Abstract
Introduction
In the context of managing patients’ expectations and satisfaction regarding visual acuity after cataract surgery, we aimed to investigate the improvement in visual acuity and patient satisfaction after small-incision lenticule extraction (SMILE) in pseudophakic (trifocal intraocular lens, IOL) patients with residual myopic refraction after cataract surgery.
Methods
Seventy-six patients (82 eyes) who underwent cataract surgery with ZEISS AT LISA tri 839MP IOL implantation were included in this retrospective study. The included patients were 56–79 years old, wanted spectacle independence, and had preoperative myopic refraction between − 1.0 and − 2.25 diopters (D) and astigmatism between − 0.75 and − 1.75 D. The treatment status of these patients was defined as trifocal IOL (n = 82). SMILE was performed in patients who were dissatisfied after cataract surgery, and these patients were followed up for 1 year on average. We evaluated visual acuity and satisfaction and further examined laser vision correction and satisfaction levels in patients who were dissatisfied after trifocal IOL implantation.
Results
The possible reasons for patient dissatisfaction were reading books, using a computer, and driving at night. After SMILE, the residual myopic refractive error (spherical) decreased significantly from − 2.08 ± 0.28 [− 2.25 to − 1.0] preoperatively to − 0.25 ± 0.20 − 0.5 to 0] 1 year postoperatively (p < 0.001). Additionally, the uncorrected distance visual acuity increased from 0.65 ± 0.08 [0.52–0.7] logMAR preoperatively to 0.09 ± 0.02 [0.05–0.1] logMAR at 1 month postoperatively (p < 0.001), 0.09 ± 0.02 [0.05–0.1] logMAR at 6 months postoperatively, and 0.06 ± 0.02 [0.05–0.1] logMAR at 12 months postoperatively (p < 0.001). Patient satisfaction measures after SMILE (reading, night driving, and using a computer) were significantly improved.
Conclusion
SMILE is a reliable method for treating residual refraction after cataract surgery, as it provides results in the shortest time without complications and increases patient satisfaction.
Trial Registration
The protocol was registered on clinicaltrials.gov (NCT04693663).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Residual refraction has been ranked at the top of the list of postoperative patient complaints after cataract surgery and is mainly caused by the inaccuracy of predicted postoperative refraction. |
The presence of residual refraction makes it difficult for the patient to see and perform daily activities and generally reduces the quality of life. |
We hypothesize that small-incision lenticule extraction (SMILE) surgery may improve the accuracy of predicted postoperative visual acuity and increase patient satisfaction without any complications. |
What was learned from the study? |
This study demonstrated that the SMILE technique is a safe and effective treatment modality for pseudophakic myopic fractures to improve patient visual outcomes and satisfaction after cataract surgery. |
This study was the first to provide new information about long-term (1 year) optical quality changes after cataract surgery using the SMILE module for residual myopic refraction. |
Introduction
Cataracts are one of the most common physiological changes affecting vision and the most common reason for visual impairment in adults aged 50 years and older [1]. This eye disease can lead to clouding of the lens inside the eye and reduced vision. Currently, the usual treatment for cataracts involves removing the opacified lens and replacing it with an artificial monofocal or multifocal intraocular lens (IOL). Cataract surgery is a refractive surgery, as it will increase vision if a lens with a suitable power is implanted in the patient’s eye.
However, it is necessary to manage patients’ expectations and satisfaction with visual acuity after cataract surgery. These treatments are only needed for postoperative adjustment of the refractive error, such as IOL exchange, overlay IOL implantation, laser-assisted in situ keratomileusis (LASIK), and photorefractive keratectomy (PRK) [2]. Additionally, corneal refractive surgeries are performed. Methods such as small-incision lenticule extraction (SMILE) and femtosecond LASIK (FS-LASIK) are practical and comfortable options for patients who are not satisfied with their visual acuity after cataract surgery [3, 4]. This study aimed to provide evidence to support SMILE as an alternative and comfortable option for both patients and surgeons for improving both the visual outcome and patient satisfaction after trifocal IOL implantation.
