Abstract
Introduction
This study developed and validated a nomogram for predicting the risk of second surgery in patients with concomitant esotropia (CE) based on a cohort in Beijing.
Methods
In this retrospective cohort study, the inpatient and outpatient medical records of 419 patients with CE who underwent surgery at the Peking University First Hospital between January 1, 2005 and December 31, 2009 were collected. A total of 357 CE cases were included. For those cases 70% were randomly assigned to the training set (n = 234) and 30% to the validation set (n = 123). Demographic and clinical variables were ascertained at hospital admission and discharge and screened using multivariate Cox proportional hazards regression analysis to construct predictive models and generate a 1-, 4-, and 8-year overall survival nomogram. This nomogram provided an estimate of the risk of second surgery in patients with surgically treated CE. Internal validation was conducted using the concordance index (C-index) and calibration curve for the training and validation sets.
Results
Six independent prognostic factors were identified, namely age at surgery, age at onset, amblyopia, deviation angles, surgical amount, and deviation angles 1 week after surgery, and these were entered into the nomogram. The proposed nomogram showed favorable discrimination and accuracy in the training and validation sets. The C-indexes of the training and validation sets were 0.84 (95% CI 0.79–0.89) and 0.80 (95% CI 0.78–0.82), respectively.
Conclusions
The proposed nomogram can serve as a predictive tool for prognostic evaluation of CE surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Concomitant esotropia (CE) is one of the most common types of strabismus in children. CE greatly influences binocular vision function and most cases of CE require surgical correction. |
Many patients need second surgeries (SS) after surgery for CE. Numerous clinical studies have discussed the risk factors that affect ocular alignment after surgery for CE, including amblyopia, high hyperopia, concomitant signs, and limited internal conversion after surgery. However, no consensus has been reached. |
Therefore, it was necessary to develop a convenient and reliable tool to elucidate the risk factors for SS after CE surgery and to assist the surgical design to avoid SS. |
In this study, we develop and validate a nomogram for predicting the risk of second surgery in patients with CE. |
The proposed nomogram can serve as a predictive tool for prognostic evaluation of CE surgery. |
Introduction
Concomitant esotropia (CE) is one of the most common types of strabismus in children, with a reported prevalence of approximately 0.3–3.8%. It generally occurs before the age of 10 years [1, 2]. Because CE persists, it greatly influences binocular vision function and appearance in children. Except for fully accommodating CE, most cases of CE require surgical correction. During the long postoperative follow-up period, some patients showed persistent residual CE. Wan [3] reported the long-term effects of CE surgery in a group of infants with a large-angle CE. The overall success rate of surgery was low (23%), and most failures were due to recurrence or residual CE. Consecutive exotropia (CX) has also been reported after CE surgery, with a prevalence of 3–29% [4]. Many cases of recurrent or residual CE or CX require second surgery (SS) to resolve. To avoid SS for CE, many clinical studies have discussed the risk factors that affect ocular alignment after surgery for CE, including amblyopia, high hyperopia, concomitant signs, age at surgery, and limited internal conversion after surgery. However, no consensus has been reached, and there are differences between individual reports [5,6,7,8]. Therefore, the risk factors for SS in CE must be elucidated. Furthermore, it remains unclear whether SS can be performed early. These are difficult and urgent problems that need to be resolved.
In recent years, the combination of machine learning technology and medical practice has become a major trend and has shown remarkable performance in predicting the occurrence and prognosis of diseases. Unfortunately, there remains a lack of research on patients with CE in this field. Therefore, it was necessary to develop a convenient and reliable tool to assist the surgical design and facilitate communication between specialists and patients regarding the prognosis. In our study, we collected demographic and clinical variables at admission and after discharge from 357 patients with CE and screened them using multivariate Cox proportional hazards regression. A nomogram of six predictors, namely age at surgery, age at onset, amblyopia, strabismus deviation angles, surgical amount, and deviation angles 1 week postoperative, was constructed. This nomogram provided an estimate of the risk of SS in patients with surgically treated CE. At the same time, the model was validated using multiple indicators, including the concordance index (C-index), the area under the receiver operating curve, and the calibration curve. Internal validation in this cohort suggested that the nomogram provides an evidence-based and personalized risk assessment, which is helpful for strabismus specialists in clinical management and prognostic assessment.
