunctival injection, SUN grading, and adverse events from baseline to 3 months postoperatively.
Statistical Analysis
Statistical analyses were performed using the statistical software R, v.1.2, in RStudio, v. 1.1.456, and the LMMstar package ® Program for Statistical Computing) [22]. Flare measures were log2-transformed to achieve normally distributed data. Data for the transformed flare measures, IOP, and the CCT data were confirmed to be normally distributed at all time points by the Shapiro–Wilk test and by visual inspection of QQ-plots.
A constrained linear mixed model with inherent baseline adjustment was applied to the log2-transformed flare values, IOP, and the central corneal thickness data to make pairwise comparisons between the three treatment groups for all time values. The model included time and the treatment–time interaction as fixed effects, with an unstructured covariance pattern to account for correlation between repeated measurements and possible changes in variance over time. The same covariance parameters were assumed for all groups. Missing data were handled by maximum likelihood estimation in the linear mixed model, which yields unbiased estimates of the time and treatment effects in the case of missing data being missing at random. Sensitivity analysis was performed using a best-case/worst-case approach by substituting 10th and 90th percentiles of the observed data for the missing data.
The parameters ‘conjunctival injection’ and ‘SUN grading’ were analyzed using descriptive statistics, including an illustration of the development of the parameter values over time displayed in a histogram, and the mean and CI of the data shown in a table. Comparisons between the groups for flare and CCT were made using Welch's t test.
Changes in conjunctival injection was evaluated from baseline to 3 months postoperatively by comparing the prevalence of significant injection (score ≥ 3). The statistical tests used were Wilcoxon's signed-rank test for ordinal data.
All p values were adjusted for multiple testing the false discovery rate (FDR) using the Benjamini–Hochberg procedure, which controls the false discovery rate (FDR). An adjusted p value < 0.05 was considered statistically significant.
Results
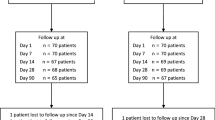
Seventy-two participants scheduled for trabeculectomy were enrolled between August 2019 and June 2021. Two participants had complications during surgery, and one participant withdrew consent after randomization (Fig. 1). This left a total of 69 eyes [30 women (43%); 39 men (57%)] to be included in the statistical analyses. The mean age was 71 (SD 9.0) years. Baseline characteristics are presented in Table 1.
Anterior Chamber Inflammation
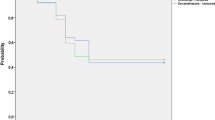
Anterior chamber flare reached a maximum 1 day after trabeculectomy. From a baseline median of 11.2 ph/ms (95% CI 10.1–12.4 ph/ms), anterior chamber flare increased by 55% (95% CI 37–73%) for DEX, 64% (95% CI 47–82%) for DICLO, and 57% (95% CI 39–75%) for DEX + DICLO. At 1 week after surgery, anterior chamber flare had decreased to 23% (95% CI 8–38%) over the baseline median for DEX, 33% (95% CI 18–48%) for DICLO, and 30% (95% CI 14–45%) for DEX + DICLO. At week 6, anterior chamber flare had nearly normalized to the baseline median plus 1% (95% CI – 11 to 12%) for DEX, 11% (95% CI 1–22%) for DICLO, and 1% (95% CI – 11 to 11%) for DEX + DICLO. Three months after surgery, anterior chamber flare had decreased to 12% (95% CI 6–23%) over the baseline median for DEX, 9% (95% CI – 2 to 20%) for DICLO, and by 7% (95% CI – 5 to 18%) for DEX + DICLO. There were no significant differences in flare at any time point between the three interventional groups (Table 2; Fig. 2).
Intraocular Pressure
The IOP was at its lowest on the first postoperative day. The pressure decreased from a baseline mean of 19.2 mmHg (95% CI 17.8–20.6 mmHg) to 6.5 mmHg (95% CI 4.5–8.5 mmHg) for DEX, 4.4 mmHg (95% CI 2.8–6.0 mmHg) for DICLO, and 4.8 mmHg (95% CI 3.2–6.3 mmHg) for DEX + DICLO. At the 3-months postoperative follow-up, the IOP for DEX had increased to 9.0 mmHg (95% CI 7.5–10.5 mmHg), 9.4 mmHg (95% CI 7.5–11.4 mmHg) for DICLO, and 11.1 mmHg (95% CI 9.10–13.0 mmHg) for DEX + DICLO. There were no significant differences in IOP between the three interventional groups at any time point (Table 2; Fig. 3).
