Abstract
Introduction
Visual impairment resulting from diseases such as neovascular age-related macular degeneration (nAMD) may cause behavioural, environmental, psychological, and logistical challenges that could act as barriers to effective uptake and sustainability of treatment with anti-vascular endothelial growth factor agents (anti-VEGFs). Understanding emotions and experiences of patients with nAMD may help inform the determinants of adherence, and could contribute to improvements in ophthalmic outcomes and quality of life.
Methods
Seventeen patients with nAMD receiving anti-VEGF injections were enrolled from three clinics: one each in France (n = 5), Germany (n = 6), and the UK (n = 6). Patients’ health information and treatment characteristics were collected. Individual phone interviews were conducted by experienced health care interviewers. Transcripts were analysed thematically.
Results
Patients (53% female) had a mean age of 77 years. Bilateral anti-VEGF injections were received by 24% (n = 4); and most (76%, n = 13) were adherent to their treatment. Patient emotions at diagnosis ranged from happiness at learning about the treatment for nAMD to being terrified of receiving an injection in the eye. Most patients mentioned feeling anxious and fearful before their first injection despite receiving reassurance. After the first injection, these feelings and apprehension abated for many, but not all. With the goal of maintaining the best possible vision, few (24%, n = 4) patients reported more than one missed appointment, and most had never considered stopping treatment. No patient reported additional assistance beyond family support; however, many had difficulties with recreational and domestic activities and had developed coping strategies.
Conclusion
This study provides insights on patients’ emotions related to their experience of nAMD and its management, highlighting the varying experiences between individuals. It shows the importance of the patient’s voice when considering patient care and management, and how the nature and timing of interventions can improve the experience of living with and managing nAMD.
Plain Language Summary
Neovascular age-related macular degeneration (nAMD), also known as wet age-related macular degeneration (wAMD), is an eye condition that is a common cause of vision loss and worsens over time without treatment. This condition mainly occurs in people aged 70 years or older. The standard of care is an injection of anti-vascular endothelial growth factor (anti-VEGF) into the eye to minimise vision loss that continues over time without treatment. To maximise the benefits of treatment, injections are required at regular intervals over time. The purpose of this study was to understand the emotions and experiences of patients with nAMD about their disease, its consequences, and its management. Seventeen patients from three countries (France, Germany, and the UK) were interviewed over the telephone. Patients reported diverse feelings and responses to their disease and treatment. Many felt nervous and anxious at diagnosis and before their first injection (despite reassurances from their doctors); however, after the first injection, these feelings and apprehension abated for many, but not all. Most patients (76%) missed fewer than two appointments in the past year, and almost all (82%) did not consider stopping treatment. Patients learned to deal with their nAMD, but many had difficulties with daily activities. Patients developed ways to manage tasks such as cooking, cleaning, knitting, and driving. The insights from this study help understand how care for patients with nAMD can be improved by addressing patients’ concerns and feelings about their disease and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although neovascular age-related macular degeneration (nAMD) affects approximately 15 million patients in Europe, few studies have evaluated patients’ views and experiences of living with the disease, which are closely associated with patient well-being and adherence to treatment. |
To gain a deeper understanding of the emotions and experiences related to several aspects of the disease, this study used semi-structured interviews with patients receiving anti-vascular endothelial growth factor (anti-VEGF) treatment for nAMD from three clinics in Europe. |
What was learned from the study? |
Patients frequently mentioned feeling anxious and frightened prior to receiving their first injection, despite receiving reassurance from their doctors. These feelings abated for many, but not all, patients following further treatment; however, some patients were worried about having different doctors every appointment, and therefore different people performing the intraocular injections. Most patients who participated in the study maintained their appointments and did not consider stopping treatment. |
Patients had diverse feelings and responses to the disease, indicating the need for a personalised approach to patient care. Patients learnt to deal with the consequences of nAMD and attributed difficulties with recreational and domestic activities to be typical of their age rather than disease state. |
This study highlights the importance of the patient’s voice when considering patient care and management, and provides insights into the determinants of adherence, and the nature and timing of interventions that may be utilised to improve the experience of living with, and managing, nAMD. |
Introduction
The gold standard treatment for neovascular age-related macular degeneration (nAMD) is intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections [1,2,3]. Treatment outcomes in routine clinical practice do not match those seen in clinical trials [1, 4], at least partly because patients in routine clinical practice receive fewer anti-VEGF injections compared with those enrolled in clinical trials [5,6,7,8]. Visual impairment can cause behavioural, environmental, psychological, and logistical barriers [9, 10] which may impact adherence to, and persistence with, treatment [11, 12], in turn contributing to poor visual outcomes, resulting in a continuation of the cycle. For example, at a more severe level, clinical depression is strongly associated with nAMD [9, 13], as well as poor adherence to treatment, lower physical activity, and poor diet [14]. Additionally, dissatisfaction with nAMD treatment or care is associated with a greater likelihood of non-adherence to therapy [11, 15, 16] and poorer outcomes. Therefore, there is an inextricable link between the broader patients’ experiences and their adherence to nAMD treatment and its outcomes.
