Abstract
Introduction
This study investigated choroidal structural changes on optical coherence tomography (OCT) using choroidal vascularity index (CVI) and choroidal thickness (CT) in patients with Best vitelliform macular dystrophy (BVMD).
Methods
This retrospective case control study included 78 patients with BVMD of different clinical stages and 242 age- and gender-matched healthy controls. Subfoveal OCT scans were analysed. Total choroidal area (TCA), luminal area (LA) and CT were measured after image segmentation and binarization. CVI, a novel marker for choroidal angioarchitecture, was defined as the ratio of LA to TCA. CVI and CT were compared between BVMD and control group, as well as among the BVMD subgroups.
Results
Mean CVI was lower in eyes with BVMD (65.0 ± 3.5%) compared to that in control eyes (67.5 ± 3.9%) and this was statistically significant (p < 0.0001). There was no significant difference in subfoveal CT between BVMD (302.88 ± 81.68 μm) and control (309.31 ± 65.46 μm) eyes (p = 0.4799). In the subgroup analysis, all stages of BVMD showed lower CVI compared to control while SFCT remained similar. Within the BVMD subgroups, CVI and subfoveal CT did not differ significantly and both were not shown to be associated with visual acuity.
Conclusion
Decreased CVI was shown in eyes with BVMD compared to control eyes, while no significant difference in subfoveal CT was seen. CVI may be helpful in the understanding of choroidal pathology in BVMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The pathophysiology of Best vitelliform macular dystrophy (BVMD), the second most common macular dystrophy, is not yet fully understood. |
We aim to investigate choroidal structural changes in various stages of Best vitelliform macular dystrophy using optical coherence tomography-based biomarkers—choroidal vascularity index and choroidal thickness. |
What was learned from this study? |
Eyes in all stages of Best vitelliform macular dystrophy showed decreased choroidal vascularity index compared to healthy control eyes, while there was no significant difference in subfoveal choroidal thickness between the two groups. |
Reduced choroidal vascularity in Best vitelliform macular dystrophy suggested a possible contribution of choroidal ischemia in the pathogenesis of BVMD. |
Choroidal vascularity index may be a more robust biomarker of choroidal angioarchitecture in hereditary macular dystrophy. |
Introduction
Best vitelliform macular dystrophy (BVMD), also known as Best disease, is the second most common macular dystrophy. The reported prevalence of BVMD ranged from 1.5 in 100,000 in Denmark [1] to 1 in 10,000 to 20,000 in the USA [2]. It is typically inherited in an autosomal dominant pattern. The disease-causing gene has been identified as BEST1, which is located on chromosome 11q13 [3]. BEST1 encodes the transmembrane protein bestrophin-1, which is primarily expressed in the retinal pigment epithelium (RPE) [4]. It is localized in the RPE basolateral plasma membrane and functions as an anion channel, a regulator of intracellular calcium signalling as well as an influencer of normal ocular development [5, 6].
Clinical manifestation of BVMD is mainly limited to the posterior pole [6]. The disease course usually progresses through five clinical stages, namely, previtelliform (stage 1), vitelliform (stage 2), pseudohypopyon (stage 3), vitelliruptive (stage 4) and atrophic stage (stage 5) [5]. Most patients with BVMD maintain good functional vision throughout the course of disease. However, choroidal neovascularization (CNV) can occur resulting in poor vision if left untreated. The incidence of CNV was reported to be about 20% in earlier studies [6, 7] and as high as 65% in a more recent study using optical coherence tomography angiography [8]. Macular hole is another rare vision-threatening complication of BVMD [9].
Even though the primary site of involvement in BVMD seems to be in the RPE [10], the pathophysiology is not fully understood. Therefore it is worthwhile to study choroid in BVMD in view of the close structural and functional relationship between RPE and choroid as well as the risk of CNV in BVMD [11]. To date, understanding of choroidal pathology in BVMD is still very limited [12] because of the rarity of the disease, physical inaccessibility of the choroid and the lack of reliable biomarkers.
With the advancement in optical coherence tomography (OCT), especially the use of enhanced depth imaging (EDI), choroidal structure can be assessed accurately and reliably in a non-invasive manner [13]. In addition, choroidal vascularity index (CVI) was developed as a novel biomarker for choroidal angioarchitecture [14]. Compared to the traditional choroidal thickness (CT), CVI takes into account changes in both the vascular and stromal area in the choroid and is proven to be a more robust marker in numerous ocular and systemic conditions [15]. In this study, we aim to investigate choroidal structural changes measured by CVI and CT in eyes with BVMD in different clinical stages by comparing to health controls.
