Abstract
Introduction
The purpose of this study was to investigate the efficacy and safety of consecutive use of 1% and 0.01% atropine compared with 0.01% atropine alone over 1 year.
Methods
A total of 207 participants aged 6–12 years with myopia of − 0.50 to − 6.00 D in both eyes were enrolled in this randomized, controlled, non-masked trial and randomly assigned (1:1) to groups A and B. Group A received 1% atropine weekly and were tapered to 0.01% atropine daily at the 6-month visit, and group B received 0.01% atropine daily for 1 year.
Results
Of the 207 participants, 109 were female (52.7%) and the mean (± standard deviation) age was 8.92 ± 1.61 years. Ninety-one participants (87.5%) in group A and 80 participants (77.7%) in group B completed the 1-year treatment. Group A exhibited less refraction progression (− 0.53 ± 0.49 D vs. − 0.74 ± 0.52 D; P = 0.01) and axial elongation (0.26 ± 0.17 mm vs. 0.36 ± 0.21 mm; P < 0.001) over 1 year compared with group B. The changes in refraction (− 0.82 ± 0.45 D vs. − 0.46 ± 0.35 D; P < 0.001) and axial length (0.29 ± 0.12 mm vs. 0.17 ± 0.11 mm; P < 0.001) during the second 6 months in group A were greater than those in group B, with 72.5% of participants presenting refraction rebound. No serious adverse events were reported.
Conclusions
The 1-year results preliminarily suggest that consecutive use of 1% and 0.01% atropine confers an overall better effect in slowing myopia progression than 0.01% atropine alone, despite myopia rebound after the concentration switch. Both regimens were well tolerated. The long-term efficacy and rebound after the concentration switch and regimen optimization warrant future studies to determine.
Trial Registration Number
Clinical Trials.gov PRS (Registration No. NCT03949101).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Consecutive use of 1% and 0.01% atropine confers an overall better effect in slowing refraction progression and axial elongation than 0.01% atropine alone over 1 year. |
Consecutive use of 1% and 0.01% atropine leads to myopia rebound after the concentration switch. |
Both regimens are tolerated. |
The long-term efficacy and rebound after the concentration switch and regimen optimization warrant future studies to determine. |
Introduction
Myopia has attracted public attention as a result of its rapidly rising trend [1]. Irreversible damage to visual acuity (VA) occurs once myopic maculopathy develops and progresses [2]. Atropine is an emerging therapy for myopia control; however, the optimal concentration and treatment strategy are yet to be defined. The efficacy, side effects, and rebound after atropine cessation occur in a dose-dependent manner, raising questions about the optimal balance between efficacy and safety [3,4,5]. Low-dose atropine has attracted widespread attention owing to its promising treatment-to-side effect ratio. However, the optimal concentration of atropine remains controversial, with 0.01% atropine being suggested in the Atropine for the Treatment of Myopia 2 (ATOM2) study [4] and 0.05% atropine being suggested in the Low-concentration Atropine for Myopia Progression (LAMP) study [6]. Reducing the frequency of 1% atropine has been proposed to render fewer side effects (discomfort, 1.2%; near blurred vision, 0.6%) [7] than daily use (discomfort, 4.5%; near blurred vision, 1.0%) [4]. In the first 6-month results of the Atropine for Children and Adolescent Myopia Progression (ACAMP) study, participants treated with weekly 1% atropine presented less myopia progression than those treated with daily 0.01% atropine, with endurable side effects [8]. In an attempt to further reduce the side effects, children who originally received 1% atropine were switched to 0.01% atropine daily for a further 6 months.
Previous studies have reported myopia rebound after cessation of moderate-to-high concentrations of atropine (0.1–1%) with daily use [3, 9, 10], which was prevented by tapering the dosage [11, 12]. However, it remains unclear whether reducing the frequency of 1% atropine to once per week (which is close to the atropine concentration of daily 0.1%) and subsequently tapering the dose to 0.01% would alleviate myopia rebound. Our previous study has demonstrated a reduction in lens power after using 1% atropine, which was closely associated with the change in spherical equivalent (SE) [13]. However, how lens power changes after cessation of 1% atropine and its correlation with myopia rebound remain to be investigated.