Methods
Study Population
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University First Hospital. Written informed consent was waived because of the use of retrospective data. We collected the inpatient and outpatient medical records of 419 patients with CE who underwent surgery at Peking University First Hospital between January 1, 2005 and December 31, 2009. Two experienced doctors abstracted and cross-checked the clinical data to determine the inclusion or exclusion of patients.
The inclusion criteria were as follows: CE, no ocular organic lesions, no neurological or developmental disorders, no history of strabismus surgery, and a follow-up period of at least 6 months. The exclusion criteria were as follows: central nervous system defects, paralytic esotropia, anatomical abnormalities of the eye, such as optic nerve hypoplasia, previous ophthalmic surgery, and previous strabismus surgery.
Among the 419 surgically treated inpatients with CE, 22 (5.2%) had a history of strabismus surgery, 17 (4.1%) were not followed up as outpatients, and 23 (5.5%) were followed up for less than 6 months. Therefore, 357 (85.2%) patients that were surgically treated for CE were included in this study. The timeline of their follow-up is shown in Fig. 1. Among these subjects, there were 99 cases (27.73%) of congenital esotropia, 202 cases (56.59%) of partially accommodative esotropia, and 56 cases (15.68%) of non-accommodative esotropia. The cohort of this study was randomly divided using a computer according to the proportion of 70% and 30%; 234 cases were included in the training set, and 123 cases were included in the validation set, as shown in Fig. 2.
Data Collection
A complete medical history was recorded for all patients upon admission, and a general examination was performed. A detailed ophthalmological examination was performed before surgery to exclude ocular abnormalities, and further strabismus specialist examinations included visual acuity, cycloplegic refraction, deviation angles of distance and near primary, upward and downward 25° gaze, extraocular muscle function, and binocular sensory function.
Potential predictive variables included the patient demographics, medical history, and clinical variables at admission and after discharge. The demographic variables included sex, age at diagnosis, age at onset, and age at surgery. The medical history included family history, history of premature birth, history of febrile convulsion, duration of strabismus, and time of spectacle correction. Clinical variables included best-corrected visual acuity, cycloplegic refraction, amblyopia, deviation angles, stereopsis, dominant eye or non-dominant eye surgery, surgical muscle, surgical method, surgical amount, accompanying signs (A-V sign, compensatory head position, dissociative vertical deviation (DVD), inferior oblique muscle overaction (IOOA), and nystagmus). The distance and near deviation angles and distance and near stereopsis were obtained at 1 day, 1 week, 1 month, 3 months, and 6 months, and the last postoperative follow-up.
Follow-Up
All patients in this study were regularly followed up in the outpatient clinic at 1 week and 1, 3, and 6 months after surgery. Patients with unstable visual acuity and ocular alignment were followed up three to four times in 12 months, and patients with stable visual acuity and ocular alignment were followed up one to two times in 12 months. During the follow-up period, when the deviation angles of both distance and near were greater than + 15 PD (prism diopters) or less than − 15 PD, we treated the patients by adjusting eyeglasses or using drugs (1% atropine ointment once per day) for 3 months. If these treatments were ineffective, we concluded that the patients needed SS. This was a positive endpoint in this study. The follow-up termination time for this study was December 31, 2012. For patients who required SS, the follow-up termination time was the date of SS confirmation. For cases that did not require SS and the last follow-up date was before December 31, 2012, censored data were confirmed by the date of the last follow-up. The cohort was followed up for 6 to 96 months, with an average of 40.42 ± 26.02 months.
Surgical Method
The surgical method was determined on the basis of the deviation angles. Three methods were used: unilateral medial rectus (MR) recession, bilateral MR recession, and bilateral MR recession combined with unilateral lateral rectus (LR) resection. The range of surgical amount was as follows: MR recession, 3–6 mm; LR resection, 5–8 mm. The surgical amount calculated in this study was the sum of muscle recession and resection.