Central Corneal Thickness
The central corneal thickness was measured at baseline, 1 week, 6 weeks, and 3 months postoperatively, see Table 2 and Fig. 4. At one week after surgery, mean central corneal thickness had decreased by 1 µm (95% CI, − 11 to 9 µm) below baseline for DEX, increased by 6 µm (95% CI − 4 to 17 µm) for DICLO, and increased by 1 µm (95% CI – 10 to 11 µm) for DEX + DICLO. At week 6, mean central corneal thickness had decreased by 7 µm (95% CI – 16 to 2 µm) below baseline for DEX, increased by 1 µm (95% CI – 8 to 11 µm) for DICLO, and decreased by 8 µm (95% CI − 17 to 2 µm) for DEX + DICLO. Three months postoperatively, mean central corneal thickness had been reduced by 5 µm (95% CI – 13 to 4 µm) below baseline for DEX, decreased by 1 µm (95% CI – 10 to 8 µm) for DICLO, and decreased by 8 µm (95% CI – 17 to 1 µm) for DEX + DICLO. No statistically significant differences between the groups were found at any time points (Table 2 and Fig. 4).
Conjunctival Injection and SUN Grading
Conjunctival injection peaked on the first postoperative day for all three groups, followed by a gradual decrease until 3 months after surgery. At baseline, 23 (35%) of participants had a significant conjunctival injection score of three or higher compared to 9 (13%) after 3 months (p < 0.001) (see Fig. 5 for an illustration of the development over time).
Conjunctival injection distribution in each group for baseline, week 1, week 6, and month 3 postoperatively; Color grading increases from 0 = white to 5 = black in increments of 1, shades of gray indicate varying degrees of conjunctival injection between the extremes, with darker shades indicating higher levels of conjunctival injection. The evaluation was unavailable for one patient at 6 weeks and two patients at 3 months postoperatively due to exclusion from the study
The SUN grading of cells in the anterior chamber peaked at day 1 postoperatively for all three groups followed by a gradual decrease over time and returned to baseline values 6 weeks after surgery (Table 3).
Adverse Events and Additional Treatment
Eye-related adverse events were registered in 43 participants (62.3%) within the first 3 months after trabeculectomy (Table 4). Each participant could experience more than one adverse event. The most common adverse event was bleb leak managed by slit lamp intervention followed by hypotony. No patient required additional anti-inflammatory treatment during the first 3 months after trabeculectomy. There were no significant differences in adverse events between the three interventional groups.
Discussion
This randomized, controlled trial aimed to investigate which regime after trabeculectomy provides the best anti-inflammatory effect in the first 3 months after surgery, determined by anterior chamber flare, IOP, central corneal thickness, conjunctival injection, and SUN grading of cells in the anterior chamber. Patients were randomized to receive either topical dexamethasone (DEX), topical diclofenac (DICLO), or a combination of the two (DEX + DICLO). We did not find any statistically significant difference between the treatment groups. We observed that DICLO eye drop therapy is non-inferior to DEX eye drop therapy in controlling early postoperative inflammation after trabeculectomy. A combination of DEX and DICLO showed no added value compared to DEX monotherapy. Additionally, none of the patients needed additional therapy to control inflammation in the first 3 months after trabeculectomy.
To the best of our knowledge, no studies have compared DICLO and DEX in controlling early postoperative inflammation after trabeculectomy. However, a recent meta-analysis [18] compiled the results of several randomized controlled trials, including participants undergoing trabeculectomy or phacotrabeculectomy. They evaluated postoperative IOP, visual acuity, visual fields, number of antiglaucomatous medications, and complete/qualified success. For patients undergoing trabeculectomy, NSAID eyedrops were non-inferior when used on their own or combined with topical steroids concerning outcome and risks. Unfortunately, the authors found insufficient evidence to recommend one anti-inflammatory modality over the other. Kent et al. found that, following trabeculectomy, similar IOP may be expected when using either prednisolone or DICLO [23]. The beneficial effects of preoperative treatment with either topical NSAID, steroid, or artificial tears following trabeculectomy have been investigated by Breusegem et al. [24]. They concluded that both NSAID and steroid lead to a reduced need for needling after trabeculectomy, and that the steroid group required less supplemental postoperative IOP-lowering medication compared to NSAID and artificial tears.