We conducted the ACAMP study to explore the efficacy and safety of consecutive use of 1% and 0.01% atropine over 1 year compared with 0.01% atropine alone. We also explored myopia rebound after switching from weekly 1% atropine to daily 0.01% atropine.
Methods
Study Participants
The ACAMP study was conducted from May 2019 to August 2020 at the Shanghai Eye Disease Prevention and Treatment Centre, China. The study design has been previously described [8]. In brief, 207 children aged 6 to 12 years with myopic refraction of at least − 0.5 diopter (D) and astigmatism of less than − 2.0 D in both eyes were enrolled in this randomized clinical trial. After exclusion of those with ocular diseases or severe systemic diseases, previous use of myopia interventions such as atropine, pirenzepine, or orthokeratology lens, or allergy to atropine and cyclopentolate, children were randomly assigned to two treatment groups in a ratio of 1:1: group A received 1% atropine sulfate eye gel (Dishan, Shenyang Xingqi Eye Hospital Co., Ltd. Shenyang, China) once weekly in both eyes for 6 months (starting with 1-week loading dose: 1% atropine once daily in both eyes) and were switched to 0.01% atropine (Myopine, Shenyang Xingqi Eye Hospital Co., Ltd. Shenyang, China) once nightly in both eyes for another 6 months; group B received 0.01% atropine sulfate eyedrops once nightly in both eyes throughout 1 year. All participants were followed at 1, 12, 24, and 48 weeks. On account of COVID-19 pandemic, 36-week follow-up visit was canceled, and 48-week follow-up visits were delayed by 3–4 weeks in 16 participants (9.4%).
Written informed consent forms were obtained from the participants and their parents or guardians. The study protocol was approved by the Ethics Committee of Shanghai General People’s Hospital, Shanghai, China (Approval number 201939), and registered at the Clinical Trials.gov PRS (Registration No. NCT03949101). All procedures were conducted in accordance with the tenets of the Declaration of Helsinki.
Sample Size
The sample size calculation of ACAMP study has been previously described [8]. Briefly, the estimated myopia progression rate for group A was assumed to be − 0.37 D/year, which was averaged from the myopia progression rate for daily 0.1% atropine in the ATOM2 study (which was close to an atropine concentration of weekly 1% atropine, − 0.31 ± 0.50 D/year) [4] and 75% of the myopia progression rate for daily 0.01% atropine in the LAMP study (− 0.59 ± 0.61 D/year) [5]. The estimated myopia progression rate for group B was set to − 0.59 D/year based on the result of daily 0.01% atropine in the LAMP study [5]. The within-group standard deviation was assumed to be 0.6 D [5]. To detect a mean difference of at least 0.5 D between treatment groups [5], 158 participants (79 per group) could achieve 90% power at a significance level of 0.05. By factoring in an attrition rate of 20%, this study required 198 subjects (99 per group).
Randomization
Eligible participants were randomly allocated to two treatment groups (1:1) on the basis of computer-generated randomization sequences with a block of six by a statistician using Statistical Analysis System (v. 9.3; SAS Institute, Cary, NC, USA), according to the priority order the children completed the baseline examination. Masking could not be carried out in this study because of the different usage between two treatment groups.
Study Procedures
Examination procedures were performed by fixed optometrists who were masked to treatment groups, as previously described [8]. Briefly, ophthalmic parameters collected at each visit included intraocular pressure using a non-contact tonometry (NT-1000; Nidek, Tokyo, Japan), best-corrected distance VA in the logarithm of the minimum angle of resolution, near VA under best-corrected distance spectacle correction at 40 cm, and near point of accommodation with best-corrected distance spectacle correction. The accommodation amplitude was calculated as the inverse of the near point of accommodation. After exclusion of the contradictions of cycloplegia, one drop of topical 0.5% proparacaine (Alcaine; Alcon, Fort Worth, TX, USA) was administered in both eyes, followed by two drops of 1% cyclopentolate (Cyclogyl; Alcon, Fort Worth, TX, USA) at a 5-min interval. A third drop of cyclopentolate was given 45 min later if the pupillary light reflex was still present or the pupil size was less than 6.0 mm. Further drops of cyclopentolate were administered if necessary. Autorefraction was performed using an autorefractor (Topcon KR 800; Optical Corp., Guangdong, China). Axial length (AL), anterior chamber depth, lens thickness, central corneal thickness, corneal power, and pupil size were measured using an IOL-Master 700 (Carl Zeiss Meditec AG, Jena, Germany).