Methods
Study Population
This study was performed in accordance with the Declaration of Helsinki. The protocol was registered on clinicaltrials.gov (NCT04693663). Approval was obtained from the national research ethics committee of the Ministry of Health, Kosovo. Written informed consent was obtained from each patient for the publication of all patient information. Each patient was informed about the SMILE surgery.
This retrospective study included 82 eyes of 76 consecutive patients who underwent cataract surgery with ZEISS AT LISA tri 839MP IOL implantation. We included patients who underwent cataract surgery at various clinics between 2017 and 2018 and YAG laser capsulotomy with or without posterior capsule opacity 3 months after cataract surgery. SMILE surgery was performed 1 month after YAG laser surgery. Patients with a history of hyperopia, less than 23 mm in axial length, and implanted ZEISS AT LISA tri 839MP IOL were included. In addition, the patients had residual myopia between − 1.0 and − 2.25 D and astigmatism between − 0.75 and − 1.75 D. The same surgeon (FS) performed all SMILE surgeries with the patient under local anesthesia at the Eye Hospital of Pristina (Kosovo). We excluded patients with a history of high myopia, retinal detachment, corneal disease, irregular corneal astigmatism, glaucoma, macular degeneration, advanced retinopathy, keratoconus, ocular inflammation, an endothelial cell count less than 1900/mm2, and/or dry eye.
Pre- and Postoperative Evaluations
Slit-lamp examination, Goldmann applanation tonometry, and visual acuity examination using an ETDRS chart with the Sloan 5 × 5 family of letters as optotypes were performed under photopic conditions using room illumination of 90 cd/m2 and mesopic illumination of 0.7 cd/m2. The uncorrected distance visual acuity (UDVA), uncorrected near visual acuity (UNVA) at 35 cm, corrected near visual acuity (CNVA) and uncorrected intermediate visual acuity (UNVA) at 70 cm, and corrected intermediate visual acuity (CNVA) were assessed by simulating normal daily life activities. Determination of the total root mean square (RMS) and Q value, keratometry, corneal topography (ATLAS, Carl Zeiss Meditec, Dublin, USA), endothelial cell count analysis (SP-3000P, Topcon, Japan), axial length of each preoperative patient measured with the ZEISS IOLMaster 500 (Carl Zeiss Meditec, Dublin, CA, USA), and fundoscopy were performed in all patients preoperatively and at 1, 6, and 12 months after SMILE surgery. All patients completed questionnaires before and 1 year after SMILE. The questionnaire assessed patients’ perceptions of vision and vision difficulties associated with daily activities such as reading, using a computer, or driving at night in the preoperative period. Another questionnaire was used to evaluate these conditions as well as to investigate patient satisfaction and quality of life postoperatively.
SMILE Surgery
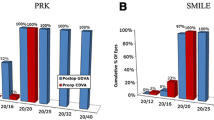
The Carl ZEISS VisuMax femtosecond laser (frequency 500 kHz) was used to treat residual myopic refractive error with the SMILE technique 4 months after cataract surgery [5,6,7,8,9]. First, topical anesthetic drops were applied to the cornea. After docking the cornea with a curved cone, a low vacuum was applied so that the patient could see the fixation light during docking. Then, an intrastromal lenticule with a diameter of 6.6 mm was created in the appropriate shape to achieve the desired refractive correction, and a cap with a minimum diameter of 7.7 mm and thickness of 10 µm was created using a femtosecond laser (spot energy 130 nJ). With the cap cut, a 2-mm incision was created, and the lenticule was opened for accessibility, manually dissected with a Mallorca 1297 spoon-type spatula, and removed with forceps. Figure 1a shows the preoperative low, high, and total RMS. Figure 1b shows the postoperative low, high, and total RMS. Figure 1c shows the preoperative Q value, and Fig. 1d shows the postoperative Q value. The postoperative therapy included antibiotic eye drops (moxifloxacin hydrochloride) and steroidal eye drops (dexamethasone), administered four times per day for 1 month.