By taking a comprehensive approach to patient care for nAMD, including considering the challenges and emotions of patients at every stage of their journey, we hypothesise that the treatment experience, and therefore visual outcomes and patient’s quality of life, could be optimised. Such an explorative approach (capturing the broadest range of responses) would be best through qualitative methodology. Despite being uncommon in ophthalmology [17], qualitative methodology allows for thorough evaluation and interpretation of important open-ended data, where patients can present their thoughts and opinions freely with minimal structuring and without loss of the nuances of expression.
The aim of this multicentre study conducted in three European countries was to gain insights into the emotions and experiences of patients with nAMD with regards to treatment, to understand the determinants of adherence, and to identify where meaningful interventions to support improved outcomes can be developed.
Methods
Study Design, HCPs, and Patient Recruitment
The study was conducted at the Departments of Ophthalmology at the Universitäts-Augenklinik in Bonn, Germany; the Centre Hospitalier Universitaire de Montpellier in Montpellier, France; and the Royal Victoria Hospital in Newcastle upon Tyne, UK, between 26 October 2020 and 29 March 2021, and followed ethical principles laid out in the Declaration of Helsinki and applicable local legislation on non-interventional studies. No treatment decisions were impacted by inclusion in the study; prescription of medicines was clearly separated from study inclusion. Health care professionals (HCPs) responsible for recruiting patients were retinal specialists with at least 5 years’ experience, who had at least 100 patients with nAMD within their clinic and regularly initiated anti-VEGF treatments and personally managed patients. HCPs identified patients with diverse treatment durations and personal circumstances. Recruited patients had been diagnosed with nAMD and had received anti-VEGF treatment. Ethical approval or waivers (as locally appropriate) were obtained at all centres and all participants gave informed consent.
Survey and Questionnaire Completion
HCPs gathered data through an online survey on each patient, including patient characteristics, disease pattern, treatment history and medical care, visits and injections, anti-VEGF treatment behaviour and treatment adherence, reasons for initiating anti-VEGF treatment, information on treatment regimens, comorbidities, and current health status. Patients completed a paper-based National Eye Institute Visual Function Questionnaire (NEI VFQ-25; measures vision-related quality of life elements that are most important for people with chronic eye disease) [18] and the Patient-reported Health Status Scale (reflective of the patient’s perception of their overall health).
Semi-structured Patient Phone Interviews
The in-depth, semi-structured patient interviews were conducted by experienced health care interviewers fluent in the patient’s native language, employed by Kantar/Cerner Enviza (Munich, Germany). Interviewers followed an interview guideline, and asked patients to expand upon their responses where appropriate. Interviews were up to 75 min in duration, and included questions about the practical and emotional effects of nAMD on the person’s life, their knowledge and understanding of nAMD, their treatment expectations, the relationship with their clinician/hospital staff, and the organisation of the clinic that they attend. Depending on the responses, follow-up questions were asked to encourage them to expand on their experiences. Interview responses from France and Germany were translated into English by an experienced medical translator for analysis.
