Abstract
Introduction
This study evaluated the changes and explored influential factors in the anterior chamber after femtosecond laser-assisted in situ keratomileusis (FS-LASIK) on the basis of patients with different degrees of myopia, refractive status, and age.
Methods
Patients underwent uneventful FS-LASIK for the treatment of ametropia. All patients with myopia were classified into a low age group (18–40 years) or high age group (> 40 years). Patients in the low age group (18–40 years) were subdivided into myopia and hyperopia groups. According to the preoperative spherical equivalent values, the myopia group was further divided into low myopia, moderate myopia, and high myopia groups. We measured the magnitude of anterior chamber depth (ACD), angle (ACA), volume (ACV), lens rise, central corneal thickness (CCT), and mean pupil power (MPP) of all patients using Sirius (version 3.2, CSO, Italy) before and 3 months post operation.
Results
A total of 140 eyes of 87 patients were evaluated. The magnitudes of ACD, ACA, and ACV decreased significantly postoperatively in both low age (P < 0.001, P < 0.001, P < 0.001, respectively) and high age group (P < 0.001, P < 0.001, P < 0.001, respectively) of patients with myopia, while an increasing tendency of ACD (2.85 ± 0.38 mm preoperatively vs 2.89 ± 0.09 mm postoperatively) and ACA (39.16° ± 7.30° preoperatively vs 39.37° ± 7.68° postoperatively) was found in hyperopia. There was no correlation between different degrees of myopia and the changes in the anterior chamber.
Conclusions
Anterior chamber parameters decreased significantly and approximately in the same degree in low and high age groups postoperatively, while an increasing tendency of ACD and ACA was found in hyperopia, indicating that it is probably a corneal magnification effect that influences changes in the anterior chamber.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The structure of the anterior chamber plays an important role in intraocular lens implantation and glaucoma screening. |
Anterior chamber parameters change after FS-LASIK surgery. |
What was learned from the study? |
We combined degrees of myopia, refractive status, and age to evaluate the anterior chamber changes after FS-LASIK. |
ACD, ACA, and ACV decreased significantly after FS-LASIK in the myopia group, while an increasing tendency of ACD and ACA was found in hyperopia. |
The change in the magnification effect of the cornea after FS-LASIK is considered to be the main reason for deviation of anterior chamber values from the actual values. |
Introduction
Femtosecond laser-assisted in situ keratomileusis (FS-LASIK) has been one of the safest and most effective treatments of myopia and myopic astigmatism so far [1,2,3,4]. Some earlier reports have focused on the changes of corneal topography, biomechanics, and visual quality after FS-LASIK [5,6,7]. However, only a few studies have been conducted on postoperative intraocular structural changes, especially the changes in the anterior chamber (AC).
Theoretically, the structure of anterior chamber is mainly determined by the posterior surface of the cornea and the anterior surface of the crystalline lens, for which the postoperative parameters of AC are supposed to be the same as those before FS-LASIK. However, some reports also found that keratoconus and keratoplasty might contribute to alterations of anterior chamber parameters [5, 6]. It is of great clinical significance to get a comprehensive understanding of the structural changes in the anterior chamber for screening of angle closure glaucoma [7] and design of intraocular surgical protocols like intraocular lens (IOL) implantation [8, 9].
At present, there is controversy about the changes of anterior chamber structure after myopic and hyperopic LASIK, and no unified explanation has been achieved. In this study, we investigate the changes of anterior chamber depth (ACD), anterior chamber angle (ACA), and anterior chamber volume (ACV) in patients after FS-LASIK, and we evaluate the changes in corneal magnification caused by LASIK correction of myopia and hyperopia, which may lead to errors in the anterior chamber depth measurement. We hope that, with a comprehensive understanding of the changes in the anterior chamber after FS-LASIK, more guidance for cataract IOL power calculation formula and glaucoma screening may be put forward.