Parents or guardians were in charge of drug administration and were required to keep medication diaries. The compliance level was classified according to the mean number of atropine uses per week. Participants with a medication adherence percentage of 80% or greater (i.e., 5.6 days/week) were considered to have good compliance and were included in the analysis [14]. Photochromatic glasses and presbyopic glasses (reading add) were provided if children experienced glare or had difficulty with near vision. Children and parents were free to report any other side effects, illness, and hospitalization during treatment. Any adverse events, regardless of whether they were related to the use of atropine, were documented.
Outcomes
The primary outcome was the change in SE over 1 year. The secondary outcomes included the changes in AL and lens power over 1 year; the changes in SE, AL, and lens power during the second 6 months; parameters associated with side effects such as the changes in accommodation amplitude, pupil size, distance VA, and near VA over 1 year and during the second 6 months. SE was calculated as the spherical power plus half of the cylindrical power. The lens power was calculated using Bennett’s equation [15]. Participants with spherical equivalent progression at least − 0.5 D over 1 year were defined as progressors; otherwise, nonprogressors.
Statistical Analysis
All data were analyzed on the basis of the intention-to-treat principle. The baseline characteristics were described as mean ± standard deviation or proportion. Changes in ocular parameters were calculated by the difference between the baseline visit and the designated follow-up visit. Chi-squared test was used to test the group differences in categorical data and Student’s t test for continuous data. The differences in ocular parameters between the baseline and each follow-up visit were analyzed using the paired t test. A generalized estimating equation model with robust standard errors for longitudinal data analysis was used to compare the different changes in ocular parameters over time with adjustment for age and sex, accounting for repeated measurements [16]. To explore the potential risk factors for progressors over 1 year in both groups and for spherical equivalent rebound during the second 6 months in group A, multiple log-binomial regression analysis was performed. Statistical analyses were performed using Statistical Analysis System. A two-sided P value less than 0.05 was considered statistically significant. Since the SE (r = 0.87, P < 0.001), AL (r = 0.97, P < 0.001), and lens power (r = 0.92, P < 0.001) of the right and left eyes at baseline were highly correlated; only the right eyes were involved in the analysis.
Results
General Characteristics of Participants
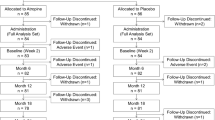
Between March 2019 and April 2020, a total of 240 children were initially assessed. Sixteen of them did not meet the inclusion criteria, 12 had a history of atropine use, and 5 declined to participate. Thus, 207 children were enrolled and randomized into groups A and B (Fig. 1). There was no significant difference between the two groups in terms of demographics, ocular parameters, and parental myopia (Table 1). Finally, 91 participants (87.5%) in group A and 80 participants (77.7%) in group B completed 1 year of treatment with good compliance (Fig. 1), with no significant difference in the dropout rate between the two groups (P = 0.07). The baseline characteristics of these 171 participants and of the 36 participants who did not complete 1 year of follow-up were similar (Table 1).
Changes in SE, AL, and Lens Power Over 1 Year
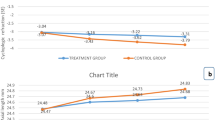
At the end of 1 year, group A presented less refraction progression (− 0.53 ± 0.49 D) and axial elongation (0.26 ± 0.17 mm) than group B (− 0.74 ± 0.52 D and 0.36 ± 0.21 mm, respectively) (both P < 0.05; Table 2, Fig. 2, and Supplementary Table 1). Over 1 year, 15.3% of the participants presented no SE progression, 25.3% of the participants progressed less than 0.5 D, 40.7% progressed between 0.5 and 1.0 D, and 18.7% progressed at least 1.0 D in group A. In group B, the progression rates were 5.0%, 26.3%, 32.5%, and 36.2%, respectively (Fig. 3). The mean 1-year change in lens power in group A (− 0.22 ± 0.43 D) was significantly lower than that in group B (− 0.35 ± 0.38 D; P = 0.03; Table 2, Fig. 2, and Supplementary Table 1).