Data Analysis
This qualitative study is exploratory in nature and HCP survey and patient questionnaire data were analysed descriptively. The sample size was considered sufficient to address the study objectives based on prior qualitative research in ophthalmology [19,20,21]. Patient interview transcripts were analysed according to the guidance of the International Society for Pharmacoeconomics and Outcomes Research [22], and using qualitative thematic analysis methodology [23], modified where appropriate to align with research in ophthalmology [20]. A grounded framework approach was taken, identifying and applying the thematic framework throughout the transcripts and collating, mapping, and interpreting the data. The thematic analysis was performed by a qualitative analysis assessor, with a second assessor reviewing more than 20% of the analysed transcripts to validate the methodology and ensure accuracy and consistency.
Definitions of Adherence and Persistence
The definitions of non-adherence used in this study align with the definitions published by Okada et al. [12] (Supplementary Table 1). Non-adherence was defined as missing two or more treatment or monitoring appointments over a period of 1 year, with an appointment considered missed if exceeding more than 2 weeks from the recommended date [12]. Patients who missed no appointments over the course of a year were considered fully adherent, and patients who missed one appointment were considered adherent [12].
Results
Patient Demographics and Disease Characteristics
Table 1 depicts the demographics and disease characteristics of the 17 patients enrolled in this study. Most patients (76%, n = 13) were adherent or fully adherent by the pre-specified definitions [12]. Most patients received unilateral injections (76%, n = 13); four (24%) received bilateral injections, and for two, their eyes were on different treatment schedules, and thus received treatment for each eye on different days. Patients reported receiving varying numbers of injections prior to their interview (ranging from 3 injections to 22 injections). Patients had similar health scores (HCP assessment of patient health status score, patient-reported health status score, NEI VFQ-25); however, one had a very low health score (0). Most (71%, n = 12) patients reported comorbidities (e.g. diabetes or high blood pressure). All patients were living at home at the time of their interview (rather than in residential care), and only 29% (n = 5) reported that they required caregiver support with daily activities at home.
Emotions and Experiences
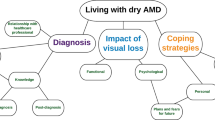
From Visual Concerns to Diagnosis and Initial Treatment
Many patients reported initially suspecting an issue with their vision other than nAMD. After scheduling a health care appointment, in general, the patient’s ophthalmologist diagnosed nAMD. One patient with a background in the medical field initially self-diagnosed nAMD while another suspected the disease, based on their prior knowledge of symptoms (Table 2). This patient had their appointments delayed multiple times, and eventually called the hospital who referred them for an ophthalmologist appointment.
Patients often received information leaflets about nAMD from their diagnosing HCP; one patient received a compact disc with additional information. However, many reported not fully understanding the disease at diagnosis. As reported, there was a reluctance to ask for further information at this stage, despite some patients feeling underinformed (Table 2). Approximately half of the respondents conducted their own research to understand nAMD and its treatment. There were other patients who had some knowledge of the disease from personal experience, including a family history, and from a neighbour. One patient reported feeling depressed when researching nAMD on the internet.
When learning about anti-VEGF treatment, emotions ranged from happiness that there was a treatment to feelings of anxiety, apprehension, and terror at having an injection into the eye. Most patients reported no immediate discussion of treatment options, outcomes, or long-term treatment plans. Initial expectations of treatment outcomes varied across the respondents, with most either expecting or hoping their vision would improve or stay the same while others were sceptical or had no expectations.
Before receiving their first injection, many patients reported feeling anxious or concerned despite receiving reassurances from their doctor. Some patients discussed their concerns with their family, but, notwithstanding these feelings, many felt that the treatment was necessary.
Example quotes from patients regarding the peri-diagnostic period are included in Table 2.
Treatment
Some patients reported discomfort during anti-VEGF injections, and some reported side effects (e.g. difficulties with vision or pain), which lasted longer than expected. After experiencing their first injection, anxiety and apprehension abated for many patients but not all. Some patients likened routine anti-VEGF treatment to a necessary activity, such as visiting the shops or the dentist (Table 3). However, one patient stopped after their third injection—they had already received two injections and reported no pain; however, after experiencing pain, bruising, and discomfort with their third injection, they developed a fear of treatment.