Methods
This was a retrospective, repeated measurements study. The study involved 140 eyes of 87 patients (age range 18–51 years) who had undergone FS-LASIK for the treatment of ametropia at Peking University Third Hospital with complete 3-month follow-up information. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the local ethics committee (Peking University Third Hospital Medical Science Research Ethics Committee, M2022138). Written informed consent was obtained from every patient.
Inclusion and Exclusion Criteria
Patients that were included in the study were candidates for ametropic refractive surgery of FS-LASIK who had stopped wearing soft contact lenses for more than 2 weeks and rigid gas permeable contact lenses (RGP) for more than 4 weeks, with normal corneal topography and no nebula or leukoma before the operation.
The exclusion criteria for the study included active eye infection or inflammation, keratoconus and suspected keratoconus, ocular operation or trauma history, retreatment, intraoperative and/or postoperative complications, and severe systemic diseases.
Case Grouping
The low age group included the patients under the age of 40 years. On the basis of their refractive status, the low age group was subclassified into two groups: myopia and hyperopia. According to the preoperative spherical equivalent (SE) values, the myopia group was further divided into three groups: low myopia (− 0.50 to − 3.00 D), moderate myopia (− 3.25 to − 6.00 D), and high myopia (− 6.25 to − 10.00 D). SE was + 1.00 to + 7.00 D in the hyperopia group.
Additionally, we included patients with myopia over 40 years old as a high age group, for comparison with the aforementioned young patients with myopia.
All patients’ astigmatism was less than 2.00 D.
Preoperative and Postoperative Assessment
A complete preoperative ophthalmological examination was performed in all patients, including uncorrected visual acuity (UCVA), best corrected visual acuity (BCVA), intraocular pressure (IOP), slit-lamp examination, IOL Master (Carl Zeiss Meditec AG, Jena, Germany), and wide-angle fundus photography (OPTS, Britain).
We measured central ACD, ACA, ACV, central corneal thickness (CCT), lens rise and mean pupil power (MPP) using a Sirius combined topographer and tomographer (version 3.2, CSO, Italy) before and 3 months post operation.
Surgical Procedure
All surgical procedures were performed by the same surgeon (Yueguo Chen). All flaps were created by the WaveLight FS200 femtosecond laser (Alcon Laboratories Inc., Fort Worth, TX, USA). The flap/canal/hinge parameters were as followed: flap thickness, 110 μm; flap diameter, 8.5–9.0 mm; side-cut angle, 90°; hinge angle, 50°; canal width, 1.5 mm. Every flap was superiorly hinged, with a superior gas canal. Flaps were lifted immediately after flap creation to perform ablation using the WaveLight EX500 excimer laser (Alcon Laboratories Inc., Fort Worth, TX, USA).
Postoperative Care and Follow-Up
The standard postoperative regimen included one drop each of 0.5% levofloxacin (Cravit, Santen, Inc.) and 0.1% fluorometholone (FML, Allergan, Inc.) QID for 2 weeks. Each patient was followed up at 1 and 7 days and 1 and 3 months after surgery. The follow-up examinations involved measurements of UCVA, BCVA, slit-lamp examination, subjective refraction, corneal topography, and tomography (Sirius, CSO, Italy).
Statistical Analysis
Data are presented as mean ± standard deviation. All statistical analyses were performed using the SPSS software (version 24.0, SPSS, Inc.). A paired t test was used to compare variables, including the baseline characteristics of the patients, the magnitude of preoperative and postoperative ACD, ACA, ACV, CCT, MPP, and lens rise. For normal distribution data, one-way analysis of variance (ANOVA) was used for comparison of changes in anterior chamber parameters among low, moderate, and high myopia groups; for non-normally distributed data, the rank sum test was used. Before we use the paired t test and the one-way ANOVA, we confirmed that the data was normally distributed. Statistical significance was set at P < 0.05.