Methods
This was a retrospective cross-sectional study carried out at tertiary eye centres in India and Italy between 2016 and 2018. Patients with BVMD, as well as normal control subjects who were age- and gender-matched, were recruited from all centres. BVMD was diagnosed clinically on the basis of characteristic funduscopic changes except in previtelliform stage, an abnormal electrooculogram (EOG, Arden ratio < 1.5) and the presence of a positive family history with at least one affected first-order relative. Subjects were excluded if they had significant media opacity, refractive error of more than − 6 dioptre spherical equivalent or abnormal fixation e.g. nystagmus. Ethics approval was obtained from the institutional review boards and written informed consent was obtained from each subject. This study adhered to the tenets of Declaration of Helsinki.
All subjects underwent complete ophthalmic examination including best corrected visual acuity measured by Snellen chart, slit-lamp biomicroscopy and dilated fundus examination. Genotype was performed in 40% of the cases. Patients’ demographics data were also recorded.
Image Acquisition
All subjects underwent EDI-OCT scans using either Spectralis (Heidelberg Engineering, Vista, CA, USA), Cirrus (Carl Zeiss Meditec, Dublin, CA) or DRI OCT-1 Atlantis (Topcon Medical Systems, Oakland, NJ) OCT machines. A volume scan of 20° × 20° containing at least 25 B-scans centring at the fovea was obtained for each eye. Individual B-scans had an average of nine frames and were 240 µm apart from the next B-scan.
Image Analysis
One eye from each subject was included in the image analysis. If both eyes from the same subject were eligible and had gradable OCT images, the right eye was chosen for analysis.
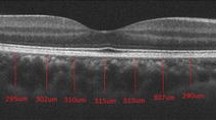
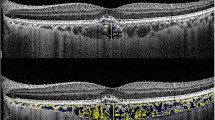
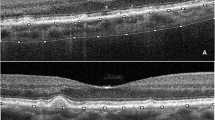
The raster scan passing through the fovea was analysed. Image binarization was performed using methods proposed by Sonoda et al. [16] with modification by our group [14, 17]. The detailed algorithm was described previously. Briefly, images were processed on ImageJ software (version 1.47; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/). The OCT scans were first converted to 8-bit images using ImageJ default setting. This was followed by application of Niblack’s auto local threshold tool to allow demarcation of choroidal luminal area (LA) and stromal area (SA). Then total choroidal area (TCA) was selected using the polygon tool by manual plotting of the upper border marked at the RPE and the lower border marked at the choroid–sclera junction. The entire length of the OCT image was used in the selection of TCA. The image was then converted back to an RGB (red, green, blue) image to allow computation of size of LA by the colour threshold tool. Finally, CVI was calculated as the ratio of LA to TCA.
Choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera.
Image grading was done by one of the authors who was a trained grader and was masked to the patients’ information. Figure 1 shows examples of OCT images before and after binarization.
Statistical Analysis
CVI and subfoveal CT in patients with BVMD and healthy controls were compared using independent sample t test. Subgroup analyses among BVMD of different clinical stages were performed using one-way analysis of variance. Relationship between visual acuity and CVI/subfoveal CT was assessed by Pearson correlation coefficient. Statistical analyses were performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
Results
Seventy-eight patients with BVMD and 242 control subjects were included in this study (approximately 1:3 case to control ratio). The mean age was 37.49 ± 19.08 years in the BVMD group and 36.71 ± 15.81 years in the control group. The proportion of female subjects was 34.6% in the BVMD group and 38.8% in the control group. There were no significant differences in the age and gender between the two groups (Table 1).
Mean CVI in eyes with BVMD was 65.0 ± 3.5% and it was significantly lower compared to that in normal eyes (67.5 ± 3.9%, p < 0.0001) (Table 2). Mean subfoveal CT was 302.88 ± 81.68 μm in the BVMD group and 309.31 ± 65.46 μm in the control group. The difference in subfoveal CT was not statistically significant (p = 0.4799) (Table 2).