Missing Data
There were no missing diagnostic and surgical data on admission in the study cohort. For young children who did not understand and cooperate in the visual acuity test and stereopsis test, a clear preference for the use of one eye was judged as having amblyopia, and stereopsis was recorded as no. There were 4.8% missing data of near and distance deviation angles 1 day postoperative, and a simple imputation approach was used to replace the missing data with the median of the cohort. During the follow-up period, 37.5% of the data were missing because the stereopsis test was not performed frequently. We deleted and did not analyze this variable, considering that imputation was more likely to cause greater bias, and it was not the main observation variable.
Statistical Analysis
Statistical analyses were performed using SPSS (version 25.0) and R (version 3.3.28) software. The significance level was set at 0.05, and all tests were two-sided. For nomogram construction and validation, the patients were randomly divided into training and validation sets; 70% were included in the training set, and 30% were included in the validation set. The characteristics of the two groups were described and compared using the chi-square test or independent sample t test. Results were expressed as a percentage and mean ± SD difference. Variables to develop the nomogram were selected by the stepwise selection method using the Akaike information criterion in the Cox proportional hazards model. The results of independent prognostic factors for the screened SS were expressed as hazard ratios (HR) and 95% confidence intervals (95% CI). Based on the predictive model with the identified prognostic factors, a nomogram that predicted 1-, 4-, and 8-year overall survival was constructed.
Internal validation was performed using the training and validation sets. The nomogram validation consisted of two parts: discrimination and calibration. The concordance index (C-index) was calculated to evaluate the discrimination ability of the prediction model. For clinical use of this model, the total points of each patient were calculated from the nomogram. Receiver operating curve (ROC) analysis was performed to calculate the optimal cutoff values determined by maximizing the Youden index (sensitivity + specificity − 1). The accuracy of the optimal cutoff values was assessed on the basis of the sensitivity, specificity, predictive value, and likelihood ratios. Calibration was evaluated using a calibration plot, and bootstraps with 1000 resamples were used to reduce overfitting.
Results
Cohort Characteristics
In total, 357 consecutive patients with CE were identified. Of these, 54 cases (15.12%) underwent SS, including 26 cases (48.15%) of congenital esotropia, 17 cases (31.48%) of partially accommodative esotropia, and 11 cases of non-accommodative esotropia (20.37%). The SS time is shown in the Fig. 3, and preoperative deviation angles are shown in the Fig. 4. These were 42 cases (77.78%) of CX with deviation angles of 23.64 ± 7.67 PD, and 12 cases (22.22%) of residual esotropia with deviation angles of 22.91 ± 5.68 PD. There were 234 (65.55%) cases included in the training set. The mean follow-up time of these cases was 38.34 ± 25.45 months, 32 (13.68%) cases underwent SS, and the 1-, 4-, and 8-year survival rates were 97.43%, 90.53%, and 86.32%, respectively. There were 123 (34.45%) cases in the validation set. The mean follow-up time of these cases was 42.34 ± 27.53 months, and 22 (17.89%) patients underwent SS. The survival rates at 1, 4, and 8 years were 98.40%, 87.80%, and 82.11%, respectively.
The characteristics of the two groups of CE cases are shown in Table 1, and there were no significant differences between the training and validation sets.
Independent Prognostic Factors in the Training Set
The results of the multivariate Cox regression analysis are listed in Table 2. Age at surgery (P = 0.028), age at onset (P < 0.001), amblyopia (P = 0.023), deviation angles (P = 0.021), surgery amount (P = 0.006), and deviation angles at near 1 week postoperative (P = 0.004) were associated with poor postoperative CE prognosis.
Prognostic Nomogram for SS
The prognostic nomogram that integrated all variables selected using multivariate Cox regression is shown in Fig. 5. The nomogram illustrated that the age of onset shared the largest contribution to prognosis, followed by deviation angles of strabismus before surgery, surgery amount, age at surgery, deviation angles of strabismus at near 1 week after surgery, and amblyopia. Each subtype within these variables was assigned a score on a point scale. By adding the total score and locating it on the total point scale, we can easily draw a straight line to determine the 1-, 4-, and 8-year survival probabilities.