Results
We ultimately analyzed 140 eyes of 87 patients and there were no complications after FS-LASIK in our study. All the patients with myopia were classified into the low age group (range 18–40 years) which had 71 eyes of 46 patients or high age group (more than 40 years) comprising 40 eyes of 26 patients. Among the low age group, there were 71 eyes of 46 patients with myopia (myopia group) and 29 eyes of 15 patients with hyperopia (hyperopia group). According to the degrees of myopia, the myopia group was divided into three subgroups: low myopia had 24 eyes of 17 patients, moderate myopia had 22 eyes of 16 patients, and the high myopic group had 25 eyes of 13 patients. There were no significant differences of baseline characteristics among the three subgroups of patients with different degrees of myopia, between the low age group and high age group of patients with myopia, or between the myopia group and hyperopia group of patients of low age (Table 1).
Figure 1a, b shows preoperative and postoperative CCT and MPP in low, moderate, and high myopia group, myopia (low age) group, high age group, and hyperopia group. CCT decreased by an average of 47.74 ± 15.90 μm, 84.59 ± 14.92 μm, and 125.60 ± 16.50 μm while MPP fell by an average of 2.55 ± 0.58 D, 4.97 ± 0.94 D, and 7.76 ± 1.31 D postoperatively in low, moderate, and high myopia groups, respectively. Mean differences above (△CCT and △MPP) were positively correlated with preoperative spherical equivalent (SE) values (R = 0.930, R = 0.973). Among all the patients with myopia, CCT and MPP decreased by 87.13 ± 36.04 μm and 5.13 ± 2.40 D in the low age group and decreased by 96.75 ± 28.95 μm and 5.18 ± 1.78 D in the high age group on average. As for the group of patients with hyperopia, CCT decreased by 14.00 ± 13.45 μm while MPP increased by 3.81 ± 2.36 D after surgery.
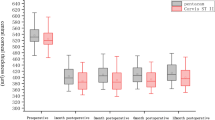
We measured ACD, ACA, ACV, and lens rise in all patients before and 3 months postoperatively (Tables 2, 3; Fig. 2a–c). AC images measured by Sirius are shown in Fig. 3. Lens rise is the difference between the position of the crystalline lens and the iridocorneal plane; a negative value means that the crystalline lens is above the iridocorneal plane.
Among the three groups of patients with different degrees of myopia, postoperative ACDs were all significantly shallower (P < 0.01) while ACAs significantly narrowed (P < 0.05) and ACVs were significantly smaller (P < 0.001) (Table 2). There were no significant differences of △ACD (P = 0.347), △ACA (P = 0.538), or △ACV (P = 0.706) among low, moderate, and high myopia groups. Besides, △CCT and △MPP were not significantly associated with the magnitude of △ACD, △ACA, or △ACV (Fig. 4a, b). Only in the low myopia group could we find significant difference between preoperative and postoperative lens rise (P = 0.001, − 0.02 ± 0.19 mm preoperatively vs − 0.07 ± 0.21 mm postoperatively, △lens rise = 0.05 ± 0.06 mm; a more negative lens rise postoperatively means the crystalline lens was moving ahead to the iridocorneal plane). There were no significant differences between pre- and postoperative lens rise in the moderate and high myopia groups.
Comparing low and high age groups of myopia (Table 3), preoperative ACD, ACA, and ACV in the high age group were all significantly less than those parameters in the low age group (P < 0.05). But in both of these two age groups, postoperative ACDs were all significantly shallower (P < 0.001) and ACAs significantly narrowed (P < 0.05) and ACVs significantly decreased after surgery (P < 0.001). Besides, there were no significant differences of △ACD (P = 0.534), △ACA (P = 0.205), or △ACV (P = 0.227) between the two age groups of myopia (Table 3). There were no significant changes in lens rise after surgery in both low and high age groups of myopia.
When considering different refractive status (Table 3), postoperative ACD, ACA, and ACV in patients with myopia were all significantly decreased as mentioned above. In the hyperopia group, there was a deepening tendency of ACD (2.85 ± 0.38 mm preoperatively vs 2.89 ± 0.098 mm postoperatively, P = 0.166) and a widening tendency of ACA (39.16° ± 7.30° preoperatively vs 39.37° ± 7.68° postoperatively, P = 0.742) after surgery, but without any significant differences. Neither ACV (P = 0.826) nor lens rise (P = 0.400) had a significant difference in hyperopia postoperatively.