Statistical Methods
SPSS 25.0 (IBM Corporation, Armonk, New York, USA) and PAST 3 (paleontological statistics) programs were used to analyze the paired corneal spherical aberration, total RMS, reading satisfaction, computer use satisfaction, night driving satisfaction, astigmatism, residual refraction after trifocal IOL implantation, and Q value samples using the bootstrap T test. UNVA (35 cm) and UNVAN (70 cm) were tested using Wilcoxon signed-ranks test and Monte Carlo simulation results to compare measurements preoperatively and 1 year postoperatively. For comparison of the preoperative UDVA (logMAR) with the UDVA at 1, 6, and 12 months postoperatively, general linear model repeated-measures analysis of variance (ANOVA) with Bonferroni’s post hoc test was used. Quantitative variables are shown in tables as the mean (standard deviation) [minimum to maximum], while categorical variables are shown as n (%). Differences with a p value less than 0.05 at a 95% confidence level were considered significant. Sampling and power calculations were performed using the G Power 3.1 program. According to the results of the analysis, the partial eta-squared value was 0.976 (effect size f = 6.37, n = 82, type I error (α) = 0.05). The post power value was calculated as 100% for single-group four-repetition Q value measurements (effect size dz = 5.18, n = 82). In addition, the post power calculated for trifocal installed cataract residual refraction measurements with two replicates in one group was 100% [type I error (α) = 0.05].
Statistical power is the probability that if a significant difference is detected between the groups, this difference is real. An 80% power level is a standard goal. The number of samples corresponding to an 80% power level is determined as the minimum number of samples to be included in the study. For Q value and trifocal installed cataract residual refraction measurements, which are the most important variables of our study, the minimum number of individuals to be included in the study was 82 at a power level of 100%.
Characteristics of Study Participants
SMILE was performed in 82 eyes of 76 patients after trifocal IOL implantation; the patient age was 68 [56–79] years, with 45 women (59.21%) and 31 men (40.78%). The mean time between the last cataract surgery and SMILE was 4 months. Unilateral SMILE surgery was performed in 70 (72.63%) patients, and bilateral SMILE surgery was performed in 6 patients (14.63%). All patients reported satisfaction during the follow-up period after SMILE.
Results
Q Value
The SMILE Q value improved from − 0.43 ± 0.04 [− 0.49 to − 0.36] preoperatively to − 0.27 ± 0.05 [− 0.34 to − 0.2] 1 year postoperatively (Fig. 2).
The corneal spherical aberration improved from 0.25 ± 0.02 [0.21–0.279] preoperatively to 0.23 ± 0.03 [0.174–0.279] μm postoperatively, which was not statistically significant (p = 0.133) (Table 1), and the total RMS improved from 0.84 ± 0.06 [0.7–0.9] preoperatively to 0.82 ± 0.08 [0.6–0.9] μm postoperatively, which was also not statistically significant (p = 0.072) (Table 1).
Questionnaire Results
All patients completed questionnaires before and after SMILE. The questionnaire assessed patients’ perceptions of vision and vision difficulties associated with daily activities such as reading, using a computer, and driving at night in the preoperative period. Another questionnaire was applied to evaluate these conditions and to investigate patient satisfaction and quality of life postoperatively. Reading satisfaction significantly improved from 78.66 ± 5.39 [70–90] preoperatively to 88.54 ± 3.56 [80–90] 1 year postoperatively (p < 0.001) (Table 1). Computer use satisfaction significantly improved from 80.12 ± 5.55 [70–90] preoperatively to 88.29 ± 3.79 [80–90] 1 year postoperatively (p < 0.001). Night driving satisfaction significantly improved from 64.27 ± 5.22 [60–80] preoperatively to 86.95 ± 4.63 [80–90] 1 year postoperatively (p < 0.001) (Table 1).