Patients were generally not aware of the treatment regimen they were receiving, but would often remark that this was not important, because they were happy to follow their HCP’s advice. Patients frequently asked to see, or were shown, optical coherence tomography scans, but many did not understand what they meant.
Generally, patients were comfortable to ask their HCP questions during appointments; however, only if they felt their HCPs had time. Some patients noted that HCPs would only offer information on request. Many patients reported a good relationship with their HCP, but concerns were raised that patients felt they did not see the primary doctor treating their nAMD frequently enough, or saw other doctors instead at some appointments. Indeed, some patients described seeing a different doctor every time. This scenario led to the feeling of being anonymous.
Most patients (88%, n = 15) were satisfied with their treatment, and understood that stability of their nAMD was a measure of treatment success. They generally reported expecting their vision to remain the same following treatment, commensurate with their current good quality of life. However, some patients felt the need for life-long injections had not been discussed at time of diagnosis (Table 3). After receiving several injections, many patients began to understand that treatment for nAMD would likely continue for the rest of their lives.
Example quotes from patients regarding treatment are included in Table 3.
Clinical Management of nAMD
Waiting times varied by clinic; one patient would request the first appointment of the day where the reported time in the injection room was short. Patients frequently reported not knowing if the next visit would be for monitoring or an injection.
Although changes in hospital set-up owing to COVID-19, or to hospital building work, were mentioned as a source of frustration, the reduction in numbers of patients in waiting rooms was noted as pleasant. Patients generally understood the requirement to keep appointments, but expressed frustration that they had to plan their lives around visits. One expressed irritation at not being able to schedule appointments in advance, and another found it difficult to arrange appointments, having to go through multiple secretaries (Table 4).
Missed appointments were infrequent. Two patients reported missing appointments owing to hospital clerical errors and both appointments were rescheduled. Three patients missed appointments because of prioritising treatment for other conditions (achilles tendonitis, pneumonia, and a suspected stroke). The two patients in the study receiving bilateral treatment shared their frustration that both eyes were treated on different schedules.
Most patients (82%, n = 14) did not consider stopping treatment; however, scenarios where patients would consider stopping treatment included feeling that treatment had no effect, the treatment side effects outweighed the benefits, reimbursement changes, difficulties in going to and from appointments, and a recommendation from their HCP (Table 4).
Example quotes from patients regarding clinical management of nAMD are included in Table 4.
The Impact of nAMD
Patients reported difficulties with driving a car, driving at night, cooking, cleaning, detailed work requiring optimal vision, and hobbies such as knitting; however, they perceived their inability to undertake these daily activities to be typical of someone of a similar age. Transport to and from the clinic was most frequently mentioned as a required source of support; however, many patients did not explicitly mention needing professional assistance in their home. Support at home was primarily provided by spouses and other family members. Patients had diverse coping strategies to help manage their nAMD, ranging from those implemented immediately following an injection (the wearing of sunglasses or dimming of lights, a spouse driving the patient home after their appointment) to long-term changes, such as requesting larger font for bills/post and visual-aid devices to help around the house (Table 5). One patient had a neighbour with nAMD, and they supported each other. Few patients used formal patient support organisations (18%, n = 3). Three patients expressed no interest, and one suggested they might be negatively impacted by listening to other patients with nAMD discuss their difficulties (Table 5). Patients were generally conscious of their overall health and reported taking steps (such as taking supplements or changing their diet) to improve their nAMD.
Patients who were able to drive valued the independence and control this gave them, despite some reporting difficulties (e.g. with driving at night). Other patients were reliant on friends or family, or hospital-organised transport for assistance in travel to and from hospital. Parking was repeatedly a major concern, regardless of whether the driver was the patient or the accompanied person. Often, the patient would leave the car to attend the appointment while the driver would find parking.
Patients from clinics in France and Germany were generally aware of the cost of the treatment and valued the reimbursement schemes. Some reported that they would not be able to pay for their treatment without such schemes. One patient received invoices for small (usually less than €10) amounts following treatment and expressed irritation at not being able to pay multiple invoices in advance. Patients from the UK clinic had their treatment costs managed by the National Health Service.