Discussion
As the population of patients undergoing FS-LASIK becomes larger and larger, it is of great interest to investigate AC changes postoperatively for their further possible optical treatment [4, 10]. Accurate assessment of anterior chamber structures has important clinical applications. First, accurate measurement of ACA and ACD is crucial for the diagnosis and follow-up of angle-closure glaucoma [7]. Second, ACD is an important parameter in the precise calculation of intraocular lens (IOL) power in cataract surgery [11, 12]; the accuracy of IOL power calculation is of great significance for postoperative visual quality of patients with cataracts. Currently, calculation formulas we have commonly used, such as Holladay 1&2, Haigis, Hoffer Q, Holladay 1, and SRK/T, involved ACD, axial length, keratometry, lens thickness, and CCT. Third, AC parameters also help to evaluate whether intraocular collamer lens (ICL) surgery is suitable [9]. Anterior chamber parameters including ACD, ACA, and ACV can be measured with the assistance of a 3D anterior segment analysis system [13]. Clinical studies on structural changes in the anterior chamber after FS-LASIK are limited and no consistent conclusions have been drawn. In this study, we compared the anterior chamber parameters before and after myopic and hyperopic FS-LASIK hoping to explore any changes and analyze the possible influencing factors. To the best of the authors’ knowledge, this is the first comprehensive study which assessed the changes in the anterior chamber after FS-LASIK on the basis of degrees of myopia, refractive status, and age, revealing that in myopia, regardless of age and refractive degree, there are significant decreases in ACD, ACA, and ACV after FS-LASIK. We also found an increasing tendency of ACD and ACA in hyperopia postoperatively.
In this study, a Sirius system was used to measure preoperative and postoperative anterior chamber parameters. A wide variety of instruments have been used to evaluate the anterior segment, including Placido disc corneal topography, Scheimpflug camera, scanning slit topography, and optical coherence tomography (OCT) [14]. Sirius uses a variety of preferred reference parameters combined with support vector machine (SVM) to obtain the corneal morphology and anterior chamber structures. Combining the advantages of Placido disc’s curvature measurement of the anterior surface of cornea and rotating Scheimpflug camera’s height measurement by imaging the anterior and posterior surface of cornea, the Sirius system can provide anterior segment tomography and corneal topographic evaluation within seconds, improving the accuracy of measurement [15]. The consistency of Sirius with that of previous Scheimpflug camera has been confirmed in a number of studies [16, 17]. In addition, we also compared our results with several studies using different devices. Wang et al. measured preoperative and postoperative central ACD in 66 eyes of young patients with myopia by using Pentacam (3.317 ± 0.219 mm and 3.252 ± 0.230 mm), Orbscan (3.100 ± 0.223 mm and 3.047 ± 0.237 mm), IOL-Master (3.247 ± 0.246 mm and 3.207 ± 0.238 mm), and A-scan ultrasonography (3.404 ± 0.220 mm and 3.331 ± 0.219 mm), and found that ACD decreased significantly after LASIK despite use of different devices [18]. The results of ACD after LASIK in their study were similar to ours in the low age group. Hashemi and Mehravaran reported a mean ACD decrease of 0.02 ± 0.06 mm with the Orbscan II and 0.06 ± 0.05 mm with the Pentacam 6 weeks after surgery, values which were comparable to ours measured with Sirius [19]. Zhou et al. used Pentacam to measure ACD after hyperopic FS-LASIK treatment [20] and found central ACD of 2.81 mm preoperatively and 2.84 mm postoperatively, values which were consistent with ours in the hyperopia group, and there was a similar increasing tendency of ACD after hyperopic FS-LASIK surgery as well.