Nomogram for predicting the 1-, 4- and 8-year survival probability of patients with concomitant esotropia. (Deviation angles = deviation angles of strabismus before surgery, Deviation angles 1wN = deviation angles of strabismus at near 1 week postoperative. To use the nomogram, an individual patient’s value is located on each factor axis, and a line is drawn upward to determine the number of points received for each factor value. The sum of these numbers is located on the total points axis, and a line is drawn downward to the survival axes to determine 1-, 4-, and 8-year survival probability. For deviation angles 1wN, 0 = − 4 to 4 means XT 4 PD to ET 4 PD; 1 = 5 to 9 means ET from 5 to 9 PD; 2 = > 10 means ET more than 10 PD; 3 = − 9 to − 5 means XT from 9 to 5 PD; 4 = <− 10 means XT more than 10 PD)
Validation of the Nomogram
The ROC curves of the training and validation sets are shown in Fig. 6a, b, respectively. The area under the curve was 0.839 for the training set and 0.829 for the validation set. The C-index was 0.84 (95% CI 0.79–0.89) in the training set and 0.80 (95% CI 0.78–0.82) in the validation set. The optimal cutoff points for the total score of the nomogram were 196 and 192 in the training and validation sets, respectively. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio for predicting the 1-year survival rate in the training and validation sets are presented in Table 3.
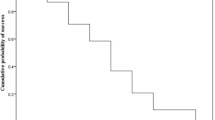
The calibration curves for predicting the 1-year survival probability of patients with CE in the training and validation sets are shown in Fig. 7a, b, respectively. Both curves showed good agreement between the nomogram predictions of the 1-year SS and the actual observations.
Discussion
There are many clinical reports on the outcome and risk factors of CE surgery, and the results of long-term orthotopic rates and related risk factors vary widely. Strabismus specialists need to try and reduce the need for SS due to residual CE or CX after CE surgery. However, no significant clinical attempts have been made to predict the probability of SS for CE. It is necessary to establish a model for predicting the risk of SS for CE so that strabismus specialists can identify high-risk patients early and adjust the treatment plan appropriately in the early stage to reduce the occurrence of SS for CE.
In this study, we attempted to develop and validate a nomogram for predicting the risk of SS for CE. We identified six independent predictors, namely amblyopia, age at surgery, age at onset, deviation angles, surgery amount, and deviation angles 1 week postoperative. To use the nomogram, an individual patient’s value is located on each factor axis, and a line is drawn upward to determine the number of points received for each factor value. The sum of these numbers is located on the total points axis, and a line is drawn downward to the survival axes to determine 1-, 4-, and 8-year survival probability. In t his study, the 1-, 4-, and 8-year orthotopic survival rates of the training set were 97.43%, 90.53%, and 86.32%, respectively. The SS rates were 2.57%, 9.47%, and 13.68%, respectively. We can tell that the orthotopic rate of esotropia surgery is high and the SS rate is low. The predictive scores of most cases are lower than 175 points, and the risk of SS is very small. Only a few patients with predictive score greater than 180 points have the risk of SS. For example, a case with a score of 130 is unlikely to require an SS.
Han and colleagues [4] reviewed 54 cases of CE who developed CX after bilateral MR recession and used a linear mixed model to analyze whether this group of cases had amblyopia, DVD, refractive error, and IOOA before surgery. The results suggest that amblyopia and DVD before CE surgery are risk factors for developing CX postoperatively. Burke [9] studied acquired CE with deviation angles of the distance-near disparity greater than 10 PD and believed that timely and adequate refractive correction and amblyopia treatment were the key factors for the success of the surgery. Lembo and colleagues [10] pointed out that accommodative CE is the most common form of CE in children, and some patients with accommodative CE often have amblyopia at the same time. They agreed that treating amblyopia first helps establish binocular visual function and stable ocular alignment after strabismus surgery. They believe that amblyopia and high hyperopia are the most common risk factors for developing CX after CE surgery and that amblyopia was found to be a predictor of SS for CE. To obtain stable results we also recommend that amblyopia should be cured as much as possible before surgery.