Example quotes from patients regarding the impact of nAMD are included in Table 5.
Discussion
This multicentre study sought to gain a deeper understanding of the experiences and emotions of patients with nAMD. Patient responses varied; most indicated nAMD and its treatment impact their daily lives, despite many patients becoming accustomed to regular visits and anti-VEGF injections. While patients generally had appropriate expectations of treatment and its outcomes, there were information gaps and concerns regarding the patient–HCP relationship, which suggest the need for a personalised approach to care. Our overall findings must be seen in the context of the heterogeneity of the population of people with nAMD, the social determinants of health, and the unique health systems in France, Germany, and the UK. These factors, together with the areas for improvement raised by this research, highlight the importance of the patient voice when considering the care of patients with nAMD.
Few previous studies have investigated patients’ experiences and emotions regarding their nAMD. Existing studies either tend to focus on experiences of the injection procedure itself [24], systematically utilise structured questionnaires and surveys with binary or simple response input [25], or aggregate current literature [26,27,28]. Such approaches provide important data; however, they may not comprehensively assess all emotional aspects of the patient’s treatment from their own perspective. McCloud and Lake assessed patient experiences with nAMD in their single-centre, single-country study in Australia in 2015 [29], capturing similar themes to those described in this study, as with Midena et al. in their single-country study in Italy in 2022 [30]. This study builds on these data by gathering the emotions and experiences of patients from different centres in Europe, focussing on every stage of their disease.
Information provided at diagnosis was often insufficient, with some patients not understanding the requirement for life-long injections until they had received several injections. Furthermore, patients were reluctant to ask for further information; they reported apprehension about receiving treatment, which abated for some, but not all, following their first injection. They spoke about not having enough contact with their primary HCP treating their nAMD and being treated by different administering physicians at various appointments, indicating that time with the primary HCP and consistency with HCPs are two tangible actions that could minimise discomfort and improve adherence. Frustration was reported by one patient at not being able to book appointments far in advance. Treatment regimens were not captured; however, this may indicate that the patient did not fully understand the regimen they are receiving (e.g. the difference between pro re nata and being able to plan appointments in advance, and treat-and-extend and more reactive modalities). Given that intraocular injections can be uncomfortable and frightening to think about, if the patient knows and understands when they will next be receiving their injection, it may allow them to mentally prepare and have confidence in the treatment and procedure. Conversely, others may feel that knowing in advance allows additional time for worry, so a personalised approach to patient communication, and continued information and education about nAMD and its treatment would be useful.
Patients with nAMD adapted their lives and lifestyle activities around their disease, and no patient reported receiving additional assistance beyond help with transport to and from appointments (either paid or family support). In fact, patients expressed the desire to not be perceived as “the typical nAMD patient”. Whilst many had difficulties with various recreational and domestic activities, notably, some patients believed that their inability to undertake activities of daily life was consistent with a person of a similar age who did not have nAMD. Given the descriptions of family assistance and coping strategies, it appears that patients may underestimate the impact of their nAMD on their daily life, with adaptations regularly becoming routine. Most patients did not want to use, or had not considered using, patient support associations.
Strengths of this study included that the interviews took place at home on the phone, in private, with an experienced health care interviewer. These factors, and the structure of the interview, were designed to allow elaboration, going off-topic, and reporting thoughts and feelings without the pressure of possibly offending their HCP. Capturing patient responses in this way allows for an understanding of the patient’s emotions and experiences in all aspects of their disease and treatment, highlighting their own areas of concern. These data may provide additional insights beyond what was expected by the patient’s treating physician and are invaluable in providing the patient voice.
Limitations include recruitment challenges resulting in a small sample size per country (particularly with patients who were non-adherent), which was further complicated by the COVID-19 pandemic. Patients may have been unwilling to participate in the study owing to a general lack of engagement with their disease or treatment, multiple comorbidities, not wanting to be perceived as complaining about their treatment or experience with the centre, or concerns over the additional burden of taking part. The results of the study may reflect the experiences of a patient cohort who are motivated and educated regarding their disease, with relatively good vision and health and who were living in their own homes within Europe. Additionally, the very nature of qualitative research and the approach taken in this study may make findings difficult to compare across different studies. Further studies could evaluate any differences in emotions and experiences between patients receiving treatment before, during, and after the COVID-19 pandemic, and between patients receiving unilateral injections, bilateral injections, and bilateral injections with each eye on a different schedule (evaluating any links between visual acuity and frequency of appointments on adherence to treatment).