We found that there were significant decreases in ACD, ACA, and ACV after FS-LASIK in both the low age group and high age group of patients with myopia, consistent with the results of previous studies [21,22,23,24], and the ACD became shallower in both two groups approximately to the same degree. Mean preoperative ACD in the high age group of myopia (2.85 ± 0.24 mm) was significantly lower than that of the low age group (3.19 ± 0.23 mm), the reason for which was the decrease in ACD with age [25, 26]. It has been reported that anterior chamber depth and the accommodation function decreased with age [25], and some studies put forward that it might be related to accommodation. The better accommodation a patient has, the thicker their crystalline lens turns out to be, thus the greater extent of decreases in ACD that are observed [27,28,29,30]. However, in our study, ACD decreased significantly in the high age group after surgery (2.85 ± 0.24 mm preoperatively vs 2.78 ± 0.23 mm postoperatively, P < 0.001, mean age = 44.7 ± 3.5 years). Thus, we did not think that accommodation is adequate enough to cause the significant decrease in postoperative ACD. Our findings were not consistent with the results of Nishimura et al., who measured the changes in ACD after LASIK by Pentacam and found that ACD decreased significantly in younger patients (3.26 ± 0.22 mm preoperatively vs 3.22 ± 0.22 mm postoperatively, P < 0.001, mean age = 30.4 ± 4.2 years), but not in older patients (mean ACD of 3.15 ± 0.34 mm preoperatively vs mean ACD of 3.15 ± 0.33 mm postoperatively, P = 0.514, mean age = 45.8 ± 5.1 years) [31]. On the other hand, Dominguez-Vicent et al. investigated 80 emmetropia candidates between 22 and 30 years of age (mean age = 25.30 ± 2.98 years) and evaluated their ACD values with accommodation stimuli ranging from − 1.0 to + 4.0 diopters (D) in 1.0-D steps, and found no significant changes in ACD during accommodation [32]. Tsorbatzoglou et al. found that accommodation-induced ACD decreases could be 0.03 ± 0.06 mm (P = 0.0004) in patients over 45 years of age [25]. In our study, the shallowing degree of ACD in both the low age and high age groups of patients after myopic FS-LASIK was approximately the same, 0.08 ± 0.08 mm and 0.07 ± 0.08 mm, respectively, which were much higher than the accommodation-induced decreases in ACD described in the aforementioned studies. Combining all of the findings noted above, we hypothesize that accommodation is not the main reason behind the significant decrease in postoperative ACD.
Considered from the aspect of refractive status, changes in ACD, ACA, and ACV after FS-LASIK were compared between patients with myopia and hyperopia. Significant decreases in ACA, ACV, and ACD were found in the myopia group, while an increasing tendency in ACA and ACD (mean ACD of 2.85 ± 0.38 mm preoperatively vs mean ACD of 2.89 ± 0.098 mm postoperatively) and no significant change in ACV were seen in the hyperopia group. Mean preoperative ACD in patients with hyperopia was significantly lower than that of myopia, since the former was usually combined with shorter axial length. At present, only Zhou et al. found no significant changes in ACD, ACA, and ACV measured by Pentacam after hyperopic FS-LASIK treatment [20]. However, their results in preoperative and postoperative ACD were comparable to ours, and there was a similar increasing tendency of ACD after surgery in Zhou et al.’s study (central, superior, inferior, nasal, and temporal ACD of 2.81, 2.28, 2.53, 2.16, and 2.61 mm preoperatively vs 2.84, 2.31, 2.54, 2.16, and 2.65 mm postoperatively, respectively) [20]. According to the statistical results, opposite changes in the anterior chamber after FS-LASIK were observed between myopia and hyperopia, for which the magnification effect by cornea after surgery was considered to be a possible explanation. Refractive surgery on the cornea is designed to correct ametropia by reshaping the anterior surface of the cornea without even touching the posterior surface, so the surgical procedure itself will not alter the structure of the anterior chamber. Additionally, given that surgical treatment for myopia and hyperopia is in an opposite manner, the opposite changes in the anterior chamber parameters suggested a great possibility of the magnification effect of the cornea. From the standpoint of magnification, Nawa et al. considered performing myopic LASIK as adding a minus lens on the cornea. Thus, decreases in the measurement of internal ocular structures, including ACD, are considered to be the result of a decreased corneal magnification effect after myopic LASIK. It has been disclosed through the Obscan that the magnification ratio of the cornea decreases by 0.35% per 1.0-D correction of myopia [23, 33, 34].