The distribution of patients in the BVMD group in terms of clinical staging is shown in Table 3. The number of patients was 6 (7.69%), 24 (30.77%), 8 (10.26%), 20 (25.64%) and 20 (25.64%) in the previtelliform (stage 1), vitelliform (stage 2), pseudohypopyon (stage 3), vitellieruptive (stage 4) and atrophic stage (stage 5), respectively. In the subgroup analysis, all clinical stages of BVMD showed lower CVI compared to control and this was statistically significant in stage 2 to stage 5 (p = 0.0353, 0.032, 0.0068 and 0.0002, for stage 2 to 5, respectively). On the other hand, subfoveal CT values were similar between all stages of BVMD and control group (Table 3).
Within the BVMD subgroups, CVI and subfoveal CT across five stages of disease did not differ significantly (p = 0.6803 and 0.4783, respectively) (Figs. 2, 3). Both CVI and subfoveal CT were also not shown to be associated with visual acuity in patients with BVMD (Pearson correlation coefficient = − 0.078 and − 0.031, respectively) (Figs. 4, 5).
Discussion
In this study, eyes with BVMD were shown to have reduced choroidal vascularity, as measured by a lower CVI, compared to age- and gender-matched healthy controls. In addition, lower CVI was also consistently found in all clinical stages of BVMD. On the other hand, there was no significant difference in subfoveal CT between BVMD and control group. Our results suggested a possible contribution of choroidal ischemia in the pathogenesis of BVMD and supported the role of CVI as a more robust biomarker of choroidal angioarchitecture in this hereditary macular dystrophy.
The understanding of the pathophysiology of BVMD is not fully complete. This is partly due to rarity of the disease and the lack of biospecimen for mechanistic studies at molecular and cellular levels during disease-relevant stages [10]. In spite of these, studies based on in vitro experiments and animal models have generated important insights into BVMD [5]. Bestrophinopathies, a heterogenous group of inherited retinal diseases including BVMD, are caused by mutations in the BEST1 gene [3]. More than 200 different BEST1 mutations have been identified to date [6]. On the molecular and cellular level, these mutations appear to result in one or more of the following abnormalities: mistrafficking of the Best1 protein, defective Best1 oligomerization, attenuation of anion channel activity and abnormal intracellular calcium signaling [5]. On the tissue and organ level, the primary sites of pathology is shown to be in the retinal pigment epithelium and the RPE–photoreceptor interface where there is excessive buildup of lipofuscin, dysfunctional cholesterol metabolism and structural changes compromising RPE–photoreceptor interaction [10].
Study of choroidal structure is meaningful in BVMD because of the structural and functional interdependence of choroid and RPE. Animal studies have shown that the RPE produces vascular endothelial growth factor (VEGF) that supports the choroid, while choroidal vessels nourish the RPE [11]. Choroidal neovascularization is also a known complication of BVMD, which happens in about 20% of patients in the course of the disease [6, 7]. In fact, there is growing interest in understanding choroid changes in ocular diseases where the primary site of pathology is at the level of RPE/photoreceptor, e.g. age-related macular degeneration [18,19,20] and retinitis pigmentosa [21,22,23]. In both cases, choroidal structural remodeling and reduced blood flow were noted, which were supportive of choroidal involvement in the pathogenesis.
Studies on choroidal changes in BVMD are rather limited in the current literature. A single histopathological case report showed marked abnormalities in RPE, photoreceptor and sub-RPE area but a normal choriocapillaris in one elderly patient with BVMD [12]. There was no detailed report on choroidal structural and blood flow assessment using conventional dye angiography or various ultrasound techniques. Advancement in EDI-OCT technology has allowed for accurate measurement of choroidal structure in vivo [13]. Choroidal thickness has been a popular surrogate biomarker for choroidal structural alteration. Battaglia Parodi et al. reported that choroidal thickness varied depending on the disease stage in BVMD, being higher in the vitelliform/pseudohypopyon stage, thinner in the atrophic stage and similar to normal controls in the previtelliform and vitelliruptive stage [24]. This was one of the larger studies in BVMD with 34 patients in the disease arm and 20 in the control arm. Two other studies with a sample size in the single digit range showed reduced choroidal thickness in BVMD [25, 26]. However it was not statistically significant in one study [25] and there was no normal control group in the other [26]. On the other hand, there was one report demonstrating increasing choroidal thickness in BVMD [27]. Of note, it was a single case report of BVMD with choroidal neovascularization and there was no mention of abnormal EOG or genotype in the diagnosis.