Distribution of mild, moderate, and severe myopia progression over time in two treatment groups. Myopia progression if less than 0.00 D (no progression), between 0.00 D and less than 0.50 D (mild progression), between 0.50 D and less than 1.00 D (moderate progression), and greater than 1.00 D (severe progression)
Comparisons of participants’ demographics and ocular parameters between nonprogressors and progressors were performed in each group (Supplementary Table 2). Nonprogressors were significantly older than progressors in group A and group B (both P < 0.05), while other baseline characteristics did not differ significantly between the two groups. In multiple log-binomial regression analyses, there was a 15% lower risk of being progressors with every year of increasing age in both groups (group A: relative risk [RR] 0.854, 95% confidence interval [CI] 0.758 to 0.964; group B: RR 0.855, 95% CI 0.850 to 0.955]; both P < 0.05; Supplementary Table 3).
Comparisons of Changes in SE, AL, and Lens Power During Second 6 Months Versus First 6 Months
In group A, greater changes in SE (− 0.82 ± 0.45 D) and AL (0.29 ± 0.12 mm) were found during the second 6 months compared to the first 6 months (0.29 ± 0.36 D and − 0.03 ± 12 mm, respectively; both P < 0.001). Most participants (74.7%) showed no SE progression during the first 6 months, whereas most participants (81.3%) experienced SE progression of at least 0.50 D during the second 6 months. In group B, the change in SE (− 0.46 ± 0.35 D) during the second 6 months was greater than that during the first 6 months (− 0.28 ± 0.34 D; P < 0.001), whereas the AL elongation was similar between two phases (Table 2, Fig. 2, Supplementary Table 1). More than one-third of participants presented SE progression of at least 0.50 D during the second 6 months (42.5%), which was higher than that during the first 6 months (28.7%; Fig. 3). Smaller reductions in lens power (0.07 ± 0.33 D vs. − 0.29 ± 0.44 D) were observed during the second 6 months compared with the first 6 months in group A (P < 0.001), while the opposite result was found in group B (− 0.24 ± 0.29 D vs. − 0.12 ± 0.38 D; P < 0.001). Compared with the 6- to 12-month changes in group B, group A presented greater SE progression, greater AL elongation, and a smaller reduction in lens power (all P < 0.001; Table 2, Fig. 2, and Supplementary Table 1).
On the basis of the mean SE progression of 0.46 D in group B during the second 6 months, SE progression of greater than 0.5 D when switching from 1% atropine to 0.01% atropine was defined as SE rebound in group A. In group A, 66 participants (72.5%) who presented with SE rebound during the second 6 months were significantly younger and were more often female than the 25 participants (27.5%) who did not present with SE rebound. Other baseline characteristics did not differ significantly between the two groups (Supplementary Table 4). Multiple log-binomial regression analyses showed that the risk of SE rebound decreased by 13% for every year of increasing age (RR 0.871, 95% CI 0.870 to 0.884; P = 0.02) and by 27% for male sex (RR 0.735, 95% CI 0.712 to 1.056; P = 0.04; Supplementary Table 5).
Adverse Events and Changes in Associated Ocular Parameters
In group A, the accommodation amplitude significantly decreased over 1 year (− 1.54 ± 2.94 D, P = 0.001), whereas pupil size did not change significantly. Opposite changes in accommodation amplitude and pupil size were observed during the second 6 months compared with during the first 6 months (both P < 0.001). The changes in accommodation amplitude (− 1.55 ± 3.11 D) and pupil size (− 0.39 ± 1.01 mm) in group B were comparable to those in group A. The distance VA and near VA did not change significantly over 1 year in either group (Table 2 and Supplementary Table 5).
At the 1-year visit, 1.1% and 2.5% of participants reported photophobia in groups A and B, respectively, with no need for photochromic glasses. No near blurred vision was reported. Over the 1-year period, allergic conjunctivitis was reported in one participant in group A and two participants in group B, dizziness occurred in two participants in group B, rubella was reported in one participant from group B, and nose bleeding occurred in two participants in group A and in one participant in group B.
Discussion
To the best of our knowledge, this study is the first randomized, controlled, non-masked trial to contrast the efficacy and safety of consecutive use of 1% atropine and 0.01% atropine with 0.01% atropine alone. However, limited by its 1-year follow-up, the clinical relevance of this consecutive-use regimen cannot be determined from this trial. Thus, future studies should be conducted to determine the long-term efficacy and rebound after the concentration switch and to optimize the regimen.