Conclusion
Key areas for improving the patient’s overall experience include a personalised approach to communication and support from their doctor and health care team, and a comprehensive approach to tackling logistical challenges. The unique insights found in this study highlight the importance of the patient’s voice when considering approaches to patient care and management, and these insights will assist in the development of meaningful and relevant interventions that support improved outcomes for patients with nAMD.
References
Lanzetta P, Loewenstein A, Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–73.
Colijn JM, Buitendijk GHS, Prokofyeva E, et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124(12):1753–63.
Bobadilla M, Pariente A, Oca AI, Pelaez R, Perez-Sala A, Larrayoz IM. Biomarkers as predictive factors of anti-VEGF response. Biomedicines. 2022;10(5):1003.
Hussain RM, Shaukat BA, Ciulla LM, Berrocal AM, Sridhar J. Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration. Drug Des Dev Ther. 2021;15:2653–65.
Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina. 2020;40(9):1673–85.
Holz FG, Minnella AM, Tuli R, et al. Ranibizumab treatment patterns in prior ranibizumab-treated neovascular age-related macular degeneration patients: real-world outcomes from the LUMINOUS study. PLoS ONE. 2020;15(12):e0244183.
Almuhtaseb H, Johnston RL, Talks JS, Lotery AJ. Second-year visual acuity outcomes of nAMD patients treated with aflibercept: data analysis from the UK Aflibercept Users Group. Eye (Lond). 2017;31(11):1582–8.
Eter N, Hasanbasic Z, Keramas G, et al. PERSEUS 24-month analysis: a prospective non-interventional study to assess the effectiveness of intravitreal aflibercept in routine clinical practice in Germany in patients with neovascular age-related macular degeneration. Graefes Arch Clin ExpOphthalmol. 2021. https://doi.org/10.1007/s00417-021-5073-8.
Zheng Y, Wu X, Lin X, Lin H. The prevalence of depression and depressive symptoms among eye disease patients: a systematic review and meta-analysis. Sci Rep. 2017;7:46453.
Jian-Yu E, Li T, McInally L, et al. Environmental and behavioural interventions for reducing physical activity limitation and preventing falls in older people with visual impairment. Cochrane Database Syst Rev. 2020;9:CD009233.
Okada M, Mitchell P, Finger RP, et al. Non-adherence or non-persistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2020;128:234–47.
Okada M, Wong TY, Mitchell P, et al. Defining nonadherence and nonpersistence to anti-vascular endothelial growth factor therapies in neovascular age-related aacular degeneration. JAMA Ophthalmol. 2021;139(7):769–76.
Cimarolli VR, Casten RJ, Rovner BW, Heyl V, Sorensen S, Horowitz A. Anxiety and depression in patients with advanced macular degeneration: current perspectives. Clin Ophthalmol. 2016;10:55–63.
Park M, Unutzer J. Geriatric depression in primary care. Psychiatr Clin North Am. 2011;34(2):469–87.
Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1281–4.
Polat O, Inan S, Ozcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47(4):205–10.
Jones RK, Jefferis JM. Is qualitative research under-represented in ophthalmology journals? Eye (Lond). 2017;31(8):1117–9.
Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–8.
Lane J, Rohan EMF, Sabeti F, et al. Impacts of impaired face perception on social interactions and quality of life in age-related macular degeneration: a qualitative study and new community resources. PLoS ONE. 2018;13(12):e0209218.
Taylor DJ, Jones L, Binns AM, Crabb DP. “You’ve got dry macular degeneration, end of story”: a qualitative study into the experience of living with non-neovascular age-related macular degeneration. Eye (Lond). 2020;34(3):461–73.
Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1–eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psych. 2006;3(2):77–101.
Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med. 2015;20(3):296–310.