Therefore, in order to explore whether the corneal magnification effect is correlated with the corrected diopters, we further compared the extent of changes in ACD among different degrees of myopia. According to the statistics results, there were no significant differences of △ACD (P = 0.347), △ACA (P = 0.538), or △ACV (P = 0.706) among the low, moderate, and high myopia groups. In other words, we did not observe any correlation between the degrees of myopia and the extent of changes in the anterior chamber. There are three possible reasons: First, Nawa et al. used Obscan to measure relevant parameters of the anterior segment while we used the Sirius system in this study. There are certain differences in the origin settings between the two devices. Second, Nishimura mentioned in the reply to Nawa that the manufacturer (Oculus) had already corrected Pentacam’s magnification effect of the cornea with ray tracing. In the same way, we presumed that the manufacturer of Sirius had also implemented certain corrections to corneal magnification effect caused by FS-LASIK, but not in a complete way. Third, the crystalline lens was observed to move forward after surgery in this study in the low myopia group, while no significant changes in lens position were observed in the moderate and high myopia groups, hyperopia group, or high age group after surgery. Thus, we considered there were some mediating factors such as accommodation that might contribute to the changes in ACD after FS-LASIK. Combining the magnification effect of the cornea as a dominant factor and the accommodation factor as a supplementary factor, we saw the phenomenon that the anterior chamber decreased in patients with myopia but not in correlation with the degree of myopia, and it tended to deepen in patients with hyperopia after FS-LASIK.
Limitations
The limitations of this study are as follows: First, we only measured the anterior chamber index values of patients 3 months postoperatively, but the literature has followed up the anterior chamber values from 1 week to 6 months after surgery, although they still come to a conclusion that is consistent with ours [18, 21]. Second, because this is a retrospective study and did not verify the effect of accommodation on the anterior chamber, relevant prospective studies need to be further improved to explore the effect of accommodation on the anterior segment before and after surgery. However, there are already many documents verifying that the accommodation does have an impact on the anterior chamber [13, 18, 25, 28,29,30]. Third, the sample size of this study is not big enough and only a single measuring device was used in our study, so more intensive as well as alternative measuring devices and multicenter studies are needed to further explore the influencing factors of postoperative changes in the anterior chamber.
Conclusion
The structure of the anterior chamber has important guiding significance for the assessment of the risk of ophthalmic diseases and the phakic IOL treatment. This study investigated changes in the anterior chamber parameters after ametropic FS-LASIK from three aspects: age, refractive status, and degrees of myopia. ACD, ACA, and ACV decreased significantly after FS-LASIK for myopia and the extent of decreases was independent of age and refractive degree. As for hyperopic FS-LASIK, ACD and ACA had an increasing tendency after surgery, but there was no significant change in ACV. We do not deny that some mediating factors such as accommodation were involved, but the change in magnification effect of the cornea after FS-LASIK is considered to be the main reason for deviation of anterior chamber values from the actual values. And Sirius does not fully compensate the corneal magnification changes induced by FS-LASIK. The influence of the magnification effect by the cornea, accommodation, and biomechanics on the anterior chamber still needs to be further clarified. Clarifying the influencing factors of anterior chamber changes after refractive surgery has important clinical significance.
References
Teus MA, Garcia-Gonzalez M. Comparison of the visual results after small incision lenticule extraction and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30(9):582.
Lin F, Xu Y, Yang Y. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30(4):248–54.