Efficacy and Safety of the Two Treatment Regimens
Children in the 0.01% atropine group showed a refraction progression of − 0.74 D and an axial elongation of 0.36 mm after 1-year treatment, which were comparable with the progression rate in the placebo group (− 0.76 to 0.81 for SE; 0.38 to 0.46 mm per year for AL) [4, 17,18,19]. Compared with other interventions, 0.01% atropine had a worse inhibitory capacity of myopia progression than orthokeratology (0.16–0.27 mm for AL [20, 21]), multifocal spectacle/contact lenses (− 0.38 D for SE [22]; 0.09–0.11 mm for AL [22, 23]), or low-level red-light therapy (− 0.20 D for SE; 0.13 mm for AL) [19]. Therefore, 0.01% atropine might not be a suitable strategy for young children with myopia who experience greater myopia progression in the natural course [24] and who are at a high risk of high myopia in adulthood [25]. Instead, a higher concentration of atropine should be considered.
In this study, consecutive use of 1% and 0.01% atropine presented an overall better control in both SE (− 0.53 D) and AL (0.26 mm) than 0.01% atropine only. There were a hyperopic shift and a reduction in AL over the first 6 months, which were mainly caused by lens power loss and choroidal thickening, respectively, based on our previous research [8, 13]. However, the myopia rebound after the concentration switch was non-negligible, leading to an overall worse capacity of myopia control compared with other interventions mentioned above [19,20,21,22,23]. Weekly treatment with 1% atropine has been proved to be endurable for most participants [8], and the pupil enlargement and impaired near vision caused by 1% atropine recovered after the concentration switch. The incidences of photophobia and near blurred vision in both groups were similar to those in the 0.01% atropine group in the LAMP study (2.1% and 1.8%, respectively) [5], confirming the safety of this regimen.
Myopia Rebound When Switching from Weekly 1% Atropine to Daily 0.01% Atropine
In the ATOM [9] and ATOM2 [10] studies, SE progression values during the first 6 months after cessation of daily 1% atropine and 0.1% atropine were − 0.76 D and − 0.34 D, respectively. These values were 3.75 times and 1.70 times the change in the placebo groups (− 0.20 D), respectively. AL elongation values in the first 6 months after cessation of daily 1% atropine and 0.1% atropine were 0.15 mm and 0.17 mm, respectively. These values were 2.14 times and 2.43 times the change observed in the placebo groups (0.07 mm), respectively. In the present study, however, the changes in SE and AL during the second 6 months in group A (− 0.82 D and 0.29 mm, respectively) were 1.78 times and 1.70 times greater than those in group B (− 0.46 D and 0.17 mm, respectively). These results indicate that the SE rebound after the concentration switch is less than that after cessation of daily 1% atropine, but comparable to that after cessation of daily 0.1% atropine. AL rebound after the concentration switch is less than that after cessation of 1% or 0.1% atropine. Notably, children in the 0.01% atropine group in this study presented greater myopia progression than those in the placebo group in the ATOM study [9], probably as a result of the greater length of time spent on near work than that when the ATOM study was conducted (more than 10 years ago). This might explain the numerically greater SE and AL rebound in our study than in the 1% atropine group in the ATOM study [9]. Polling et al. observed no AL rebound among children with myopia after initial treatment with 0.5% atropine for 1 year followed by tapering to 0.25%, and further to 0.1% and 0.01% every 6 months [11]. These results suggest that a better way to taper the dose of weekly 1% atropine to reduce myopia rebound is worthy of investigation.
The true mechanism underlying myopia rebound remains unrevealed. Muscarinic agonists activated human and mouse scleral fibroblast cell proliferation via MEK-ERK-MAPK cascade, which could be abolished by atropine in a concentration-dependent manner [26]. Also, muscarinic receptors in the sclera could be regulated after atropine treatment, associated with the concentration of atropine [27, 28]. Thus, the scleral proliferation could be influenced by atropine concentration switch through changing cascade reaction and receptor density in the sclera, which might partly explain the AL rebound. Lens power showed a marked decrease during the first 6 months (− 0.29 ± 0.44 D), but it did not change among children without SE rebound after switching to 0.01% atropine (− 0.05 ± 0.26 D) and significantly increased among children with SE rebound (0.12 ± 0.34 D). These findings suggest that lens power might lose its compensation ability for axial elongation [29] after the concentration switch, and even rebound to promote myopic shift. Theoretically, an increase in AL of 1 mm would lead to an average of approximately 3.0 D of myopia without counterbalancing by a change in lens power. Thus, the unchanged or even increased lens power after the concentration switch might partly explain the SE rebound in the present study and the mismatch between SE rebound (− 0.76 D) and AL rebound (0.15 mm) during the first 6-month cessation period in the ATOM study [9].