The efficacy of DICLO and other NSAIDs in controlling early postoperative inflammation is well established in cataract surgery. Regarding flare, Erichsen et al. [25] compared the efficacy and safety of five different anti-inflammatory regimens on early postoperative inflammation after cataract surgery. They reported no difference between NSAID and steroid eye drops. Similarly, Ylien et al. [26] investigated topical DICLO against DEX eyedrops and their combination and found no significant difference in flare measurements between the groups. The same conclusion applies to Laurell et al. [27], who compared DICLO, DEX, and placebo. Miyake et al. [28] found that anterior chamber flare was significantly less in the NSAID group than in the steroid group. In addition to the above, several meta-analyses have been in favor of NSAID eye drops in managing postoperative inflammation after cataract surgery [15, 29,30,31].
The efficacy of NSAIDs compared to steroids in glaucoma procedures has been investigated with different results. A study of patients with primary angle closure glaucoma demonstrated that NSAID eye drop therapy was non-inferior to steroid eye drop therapy in controlling inflammation after laser peripheral iridotomy [32]. In a selective laser trabeculoplasty trial [33], similar results were found, with no significant difference in inflammatory scores between the groups.
Our study showed that all three anti-inflammatory treatments effectively suppressed the inflammatory response induced by the surgical procedure. We observed that 35% of our study participants had a conjunctival injection score of 3 or higher preoperatively. This number was reduced to 13% 3 months after surgery (p < 0.001). This may be explained by a preoperative use of pressure-lowering eye drops, as they have been reported to have a detrimental effect on the conjunctiva [34]. Conjunctival hyperemia has epidemiologically been reported to have a prevalence of 38% in patients using antiglaucomatous eye drops [34].
Inflammation and wound healing are complex processes. Steroid is an effective anti-inflammatory drug with beneficial effects in the postoperative period after trabeculectomy, but may cause elevated IOP, cataract development, or increase the risk of infections [35, 36]. Complications associated with topical treatment with NSAID are mostly discomfort when the drop is applied, redness, blurred vision just after installation, and corneal melts, which have been reported in rare cases [37]. Long-term use of topical NSAIDs, like any other medicine, exposes patients to the risk of side effects. NSAID-induced corneal melt (NICM) is a serious adverse event typically occurring in patients with compromised cornea due to ocular surgery, diabetes, or systemic immune diseases [38]. These patients often have an existing epithelial defect. Following surgery, topical NSAID may act as a catalyst transforming the epithelial defect into a melt [38]. NICM is still debated, and has been variously reported in the literature. Even though it has been observed in some case reports [37, 38], no incidences of NICM were detected in two randomized controlled trials [39, 40], and a retrospective phacoemulsification study of over 4700 eyes investigating the safety and effectiveness of NSAID eye drops [41]. Corneal melts associated with NSAID are allegedly extremely rare following cataract surgery, but, due to the more prolonged treatment after trabeculectomies, the risk may be higher. No cases of corneal thinning were seen, and nor did we note significant differences in adverse events in any of the treatment arms in our study. Additionally, 3 months postoperatively, all groups had a non-significant decrease in CCT. The reduction was unaffected by the choice of anti-inflammatory prophylaxis after surgery [42], which is consistent with a phacotrabeculectomy trial in which postoperative NSAIDs were administered for 9 weeks [43]. Contrary to earlier reports [37, 38], we found that topical DICLO did not cause corneal thinning, demonstrating that concern for postoperative NICM should not be used as an argument for not prescribing it postoperatively; nevertheless, it is important to be aware that this can occur [44].
The main strengths of our trial include its randomized design and large sample size. The study could not be fully masked due to one group receiving two types of eyedrops. Instead, all statistical analyses were performed in a blinded manner. Estimating postsurgical inflammation with laser flare photometry is an established method, but may have limitations as measurements may be affected by postsurgical corneal edema, small pupils, and protein composition in the anterior chamber [45].