Mohan and Sharma [11] reviewed the long-term effects of 47 cases of partial refractive accommodative esotropia surgery followed up for at least 10 years; the success rate of surgery was 49%. The age of onset was older (2.87 ± 1.31 years), the duration of strabismus was shorter, the patients maintained good ocular alignment, had better stereopsis, and there was no statistically significant difference in refraction between cases with CX and those with optimal alignment. Mohan and Sharma believed that the effect of refraction on postoperative ocular alignment stability was not significant, but younger age of onset was a risk factor for poor surgical prognosis. Wan and colleagues [3] reported the long-term effect of infant CE surgery, noting the overall success rate of the treatment was 23%, mainly as a result o the young age of onset, both within 6 months after birth. In this study, age of onset was one of the predictors and was the factor making the highest relative contribution to the risk score. The younger the age of onset, the higher the risk score for SS. CE in infants is also known as congenital CE and can be diagnosed at birth. It is difficult to obtain good binocular visual function, even if it is maintained in an optimal postoperative alignment. However, the long-term SS rate is high and requires special attention.
In this study, age at the time of surgery was also a predictor of SS. The younger the age at surgery, the lower the score; and the older the age at surgery, the higher the score and the greater risk of SS. Bhate and colleagues [12] summarized the results of a study on the timing of CE surgery in infants, collecting data including demographics, clinical presentation, duration of strabismus, complications, and surgical outcomes. They believe that very early surgery, i.e., surgery within 6 months after the onset of infant CE, was beneficial to the infant’s cortical development, the establishment of binocular vision, and the maintenance of ocular alignment stability. In a recent study by Yagasaki and colleagues [13], compared with early (9–24 months) or late (more than 24 months) surgery, very early surgery (less than 8 months) can achieve better binocular vision. Muz and Sanac [14] recently followed up 79 infant patients with CE for more than 60 months and found that 37% had measurable stereopsis postoperatively at 6–11 months, while in late (11–17 months) and very late groups (18–27 months) it was 3.8%. Arslan et al. [15] reported that early surgery for infant CE helped reduce the severity and incidence of DVD. They noted that the DVD incidence was twice as high in the late surgery group than in the early surgery group (6–24 months). In conclusion, early surgery can contribute to better stereopsis and reduce the incidence and severity of postoperative DVD and IOOA.
Chen and colleagues [6] believed that it was easier to obtain good ocular alignment and stereopsis in early CE surgery, and the eye position at 1 month after surgery may be used to predict the final eye position and stereopsis results. The results of this study showed that the near-eye position 1 week postoperatively could be used to predict the long-term eye position. In this study, some data on the 1-day eye position were missing, which may be due to the obvious discomfort of the young children on the first day after surgery and their unwillingness to open their eyes to cooperate with the examination. The results of the 1-week eye position were accurate and complete in our study and were divided into five grades, with the highest PD score of deviation angles less than − 10 PD, suggesting that exophoria should be avoided as much as possible after CE surgery once there was a high probability that CX will develop. Once established at greater risk of SS at 1-week post operation, these patients were then reviewed more frequently to prevent recurrence versus those who were at lesser risk. We will shorten the interval of follow-up, and adopt the corresponding methods as much as possible to prevent further expansion of the deviation angles and avoid SS. For example, for some cases of partially accommodative esotropia, if the exotropia of − 5 PD to − 9 PD occurs 1 week after the operation, the degree of hyperopia glasses should be reduced, and if there is still a large esotropia, the degree of hyperopia glasses should be increased. These can reduce the incidence of SS to a certain extent.
The surgery amount was also one of the predictors in the nomogram established in this study. The greater the surgery amount, the higher the score and the increased risk of long-term development of CX. Some doctors believe that bilateral MR recession was the first choice for CE in children, and even for large-angle CE, it was recommended to choose bilateral MR recession for the first surgery to reduce the risk of developing CX [16].