In the present study, the adjusted analysis revealed that the risk of SE rebound after concentration switch decreased by 13% for every year of increasing age and by 27% for male sex. However, the underlying reasons for younger age and female sex being risk factors for myopia rebound remain unclear. Young age [24, 25] and sex [30, 31] have been revealed to be associated with myopia progression. Possible explanations include heredity [32], different stages of puberty between sexes [33], and social factors (e.g., education [34], differential engagement in outdoor and nearwork activities [35]), which might also involve in the process of myopia rebound. In addition, the levels of growth hormone [36] and estrogen [37, 38] might influence the myopia rebound by regulating the muscarinic receptor activity or density and subsequently affecting the reactivity of sclera to atropine treatment and cessation. Therefore, these possible risk factors are worthy of investigation to reveal the mechanisms.
Limitations
The present study has several limitations. First, 1-year follow-up period may not be enough to provide sufficient information on the efficacy and safety of weekly 1% atropine, as well as the rebound after cessation. Second, previous studies clearly showed myopia rebound of moderate-to-high concentration of atropine [9, 10], rendering complete cessation of 1% atropine unethical; therefore, the myopia rebound in the present study was not strictly evaluated since participants continued with 0.01% atropine. Other limitations include lack of a placebo-controlled trial, the possible inaccuracy of the eye drop administration from the medication diaries, measurement of pupil size under normal circumstances (estimated to 150 lx), and omission of other known risk factors of myopia such as the onset of education, time for outdoor activities and nearwork, which have been mentioned in the previous study [8].
Conclusions
The 1-year results of the ACAMP study show that consecutive use of 1% and 0.01% atropine confers an overall better efficacy than 0.01% atropine alone in children with low-to-moderate myopia, despite myopia rebound after the concentration switch. Both regimens were well tolerated without serious adverse events. Although the clinical relevance of consecutive-use regimen cannot be determined from our trial, this study gives clinicians some preliminary inspiration that a consecutive regimen is an alternative way to achieve good myopia control and high myopia prevention when children with myopia experience rapid progression and a poor response to a low concentration of atropine, warranting future studies to evaluate the long-term efficacy and rebound after the concentration switch and to optimize the regimen.
References
Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102:855–62.
Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135:624–30.
Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119:347–54.
Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24.
Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127:910–9.
Foo LL, Htoon H, Farooqui SZ, Chia A. Part-time use of 1% atropine eye drops for prevention of myopia progression in children. Int Ophthalmol. 2020;40:1857–62.
Ye L, Shi Y, Yin Y, et al. Effects of atropine treatment on choroidal thickness in myopic children. Invest Ophthalmol Vis Sci. 2020;61:15.
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–9.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157:451–7(e1).
Polling JR, Tan E, Driessen S, et al. A 3-year follow-up study of atropine treatment for progressive myopia in Europeans. Eye (Lond). 2020;34:2020–8.
Zhu Q, Tang Y, Guo L, et al. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci. 2020;17:176–81.
Ye L, Li S, Shi Y, et al. Comparisons of atropine versus cyclopentolate cycloplegia in myopic children. Clin Exp Optom. 2021;104:143–50.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
Rozema JJ, Atchison DA, Tassignon MJ. Comparing methods to estimate the human lens power. Invest Ophthalmol Vis Sci. 2011;52:7937–42.
Duenas M, Salazar A, Ojeda B, Arana R, Failde I. Generalized estimating equations (GEE) to handle missing data and time-dependent variables in longitudinal studies: an application to assess the evolution of health related quality of life in coronary patients. Epidemiol Prev. 2016;40:116–23.
Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178.
Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104:1535–41.
Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129:509–19.
He M, Du Y, Liu Q, et al. Effects of orthokeratology on the progression of low to moderate myopia in Chinese children. BMC Ophthalmol. 2016;16:126.
Lyu Y, Ji N, Fu AC, et al. Comparison of administration of 0.02% atropine and orthokeratology for myopia control. Eye Contact Lens. 2021;47:81–5.