Conclusions
Our results have shown no statistical difference between postoperative treatment with diclofenac eye drops in trabeculectomies compared with conventional dexamethasone drops. Topical diclofenac and dexamethasone effectively control early postoperative inflammation, and may both be considered for glaucoma patients undergoing trabeculectomy. NSAIDs are not in routine use after trabeculectomy, and their effect in preventing fibrosis of the bleb has not been entirely investigated [46]. However, our results and the mentioned studies suggest that diclofenac can be considered a usable alternative to dexamethasone in postoperative treatment after trabeculectomy, but studies with longer follow-up times are required.
References
Bourne RRA, Steinmetz JD, Saylan M, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the Global Burden of Disease Study. Lancet Glob Heal. 2021;9:e144–60.
Tham Y-CC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Heal Organ. 2004;82:887–8.
Friedman DSS, Wolfs RC, O’Colmain BJ, et al. Prevalence of Open-Angle Glaucoma Among Adults in the United States. Arch Ophthalmol. 2004;122:532.
Lim R. The surgical management of glaucoma: a review. Clin Exp Ophthalmol. 2022;50:213–31.
Cordeiro MF, Siriwardena D, Chang L, et al. Wound healing modulation after glaucoma surgery. Curr Opin Ophthalmol. 2000;11:121–6.
Masoumpour MB, Nowroozzadeh MH, Razeghinejad MR. Current and future techniques in wound healing modulation after glaucoma filtering surgeries. Open Ophthalmol J. 2016;10:68–85.
Schwartz K, Budenz D. Current management of glaucoma. Curr Opin Ophthalmol. 2004;15:119–26.
Vote B, Fuller JR, Bevin TH, et al. Systemic anti-inflammatory fibrosis suppression in threatened trabeculectomy failure. Clin Exp Ophthalmol. 2004;32:81–6.
Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–46.
Bettin P. Postoperative management of penetrating and nonpenetrating external filtering procedures. Dev Ophthalmol. 2012;50:48–63.
Panarelli JF, Nayak NV, Sidoti PA. Postoperative management of trabeculectomy and glaucoma drainage implant surgery. Curr Opin Ophthalmol. 2016;27:170–6.
Dada T, Bhartiya S, Baig NB. Cataract surgery in eyes with previous glaucoma surgery: pearls and pitfalls. J Curr Glaucoma Pract. 2013;7:99.
Bartlett JD, Woolley TW, Adams CM. Identification of high intraocular pressure responders to topical ophthalmic corticosteroids. J Ocul Pharmacol. 1993;9:35–45.
Kessel L, Tendal B, Jørgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops a systematic review. Ophthalmology. 2014;121:1915–24.
Wielders LHPP, Schouten JJSAG, Nuijts RMMAR. Prevention of macular edema after cataract surgery. Curr Opin Ophthalmol. 2018;29:48–53.
Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery. Surv Ophthalmol. 2012;57:530–50.
Almatlou AEA, Almatlouh A, Bach-Holm D, et al. Steroids and non-steroidal anti-inflammatory drugs in the postoperative regime after trabeculectomy: which provides the better outcome? A systematic review and meta-analysis. Acta Ophthalmol. 2019;97:146–57.
Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377.
Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform. 2019;95: 103208.
Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16.
Ozenne B, Forman J. LMMstar: Repeated measurement models for discrete times. R package version 0.3.2. 2022.
Kent AR, Dubiner HB, Whitaker R, et al. The efficacy and safety of diclofenac 0.1% versus prednisolone acetate 1% following trabeculectomy with adjunctive mitomycin-C. Ophthalmic Surg Lasers. 1998;29:562–9.
Breusegem C, Spielberg L, Van GR, et al. Preoperative nonsteroidal anti-inflammatory drug or steroid and outcomes after trabeculectomy: a randomized controlled trial. Ophthalmology. 2010;117:1324–30.
Erichsen JH, Forman JL, Holm LM, et al. Effect of anti-inflammatory regimen on early postoperative inflammation after cataract surgery. J Cataract Refract Surg. 2021;47:323–30.
Ylinen P, Holmström E, Laine I, et al. Anti-inflammatory medication following cataract surgery: a randomized trial between preservative-free dexamethasone, diclofenac and their combination. Acta Ophthalmol. 2018;96:486–93.
Laurell CG, Zetterström C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol. 2002;86:1380.
Miyake K, Ota I, Miyake G, et al. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1581–8.