Distant Visual Acuity
The UDVA increased from 0.65 ± 0.08 [0.52–0.7] logMAR preoperatively to 0.09 ± 0.02 [0.05–0.1] logMAR 1 month postoperatively (p < 0.001), 0.09 ± 0.02 [0.05–0.1] logMAR 6 months postoperatively, and 0.06 ± 0.02 [0.05–0.1] logMAR 12 months postoperatively (p < 0.001) (Fig. 3). Preoperative corrected distance visual acuity, postoperative uncorrected distance visual acuity (Fig. 4a), and attempted SEQ (D) values are presented in Fig. 4b and Table 2.
After SMILE, the residual myopic refractive error (spherical) decreased significantly from − 2.08 ± 0.28 [− 2.25 to − 1.0] preoperatively to − 0.25 ± 0.20 [− 0.5 to 0] 1 year postoperatively (p < 0.001) (Table 1). Additionally, astigmatism decreased from − 1.23 ± 0.37 [− 1.75 to − 0.75] preoperatively to − 0.37 ± 0.13 [− 0.5 to − 0.25] 1 year postoperatively (p < 0.001) (Fig. 4c) and (Table 1).
Near Visual Acuity
In all patients, the UNVA (35 cm) did not change postoperatively; it was J2 (Fig. 5). However, the UNVA (70 cm) increased from J5 to J3 (Fig. 6). This study included patients with a history of hyperopia and AT LISA tri839MP trifocal IOL (Carl Zeiss Meditec) implantation. This IOL is a one-piece diffractive, aspheric, plate-type collapsible, hydrophilic acrylate IOL with a surface of principal and phase regions. This lens provides + 3.33 and + 1.66 D to add power for near and intermediate vision, respectively, reducing negative visual symptoms and improving retinal image quality.
In this study, we benefited from this feature of the trifocal lens and increased the distance and intermediate vision ratio without compromising the comfort of near vision. In addition, since the axial lengths of the patients were hyperopic, SMILE surgery was performed without drastically increasing the Q value and without drastically decreasing the total RMS.
Discussion
Trifocal IOLs have been developed to treat cataracts to help increase patients’ far, intermediate, and near vision. However, the emergence of refractive error with this intervention can lead to patient dissatisfaction [10,11,12,13,14,15,16]. Irrespective of the location at which the cataract surgery is performed and the technology used, additional refractive surgery is needed to increase patient satisfaction. Therefore, we propose a new and comfortable treatment using SMILE technology for patients with residual myopic refraction causing dissatisfaction after trifocal IOL implantation. This study demonstrates that a residual myopic refractive error, one reason for dissatisfaction, can be corrected easily and efficiently using SMILE surgery.
A potential solution to increase patient satisfaction after cataract surgery is a lens-based procedure (IOL exchange or piggyback IOL implantation) [2, 17,18,19,20,21,22,23,24,25], in which the original cataract wound is reopened to repair the residual refractive error immediately after cataract surgery. Subsequently, another IOL can be implanted (IOL replacement), changing the lens immediately after the first surgery, but there is a risk of posterior capsule defect formation, glaucoma, zonular defect formation, endothelial damage, or postoperative pigment dispersion, which occurs when the dorsal lens rubs against the posterior surface of the iris; furthermore, endophthalmitis can occur during these procedures. Hence, laser treatment is more appropriate for these patients. Moreover, LASIK and PRK often provide better predictability and accuracy than IOL exchange or lens overlay techniques, especially regarding astigmatic outcomes, and prevent several risks associated with subsequent intraocular surgeries. Both PRK and LASIK have been used to correct residual refractive error after cataract surgery [4, 26,27,28,29,30]. However, LASIK has been shown to cause more severe and permanent damage to corneal sensation, corneal barrier function, and tear film stability than PRK. Moreover, sometimes, the residual refractive error remains after LASIK, necessitating further lens replacement. In our series, aberrations such as coma and astigmatism were not observed in any of the patients after SMILE. However, Seiler et al. [27] found such aberrations in 12.5% of treated eyes, and De Vries et al. [14] showed that the wavefront anomalies thought to be responsible for conditions reducing vision, such as high-grade coma and astigmatism, occurred in a similar percentage of eyes and could not be corrected with wavefront-optimized ablation. ZEISS AT LISA tri 839MP IOL implantation helps increase patients’ far, intermediate, and near vision, and these IOLs show greater central power than peripheral power. This study demonstrates two important concepts, i.e., success was achieved by following the path without excessively increasing the Q value or excessively reducing the total RMS (Fig. 1). Seiler et al. [27] reported that 96% of patients were completely independent of glasses, while four patients used reading glasses from time to time. However, in our study, none of the patients needed to use glass for distance or near vision after SMILE.