Lam CSY, Tang WC, Tse DY, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020;104:363–8.
Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of misight lenses for myopia control. Optom Vis Sci. 2019;96:556–67.
Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46:51–7.
Hu Y, Ding XH, Guo XX, Chen YX, Zhang J, He MG. Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a Chinese cohort. JAMA Ophthalmol. 2020;138:1129–34.
Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis. 2009;15:1277–93.
Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic receptors in mouse scleral fibroblasts: prior to and after induction of experimental myopia with atropine treatment. Mol Vis. 2011;17:680–92.
Liu Q, Wu J, Wang X, Zeng J. Changes in muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs. Mol Vis. 2007;13:1234–44.
Iribarren R. Crystalline lens and refractive development. Prog Retin Eye Res. 2015;47:86–106.
Wu JF, Bi HS, Wang SM, et al. Refractive error, visual acuity and causes of vision loss in children in Shandong, China. The Shandong Children Eye Study. PLoS ONE. 2013;8: e82763.
He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–9.
Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI—myopia genetics report. Invest Ophthalmol Vis Sci. 2019;60:M89–105.
Yip VC, Pan CW, Lin XY, et al. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012;53:7961–6.
Bez D, Megreli J, Bez M, Avramovich E, Barak A, Levine H. Association between type of educational system and prevalence and severity of myopia among male adolescents in Israel. JAMA Ophthalmol. 2019;137:887–93.
He X, Sankaridurg P, Wang J, et al. Time outdoors in reducing myopia: a school-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022. https://doi.org/10.1016/j.ophtha.2022.06.024.
Popova J, Robeva A, Zaharieva S. Muscarinic receptor activity change after prolonged treatment with growth hormone and somatostatin. Comp Biochem Physiol C Comp Pharmacol Toxicol. 1990;96:119–23.
Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FM. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor alpha in rat hippocampus. Eur J Pharmacol. 2010;634:192–200.
Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DG. Estrogen therapy and brain muscarinic receptor density in healthy females: a SPET study. Horm Behav. 2007;51:249–57.
Acknowledgements
The authors thank Shanghai Medoo Tech Co., Ltd. for the technological support of this trial.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81703287), Shanghai Health Committee, Clinical Research (Project No. 2019240241), Shanghai Shenkang Hospital Clinical Research Program (Project No. SHDC12019X18, SHDC12020127), National Key R&D Program of China (Project No. 2019YFC0840607), National Science and Technology Major Project of China (Project No. 2017ZX09304010). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; decision to submit the manuscript for publication; and the funding the journal’s Rapid Service Fee, which was funded by the authors.
Author Contributions
Jianfeng Zhu and Jiangnan He had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Xun Xu; Jianfeng Zhu, Jiangnan He. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Luyao Ye, Hannan Xu. Critical revision of the manuscript for important intellectual content: Xun Xu, Jianfeng Zhu, Jiangnan He, Luyao Ye. Statistical analysis: Luyao Ye, Hannan Xu, Ya Shi. Obtained funding: Xun Xu, Jianfeng Zhu, Jiangnan He. Administrative, technical, or material support: Jianfeng Zhu; Jiangnan He; Shanshan Li. Supervision: Xun Xu, Jianfeng Zhu, Jiangnan He.
Disclosures
Luyao Ye, Hannan Xu, Ya Shi, Yao Yin, Tao Yu, Yajun Peng, Shanshan Li, Jiangnan He, Jianfeng Zhu and Xun Xu have nothing to disclose.
Compliance with Ethics Guidelines
Written informed consent forms were obtained from the participants and their parents or guardians. The study protocol was approved by the Ethics Committee of Shanghai General People’s Hospital, Shanghai, China (Approval number 201939), and registered at the Clinical Trials.gov PRS (Registration No. NCT03949101). All procedures were conducted in accordance with the tenets of the Declaration of Helsinki.
Data Availability
The data are available upon request from the corresponding author.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ye, L., Xu, H., Shi, Y. et al. Efficacy and Safety of Consecutive Use of 1% and 0.01% Atropine for Myopia Control in Chinese Children: The Atropine for Children and Adolescent Myopia Progression Study. Ophthalmol Ther 11, 2197–2210 (2022). https://doi.org/10.1007/s40123-022-00572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00572-1