Senra H, Balaskas K, Mahmoodi N, Aslam T. Experience of anti-VEGF treatment and clinical levels of depression and anxiety in patients with wet age-related macular degeneration. Am J Ophthalmol. 2017;177:213–24.
Finger RP, Daien V, Eldem BM, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration—a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20(1):294.
Thier A, Holmberg C. The patients’ view: age-related macular degeneration and its effects—a meta-synthesis. Disabil Rehabil. 2022;44(5):661–71.
Bennion AE, Shaw RL, Gibson JM. What do we know about the experience of age related macular degeneration? A systematic review and meta-synthesis of qualitative research. Soc Sci Med. 2012;75(6):976–85.
McCloud C, Lake S. Understanding the patient’s lived experience of neovascular age-related macular degeneration: a qualitative study. Eye. 2015;29(12):1561–9.
Midena E, Varano M, Pilotto E, et al. Real-life patient journey in neovascular age-related macular degeneration: a narrative medicine analysis in the Italian setting. Eye (Lond). 2022;36(1):182–92.
Acknowledgements
The authors thank the patients in this study for their time and insights.
Funding
The study was conducted by Kantar/Cerner Enviza, and was funded by Bayer Consumer Care AG, Basel, Switzerland. The journal’s Rapid Service fee was funded by Bayer Consumer Care, AG, Basel, Switzerland. Both HCPs and patients were compensated for their time.
Medical Writing Assistance
Medical writing support and thematic analysis, under the direction of the authors, was provided by Luke Shelton, PhD, and Sarah Feeny, BMedSci, of ApotheCom (UK), funded by Bayer Consumer Care AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidance (Ann Intern Med 2015;163:461–464).
Author Contributions
S. James Talks, Vincent Daien, and Robert P. Finger were involved in patient enrolment and data collection. S. James Talks, Vincent Daien, Robert P. Finger, Anna Biberger, Ceri Hirst, and Michelle Sylvanowicz were involved in the design of the study. All authors were involved in the analysis and interpretation of the data, and the preparation, review, and approval of the manuscript for submission. S. James Talks takes responsibility for the overall content of the manuscript as guarantor. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Prior Presentation
Data from this manuscript were previously presented in short oral presentation format at the European Society of Retina Specialists (EURETINA)’s 2021 congress.
Disclosures
S. James Talks: Advisory board member, speaker fees, and research support: Bayer, Novartis; research grants: Roche, Boehringer Ingelheim; Vincent Daien: Consultant: Bayer, Horus Pharma, Novartis, Théa; Paul Mitchell: Consultant: Allergan, Bayer, Novartis; Steering Committee member for Bayer; Tariq Aslam: Consultant: Novartis, Bayer, Laboratoires, Théa Pharmaceuticals, Bausch & Lomb, Oraya; Jane Barratt: Consultant: Bayer; Anna Biberger: Employee: Cerner Enviza (formerly Kantar); Ecosse L. Lamoureux: Nothing to disclose; Ceri Hirst: Employee: Bayer Consumer Care AG; Michelle Sylvanowicz: Employee: Bayer Consumer Care AG; Robert P. Finger: Grant: Novartis, CentreVue, Heidelberg Engineering, Zeiss; Consultant: Novartis, Bayer, Roche/Genentech, Ellex, Alimera, Allergan, Santhera, Inositec, Opthea; Support for travel: Novartis.
Compliance with Ethics Guidelines
This non-interventional study complied with the ethical standards of the Declaration of Helsinki. All treatment decisions fell within the clinical practice of the individual centres. Prescription of medicines was clearly separated from the decision to include the patient in the study, and no additional diagnostic or monitoring processes were required for participation in the study.
Data Availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymised patient-level data and supporting documents from clinical studies to conduct further research that can help to advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the ‘Study sponsors’ section of the portal. Data access will be granted to anonymised patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Talks, S.J., Daien, V., Mitchell, P. et al. The Patient Voice in Neovascular Age-Related Macular Degeneration: Findings from a Qualitative Study. Ophthalmol Ther 12, 561–575 (2023). https://doi.org/10.1007/s40123-022-00631-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00631-7