CVI is an emerging biomarker for the quantification of vascular alteration in choroid [14, 15, 17]. As it takes into account both the vascular and stromal components of the choroid, it gives additional information compared to the traditional choroidal thickness measurement. A review of the current status of CVI utility has been published elsewhere [15]. In brief, CVI has been validated as a non-invasive, sensitive and robust biomarker in numerous retinal and choroidal diseases. Concerning BVMD, our group previously reported lower CVI in a heterogenous cohort of hereditary retinal dystrophies including one case of BVMD [23]. The result of the current study is in keeping with our previous report with a much larger sample size.
It is debatable whether reduced CVI shown in our study represents choroidal ischemia that contributes to disease pathogenesis; however, this hypothesis is certainly plausible. In a pilot study that evaluated whether sildenafil could treat age-related and vitelliform macular degeneration by reducing choroidal ischemia, one patient with BVMD reported significant visual improvement as well as improvement in photoreceptor and ellipsoid layers on OCT [28]. Numerous studies on the much more common age-related macular degeneration had suggested choroidal ischemia was associated with the development of choroidal neovascularization (CNV) [19, 20, 29, 30]. BVMD is also known to be complicated by CNV and therefore choroidal ischemia might be a common pathogenetic factor in age-related macular degeneration and BVMD.
Our study has a few limitations. Firstly, even though we have included one of the largest BVMD patient cohorts to date, the sample size in each clinical stage remained small because of the rarity of the disease. The lack of association between CVI/CT and disease stage could be real or due to inadequate power. Future study with larger sample size would be required to address this aspect. We were also unable to assess the association between CVI/CT and development of CNV because of a lack of CNV cases. Secondly, CVI was measured only on the subfoveal OCT image. There could be variations in choroidal vascularity in other parts of the macula. Future volumetric CVI analysis may provide more accurate assessment and reduce sampling error. Thirdly, clinical and structural correlation was only performed with visual acuity because of the lack of other clinical data. Association between CVI and markers such as fundus autofluorescence and perimetry would provide a more complete picture of the structural and functional significance of CVI. Data on refractive error was not available for comparison between the two groups but eyes with refractive error of more than − 6 dioptre spherical equivalent were excluded. Fourthly, the presence of vitelliform material in patients with BVMD may cause projection artefact in OCT scans and could theoretically affect CVI assessment. It was difficult to confirm or quantify such an effect based on our image analysis. However, a manual review of the OCT images did not reveal any obvious segmentation or binarization error in patients with BVMD. Lastly, the absolute CVI difference (2.5%) between BVMD and control eyes was small considering the mean CVI in both groups (65% and 67.5%) but it was in a range similar to that reported in other hereditary retinal diseases, e.g. retinitis pigmentosa [22] and Stargardt disease [31]. As CVI was primarily a research tool at this moment, its clinical significance in terms of disease diagnosis and prognosis in BVMD remained to be determined and further studies would be required.
Conclusion
Eyes with BVMD showed reduced CVI and similar CT compared to normal controls. CVI is potentially a more robust biomarker in assessing choroidal structural changes in BVMD. Reduced choroidal vascularity suggested a role of choroidal ischemia in the pathogenesis of BVMD that choroidal vascular changes might contribute to or modify the primary pathology at the RPE–photoreceptor interface. Future research on the longitudinal CVI changes in BVMD and its association with CNV development are warranted.
References
Bitner H, Schatz P, Mizrahi-Meissonnier L, Sharon D, Rosenberg T. Frequency, genotype, and clinical spectrum of best vitelliform macular dystrophy: data from a national center in Denmark. Am J Ophthalmol. 2012;154(2):403-12.e4.
Dalvin LA, Pulido JS, Marmorstein AD. Vitelliform dystrophies: prevalence in Olmsted County, Minnesota, United States. Ophthal Genet. 2017;38(2):143–7.
Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19(3):241–7.
Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2000;97(23):12758–63.
Johnson AA, Guziewicz KE, Lee CJ, et al. Bestrophin 1 and retinal disease. Prog Retin Eye Res. 2017;58:45–69.
Boon CJ, Klevering BJ, Leroy BP, Hoyng CB, Keunen JE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28(3):187–205.
Agarwal A. Gass’ atlas of macular diseases. 5th ed. Philadelphia: Saunders; 2012.