Deviation angles were also a predictor in the predictive model established in this study. Larger deviation angles have a lower score and a lower risk of SS. The predictors in this study showed that the risk of SS is high with small deviation angles and with large surgical amount, indicating that surgery for esotropia should be relatively conservative in surgical design. In the case of small deviation angles for esotropia, more attention should be paid to the conservative surgery amount. In clinical practice for large deviation angle esotropia, doctors are obviously more careful not to make the surgical amount too large, but it is easy to relax vigilance for small-angle esotropia. Bachar Zipori and colleagues [5] reviewed 171 failed cases of bilateral MR recession in CE and concluded that esotropia greater than 60 PD was an important risk factor for SS. Rajavi and and colleagues [17] observed 157 cases of CE, 89 cases with deviation angles within 10 PD postoperative, 68 cases with deviation greater than 10 PD or a history of reoperation, and considered CE esotropia greater than 30 PD as a risk factor for SS. Yabas Kiziloglu et al. [18] reviewed the data of 62 infants with CE who underwent surgery and were followed up for at least 2 years. The success rate of one surgery was 56.3%, and 38.7% of the 27 patients required an SS. Cases with larger preoperative deviation angles had residual CE postoperatively, which was in contrast to the results of previous studies. However, considering the small sample size of this study cohort, selection bias may have contributed to this result.
In addition to the six risk predictors screened in this study, there were many other potential risk factors for CE surgery. Many authors [19,20,21] have discussed potential factors, such as the distance-near disparity of deviation angles, whether it was accompanied by signs such as IOOA and DVD, whether vertical strabismus was performed at the same time, the time of wearing glasses before surgery, the duration of strabismus, the surgical method, anisometropia, and insufficient vergence function. However, the results of these factors are quite different in different studies. Burke [9] also pointed out that since many articles on the effect of CE surgery are retrospective, there are large differences in the sample size, follow-up time, case classification, and observation indicators, which affect the reliability of the results. For example, many articles do not classify esotropia subtypes and do not consider whether different subtypes have significantly different treatment outcomes. This was also why there was a lack of risk prediction models for CE surgery, and this study was only a preliminary attempt. In the future, more large-sample, prospective clinical studies of esotropia classification into subtypes are needed to obtain more reliable factors that can help predict surgical effects.
This study has attempted to establish a nomogram for predicting the risk of SS after CE surgery and has a number of strengths. First, the scores of most predictors were consistent with the results of the current articles, indicating that the scores were representative. Second, we performed an internal validation of the nomogram, and the discrimination and precision of the nomogram were better, indicating that our nomogram was less dependent on samples. In addition, the sensitivity and specificity of the model were above 80%, which has reference value for clinical practice.
However, our study also has several limitations. First, all patients were seen at one hospital. We randomized the patients into two subgroups, two-thirds for nomogram construction and one-third for nomogram validation, in the absence of an external cohort. Although this was a generally accepted method of nomogram building and validation, external validation from different medical institutions is still the preferred method. Second, there were missing data for stereopsis as a result of the retrospective cohort study design, which may have resulted in selection bias. Third, not all potential prognostic factors were included in the nomogram, so it cannot make accurate predictions. Although deviation angles were included in the nomogram as a prognostic factor, the scoring results differed from those in other studies, which may result in less accurate predictions.
Conclusion
We developed and validated an easy-to-use nomogram for predicting the risk of SS for CE. This nomogram may help guide the surgical design and help strabismus specialists better explain the surgical risks to patients and their families. Replication of our results and independent validation of the nomogram in a larger cohort are required before it can be applied to daily practice.
References
Pi LH, Chen L, Liu Q, et al. Prevalence of eye diseases and causes of visual impairment in school-aged children in Western China. J Epidemiol. 2012;22(1):37–44.
Chia A, Dirani M, Chan YH, et al. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Invest Ophthalmol Vis Sci. 2010;51(7):3411–7.
Wan MJ, Chiu H, Shah AS, Hunter DG. Long-term surgical outcomes for large-angle infantile Esotropia. Am J Ophthalmol. 2018;189:155–9.
Han SY, Han J, Rhiu S, Lee JB, Han SH. Risk factors for consecutive exotropia after esotropia surgery. Jpn J Ophthalmol. 2016;60(4):333–40.
Bachar Zipori A, Spierer O, Sherwin JC, Kowal L. Why bilateral medial rectus recession fails? Factors associated with early repeated surgery. Int Ophthalmol. 2020;40(1):59–66.