Binder PS. One thousand consecutive IntraLase laser in situ keratomileusis flaps. J Cataract Refract Surg. 2006;32(6):962–9.
Zhang Y, Shen Q, Jia Y, Zhou D, Zhou J. Clinical outcomes of SMILE and FS-LASIK used to treat myopia: a meta-analysis. J Refract Surg. 2016;32(4):256–65.
Emre S, Doganay S, Yologlu S. Evaluation of anterior segment parameters in keratoconic eyes measured with the Pentacam system. J Cataract Refract Surg. 2007;33(10):1708–12.
Ort A, Gunes A, Kandemir B, et al. Evaluation of the cornea and anterior chamber morphologic changes after penetrating keratoplasty in patients with keratoconus. Eye Contact Lens. 2017;43(4):236–9.
Sharma R, Sharma A, Arora T, et al. Application of anterior segment optical coherence tomography in glaucoma. Surv Ophthalmol. 2014;59(3):311–27.
Lim DH, Lee MG, Chung ES, Chung TY. Clinical results of posterior chamber phakic intraocular lens implantation in eyes with low anterior chamber depth. Am J Ophthalmol. 2014;158(3):447-454 e441.
Elmohamady MN, Abdelghaffar W. Anterior chamber changes after implantable collamer lens implantation in high myopia using Pentacam: a prospective study. Ophthalmol Ther. 2017;6(2):343–9.
Wen D, McAlinden C, Flitcroft I, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. 2017;178:65–78.
Chung J, Bu JJ, Afshari NA. Advancements in intraocular lens power calculation formulas. Curr Opin Ophthalmol. 2022;33(1):35–40.
Savini G, Hoffer KJ, Carbonelli M, Ducoli P, Barboni P. Influence of axial length and corneal power on the astigmatic power of toric intraocular lenses. J Cataract Refract Surg. 2013;39(12):1900–3.
Marchini G, Pedrotti E, Modesti M, Visentin S, Tosi R. Anterior segment changes during accommodation in eyes with a monofocal intraocular lens: high-frequency ultrasound study. J Cataract Refract Surg. 2008;34(6):949–56.
Naderan M, Shoar S, Naderan M, Kamaleddin MA, Rajabi MT. Comparison of corneal measurements in keratoconic eyes using rotating Scheimpflug camera and scanning-slit topography. Int J Ophthalmol. 2015;8(2):275–80.
Lanza M, Iaccarino S, Cennamo M, Lanza A, Coen G. New Scheimpflug camera device in measuring corneal power changes after myopic laser refractive surgery. Cont Lens Anterior Eye. 2015;38(2):115–9.
Hernandez-Camarena JC, Chirinos-Saldana P, Navas A, et al. Repeatability, reproducibility, and agreement between three different Scheimpflug systems in measuring corneal and anterior segment biometry. J Refract Surg. 2014;30(9):616–21.
Wang Q, Ding X, Savini G, et al. Anterior chamber depth measurements using Scheimpflug imaging and optical coherence tomography: repeatability, reproducibility, and agreement. J Cataract Refract Surg. 2015;41(1):178–85.
Wang L, Guo HK, Zeng J, Jin HY. Analysis of changes in crystalline lens thickness and its refractive power after laser in situ keratomileusis. Int J Ophthalmol. 2012;5(1):97–101.
Hashemi H, Mehravaran S. Corneal changes after laser refractive surgery for myopia: comparison of Orbscan II and Pentacam findings. J Cataract Refract Surg. 2007;33(5):841–7.
Zhou X, Li T, Chen Z, Niu L, Zhou X, Zhou Z. No change in anterior chamber dimensions after femtosecond LASIK for hyperopia. Eye Contact Lens. 2015;41(3):160–3.
Kim BK, Mun SJ, Yang YH, Kim JS, Moon JH, Chung YT. Comparison of anterior segment changes after femtosecond laser LASIK and SMILE using a dual rotating Scheimpflug analyzer. BMC Ophthalmol. 2019;19(1):251.