Battaglia Parodi M, Arrigo A, Bandello F. Optical coherence tomography angiography quantitative assessment of macular neovascularization in best vitelliform macular dystrophy. Investig Ophthalmol Visual Sci. 2020;61(6):61.
Liu J, Xuan Y, Zhang Y, Liu W, Xu G. Bilateral macular holes and a new onset vitelliform lesion in Best disease. Ophthalmic Genet. 2017;38(1):79–82.
Guziewicz KE, Sinha D, Gómez NM, et al. Bestrophinopathy: an RPE-photoreceptor interface disease. Prog Retin Eye Res. 2017;58:70–88.
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106(44):18751–6.
Frangieh GT, Green WR, Fine SL. A histopathologic study of Best’s macular dystrophy. Arch Ophthalmol. 1982;100(7):1115–21.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500.
Agrawal R, Salman M, Tan KA, et al. Choroidal vascularity index (CVI)—a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS ONE. 2016;11(1):e0146344.
Agrawal R, Ding J, Sen P, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retinal Eye Res. 2020;2020:100829.
Sonoda S, Sakamoto T, Yamashita T, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55(6):3893–9.
Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090.
McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43(6):1986–93.
Koh LH, Agrawal R, Khandelwal N, Sai Charan L, Chhablani J. Choroidal vascular changes in age-related macular degeneration. Acta Ophthal. 2017;95(7):e597–e601.
Wei X, Ting DSW, Ng WY, Khandelwal N, Agrawal R, Cheung CMG. Choroidal vascularity index: a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. 2017;37(6):1120–5.
Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17(2):175–205.
Tan R, Agrawal R, Taduru S, Gupta A, Vupparaboina K, Chhablani J. Choroidal vascularity index in retinitis pigmentosa: an OCT study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(3):191–7.
Wei X, Mishra C, Kannan NB, et al. Choroidal structural analysis and vascularity index in retinal dystrophies. Acta Ophthalmol. 2019;97(1):e116–21.
Battaglia Parodi M, Sacconi R, Iacono P, Del Turco C, Bandello F. Choroidal thickness in best vitelliform macular dystrophy. Retina. 2016;36(4):764–9.
Chhablani J, Nayaka A, Rani PK, Jalali S. Choroidal thickness profile in inherited retinal diseases in Indian subjects. Indian J Ophthalmol. 2015;63(5):391–3.
Yeoh J, Rahman W, Chen F, et al. Choroidal imaging in inherited retinal disease using the technique of enhanced depth imaging optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1719–28.
Batıoğlu F, Yanık Ö, Demirel S, Çağlar Ç, Özmert E. A case of best disease accompanied by pachychoroid neovasculopathy. Turkish J Ophthalmol. 2019;49(4):226–9.
Coleman DJ, Lee W, Chang S, et al. Treatment of macular degeneration with sildenafil: results of a two-year trial. Ophthalmologica. 2018;240(1):45–54.
Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(1):358–63.
Berenberg TL, Metelitsina TI, Madow B, et al. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32(1):25–31.
Ratra D, Tan R, Jaishankar D, et al. Choroidal structural changes and vascularity index in Stargardt disease on swept source optical coherence tomography. Retina. 2018;38(12):2395–400.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Xin Wei, Rupak Roy, Kumar Saurabh, Parveen Sen, Muna Bhende, Komal Shelke, Lucia Finocchio, Andrea Sodi, Gianni Virgili, Alessandro Invernizzi, Anna Paola Salvetti, Chitaranjan Mishra and Rupesh Agrawal all made substantial contributions to the study conception and design.
Disclosures
Xin Wei, Rupak Roy, Kumar Saurabh, Parveen Sen, Muna Bhende, Komal Shelke, Lucia Finocchio, Andrea Sodi, Gianni Virgili, Alessandro Invernizzi, Anna Paola Salvetti, Chitaranjan Mishra and Rupesh Agrawal all confirm that they have no relevant financial or non-financial interests to disclose.
Compliance with Ethics Guidelines
Ethics approval was obtained from the institutional review boards. This study adhered to the tenets of Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wei, X., Roy, R., Saurabh, K. et al. Optical Coherence Tomography-Based Choroidal Structural Analysis and Vascularity Index in Best Vitelliform Macular Dystrophy. Ophthalmol Ther 11, 2141–2152 (2022). https://doi.org/10.1007/s40123-022-00567-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00567-y