Chen YW, Lin SA, Lin PW, Huang HM. The difference of surgical outcomes between manifest exotropia and esotropia. Int Ophthalmol. 2019;39(7):1427–36.
Li B, Sharan S. Post-operative analysis of pediatric esotropia associated with high hypermetropia. BMC Ophthalmol. 2019;19(1):140.
Jiang D, Han D, Zhang J, Pei T, Zhao Q. Clinical study of the influence of preoperative wearing time on postoperative effects in children with partially accommodative esotropia. Medicine (Baltimore). 2018;97(19):e0619.
Burke JP. Distance-near disparity esotropia: can we shrink the gap? Eye (Lond). 2015;29(2):208–13.
Lembo A, Serafino M, Strologo MD, et al. Accommodative esotropia: the state of the art. Int Ophthalmol. 2019;39(2):497–505.
Mohan K, Sharma SK. Long-term motor and sensory outcomes after surgery for the nonaccommodative component of partially refractive accommodative esotropia. J AAPOS. 2018;22(5):356–60.
Bhate M, Flaherty M, Martin FJ. Timing of surgery in essential infantile esotropia - what more do we know since the turn of the century? Indian J Ophthalmol. 2022;70(2):386–95.
Yagasaki T, Yokoyama Y, Tsukui M. Relationship between stereopsis outcome and timing of surgical alignment in infantile esotropia. J AAPOS. 2020;24(2):78.e1–e5.
Muz OE, Sanac AS. Effects of surgical timing on surgical success and long-term motor and sensory outcomes of infantile esotropia. J Pediatr Ophthalmol Strabismus. 2020;57(5):319–25.
Arslan U, Atilla H, Erkam N. Dissociated vertical deviation and its relationship with time and type of surgery in infantile esotropia. Br J Ophthalmol. 2010;94(6):740–2.
Wallace DK, Christiansen SP, Sprunger DT, et al. Esotropia and exotropia preferred practice Pattern®. Ophthalmology. 2018;125(1):P143–83.
Rajavi Z, Ferdosi AA, Eslamdoust M, et al. The prevalence of reoperation and related risk factors among patients with congenital esotropia. J Pediatr Ophthalmol Strabismus. 2013;50(1):53–9.
Yabas Kiziloglu O, Ziylan S, Simsek I. Long term motor and sensory outcome after surgery for infantile esotropia and risk factors for residual and consecutive deviations. Semin Ophthalmol. 2020;35(1):27–32.
Bryselbout S, Promelle V, Pracca F, Milazzo S. Clinical and surgical risk factors for consecutive exotropia. Eur J Ophthalmol. 2019;29(1):33–7.
Lee D, Kim WJ, Kim MM. Surgical outcomes and occurrence of associated vertical strabismus during a 10-year follow-up in patients with infantile esotropia. Indian J Ophthalmol. 2021;69(1):130–4.
Rajavi Z, Norouzi S, Sabbaghi H, Yaseri M, Abdi S, Faghihi M. The effect of inferior oblique muscle weakening on horizontal alignment. J Curr Ophthalmol. 2019;31(3):298–304.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fees were funded by the authors.
Author Contributions
Conception and design: Haihua Liu, Yiwen Cao, Jinfang Wu; Data collection: Haihua Liu, Ruiying Li; Analysis and interpretation: Haihua Liu, Yiwen Cao; Overall responsibility: Haihua Liu, Jinfang Wu.
Disclosures
All named authors confirm that they have no proprietary or commercial interest in any materials discussed in this article.
Compliance with Ethics Guidelines
Human subjects were included in this study. The human ethics committees at the Peking University First Hospital approved the study (PN:2022–331). All research adhered to the tenets of the Declaration of Helsinki. The institutional review board waived the requirement for informed consent owing to the retrospective nature of the study.
Data Availability
Data from this study are available upon reasonable request to the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, H., Cao, Y., Li, R. et al. Development and Validation of a Nomogram for Predicting Second Surgery in Patients with Concomitant Esotropia. Ophthalmol Ther 11, 2169–2182 (2022). https://doi.org/10.1007/s40123-022-00573-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00573-0