Cairns G, Ormonde SE, Gray T, et al. Assessing the accuracy of Orbscan II post-LASIK: apparent keratectasia is paradoxically associated with anterior chamber depth reduction in successful procedures. Clin Exp Ophthalmol. 2005;33(2):147–52.
Nawa Y, Yamashita J, Tomita M. Decreased anterior chamber depth after myopic LASIK. J Cataract Refract Surg. 2010;36(5):873–4 (author reply 874).
Wang J, Lopes BT, Li H, et al. Unintended changes in ocular biometric parameters during a 6-month follow-up period after FS-LASIK and SMILE. Eye Vis (Lond). 2021;8(1):9.
Tsorbatzoglou A, Nemeth G, Szell N, Biro Z, Berta A. Anterior segment changes with age and during accommodation measured with partial coherence interferometry. J Cataract Refract Surg. 2007;33(9):1597–601.
Savini G, Carbonelli M, Barboni P, Hoffer KJ. Repeatability of automatic measurements performed by a dual Scheimpflug analyzer in unoperated and post-refractive surgery eyes. J Cataract Refract Surg. 2011;37(2):302–9.
Richdale K, Bullimore MA, Sinnott LT, Zadnik K. The effect of age, accommodation, and refractive error on the adult human eye. Optom Vis Sci. 2016;93(1):3–11.
Esteve-Taboada JJ, Dominguez-Vicent A, Monsalvez-Romin D, Del Aguila-Carrasco AJ, Montes-Mico R. Non-invasive measurements of the dynamic changes in the ciliary muscle, crystalline lens morphology, and anterior chamber during accommodation with a high-resolution OCT. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1385–94.
Ramasubramanian V, Glasser A. Can ultrasound biomicroscopy be used to predict accommodation accurately? J Refract Surg. 2015;31(4):266-U155.
Shao Y, Jiang Q, Hu D, et al. Axial elongation measured by long scan depth optical coherence tomography during pilocarpine-induced accommodation in intraocular lens-implanted eyes. Sci Rep. 2018;8(1):1981.
Nishimura R, Negishi K, Dogru M, et al. Effect of age on changes in anterior chamber depth and volume after laser in situ keratomileusis. J Cataract Refract Surg. 2009;35(11):1868–72.
Dominguez-Vicent A, Monsalvez-Romin D, Del Aguila-Carrasco AJ, Ferrer-Blasco T, Montes-Mico R. Changes in the anterior chamber during accommodation assessed with a Scheimpflug system. J Cataract Refract Surg. 2014;40(11):1790–7.
Nawa Y. Corneal magnification. Ophthalmology. 2008;115(3):588 (author reply 588).
Nawa Y, Masuda K, Ueda T, Hara Y, Uozato H. Evaluation of apparent ectasia of the posterior surface of the cornea after keratorefractive surgery. J Cataract Refract Surg. 2005;31(3):571–3.
Acknowledgements
Funding
This work was supported by a grant from The Key Clinical Innovation Program of Peking University Third Hospital, Category A (Grant No. Y65495-05). The journal’s Rapid Service Fee was funded by the authors.
Author Contributions
Zizhen Wang made substantial contributions to conception, reviews the literatures, collects the data, and drafted the manuscript; Haowen Ma conducted the statistical analysis; Haowen Ma, Yu Zhang and Yifei Yuan revised the review; Yueguo Chen gave final approval of the version to be submitted.
Disclosures
Zizhen Wang, Haowen Ma, Yu Zhang, Yifei Yuan, Yan Liu, Yueguo Chen have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the local ethics committee (Peking University Third Hospital Medical Science Research Ethics Committee, M2022138). Written informed consent was obtained from every patient.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Z., Ma, H., Zhang, Y. et al. Changes in Anterior Chamber After Myopic and Hyperopic FS-LASIK. Ophthalmol Ther 11, 2243–2257 (2022). https://doi.org/10.1007/s40123-022-00579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00579-8