Wielders LHP, Lambermont VA, Schouten JSAG. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol Case Reports. 2015;160:968–81.
Lim BX, Lim CHL, Lim DK, et al. Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD006683.PUB3.
Juthani VV, Clearfield ECR. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation aer uncomplicated cataract surgery (review) non-steroidal anti-inflammatory drugs versus corticosteroids for. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD010516.pub2.
Gayam K, Ramulu PY, Rengaraj V, et al. Safety and efficacy of 0.1% nepafenac versus 1% prednisolone acetate eye drops after laser peripheral iridotomy: a prospective, randomized trial. Ophthalmol Glaucoma. 2020;3:174–80.
Groth SL, Albeiruti E, Nunez M, et al. Steroids after laser trabeculoplasty (SALT) trial: impact of short-term anti-inflammatory treatment on SLT efficacy. Ophthalmology. 2019;126:1511.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418.
Starita RJ, Fellman RL, Spaeth GL, et al. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938–46.
Araujo SV, Spaeth GL, Roth SM, et al. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102:1753–9.
Flach AJ. Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc. 2001;99:202–5.
Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: Clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol. 2020;65:1–11.
Flach AJ, Dolan BJ, Donahue ME, et al. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology. 1998;105:1775–9.
Solomon KD, Cheetham JK, DeGryse R, et al. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology. 2001;108:331–7.
Hoffman RS, Braga-Mele R, Donaldson K, et al. Cataract surgery and nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2016;42:1368.
Kamal Junejo M, Mahar Pak PJ. Change in central corneal thickness after trabeculectomy. Pak J Ophthalmol Orig Artic Ophthalmol. 2022;33:213.
Levkovitch-Verbin H, Katz G, Kalev-Landoi M, et al. Postoperative treatment with topical diclofenac versus topical dexamethasone after combined phacotrabeculectomy with mitomycin C. J Glaucoma. 2013;22:177–82.
Topical NSAIDs: Best Practices for Safe Use-American Academy of Ophthalmology. 2022. https://www.aao.org/eyenet/article/topical-nsaids-best-practices-safe-use. Accessed 29 Nov 2022.
Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. 2005;50:27–47.
Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–33.
Acknowledgements
The authors thank all the participants for participating in this clinical trial, and Julie L. Forman, MSc, PhD at the Section of Biostatistics, University of Copenhagen, for her assistance in the statistical analysis and evaluation performed in this study.
Funding
This research was funded by Fight for Sight Denmark, The Danish Eye Research Foundation, Synoptik Foundation, Gangsted Fond, Fabrikant Einar Willumsens Fond, Aase og Ejnar Danielsens Fond and by the Henry og Astrid Møllers Fond. Funding organizations had no role in the design or conduct of this research. No funding or sponsorship was received for the publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: Afrouz Ahmadzadeh, Daniella Bach-Holm and Line Kessel. Acquisition or interpretation of data: All authors. Statistical analysis: Afrouz Ahmadzadeh and Bo Simmendefeldt Schmidt. Writing - original draft preparation: Afrouz Ahmadzadeh. Writing - critical revision and editing: All authors. Funding acquisition: Afrouz Ahmadzadeh, Daniella Bach-Holm and Line Kessel.
Disclosures
Afrouz Ahmadzadeh, Bo Simmendefeldt Schmidt, Daniella Bach-Holm and Line Kessel declare that they have no competing interests.
Compliance with Ethics Guidelines
The study was approved by the Committee on Health Research Ethics (Journal nr.: H-18056701), the Danish Medicines Agency (Journal nr.: 2018082465), and The Danish Data Protection Agency (VD- 2018-477, I-Suite nr.: 6736). The study was registered in the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT, 2018-001855-10) and at www.clinicaltrials.gov (NCT04054830) before initiation. The study was conducted following the tenets of the Declaration of Helsinki of 1964 and its later amendments. Written informed consent was obtained from participants before the screening visit.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ahmadzadeh, A., Schmidt, B.S., Bach-Holm, D. et al. Early Inflammation Control After Trabeculectomy by Steroid and Non-steroidal Eye Drops: A Randomized Controlled Trial. Ophthalmol Ther 12, 969–984 (2023). https://doi.org/10.1007/s40123-022-00636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00636-2