The strengths of this study are that the sample size was sufficient to investigate the differences between the patients and the long follow-up period. Furthermore, to our knowledge, this is the first attempt to implement SMILE surgery as a solution for correcting residual refractive error after trifocal IOL implantation. This study also has some notable limitations.
First, as there was no SMILE hyperopic module when we started the study, we could not include hypermetropic residual refraction in this study, and some patients were satisfied with their near vision without glasses or corrected distance vision after cataract surgery, while others used correction for near vision (monovision). No comparison was made with patients who underwent LASIK as another means of correcting postoperative myopia. A comparative study involving SMILE with selective wavefront-guided LASIK provides clearer evidence as to whether SMILE will be superior to selective wavefront-guided LASIK in residual refractive error after trifocal IOL implantation.
Conclusion
This treatment modality should be applied in the future, followed by an investigation of the residual refractive error, visual outcomes, and satisfaction of patients from different geographic regions, cultures, and socioeconomic statuses. Such future studies with larger samples are also warranted to confirm our results. In conclusion, the SMILE technique is a safe and effective treatment modality for pseudophakic myopic fractures to improve the visual outcomes and satisfaction of patients after cataract surgery. With this surgical technique, there is no need for glasses, and the improvement of vision in a short time increases patient satisfaction.
References
Lasch K, Marcus JC, Seo C, et al. Development and validation of a visual symptom-specific patient-reported outcomes instrument for adults with cataract intraocular lens implants. Am J Ophthalmol. 2022;237:91–103.
Fernández-Buenaga R, Alió JL, Pérez Ardoy AL, Quesada AL, Pinilla-Cortés L, Barraquer RI. Resolving refractive error after cataract surgery: IOL exchange, piggyback lens, or LASIK. J Refract Surg. 2013;29:676–83.
Semiz F, Lokaj AS, Caliskan G, et al. Perception of trifocal IOL performance in young adults with high astigmatism and hyperopia and its improvement using small incision lenticule extraction. Acta Inform Med. 2021;29:118–24.
Alfonso JF, Fernández-Vega L, Montés-Micó R, Valcárcel B. Femtosecond laser for residual refractive error correction after refractive lens exchange with multifocal intraocular lens implantation. Am J Ophthalmol. 2008;146:244–50.
Semiz F, Musa NH, Lokaj AS, Semiz O. Corneal lenticule implantation in keratoconus disease with relex smile surgery. Acta Sci Ophthalmol. 2020;3:36–44.
Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. 2011;95:335–9.
Sekundo W, Messerschmidt-Roth A, Reinstein DZ, Archer TJ, Blum M. Femtosecond lenticule extraction (FLEx) for spherocylindrical hyperopia using new profiles. J Refract Surg. 2018;34:6–10.
Reinstein DZ, Carp GI, Archer TJ, Gobbe M. Outcomes of small incision lenticule extraction (SMILE) in low myopia. J Refract Surg. 2014;30:812–8.
Ganesh S, Brar S, Pandey R, Pawar A. Interface healing and its correlation with visual recovery and quality of vision following small incision lenticule extraction. Indian J Ophthalmol. 2018;66:212–8.
Kim JW, Eom Y, Yoon EG, et al. Increased near vision spectacle dependence of patients with preoperative myopia after mix-and-match implantation of trifocal EDOF and trifocal IOLs. J Refract Surg. 2021;37:746–53.
Paul C, Gläser S, Kiraly L, Bechmann M, Sel S, Sekundo W. Patient-reported quality of life and satisfaction after refractive lens extraction using a diffractive trifocal IOL: a multicenter retrospective cohort study. J Refract Surg. 2021;37:768–74.
Benedi-Garcia C, Vinas M, Lago CM, et al. Optical and visual quality of real intraocular lenses physically projected on the patient’s eye. Biomed Opt Express. 2021;12:6360–74.
Fabian E, Birkl M, Benstetter F, Eberwein P, Seher U, Pfeiler T. Quality assurance in cataract and lens surgery with special consideration of subjective patient reported outcome measures and clinical reported outcome measures. Klin Monbl Augenheilkd. 2021;239:293–301.
De Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859–65.
Alio JL, Plaza-Puche AB, Férnandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62:611–34.
Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43:737–47.
Asena BS. Visual and refractive outcomes, spectacle independence, and visual disturbances after cataract or refractive lens exchange surgery: comparison of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2019;45:1539–46.
McNeely RN, Pazo E, Millar Z, et al. Threshold limit of postoperative astigmatism for patient satisfaction after refractive lens exchange and multifocal intraocular lens implantation. J Cataract Refract Surg. 2016;42:1126–34.
Brito P, Salgado-Borges J, Neves H, Gonzalez-Meijome J, Monteiro M. Light-distortion analysis as a possible indicator of visual quality after refractive lens exchange with diffractive multifocal intraocular lenses. J Cataract Refract Surg. 2015;41:613–22.
Fernández-García JL, Llovet-Rausell A, Ortega-Usobiaga J, et al. Unilateral versus bilateral refractive lens exchange with a trifocal intraocular lens in emmetropic presbyopic patients. Am J Ophthalmol. 2021;223:53–9.
Ruíz-Mesa R, Carrasco-Sánchez D, Díaz-Alvarez SB, Ruíz-Mateos MA, Ferrer-Blasco T, Montés-Micó R. Refractive lens exchange with foldable toric intraocular lens. Am J Ophthalmol. 2009;147(990–6):996.e1.
Djodeyre MR, Ortega-Usobiaga J, Beltran J, Druchkiv V, Baviera-Sabater J, Bouza-Miguens C. Bilateral refractive lens exchange with trifocal intraocular lens for hyperopia in patients younger than 40 years: a case-control study. J Refract Surg. 2021;37:524–31.
Kahraman G, Amon M. New supplementary intraocular lens for refractive enhancement in pseudophakic patients. J Cataract Refract Surg. 2010;36:1090–4.
Khan MI, Muhtaseb M. Performance of the Sulcoflex piggyback intraocular lens in pseudophakic patients. J Refract Surg. 2011;27:693–6.
De Ortueta D. Transepithelial photorefractive keratektomy after a clear lens exchange. Vision (Basel). 2021;5:8.
Muftuoglu O, Prasher P, Chu C, et al. Laser in situ keratomileusis for residual refractive errors after apodized diffractive multifocal intraocular lens implantation. J Cataract Refract Surg. 2009;35:1063–71.
Seiler TG, Wegner A, Senfft T, Seiler T. Dissatisfaction after trifocal IOL implantation and its improvement by selective wavefront-guided LASIK. J Refract Surg. 2019;35:346–52.
Li SM, Kang MT, Wang NL, Abariga SA. Wavefront excimer laser refractive surgery for adults with refractive errors. Cochrane Database Syst Rev. 2020;12:CD012687.
Barsam A, Allan BD. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD007679.pub4.
Fan YY, Sun CC, Chen HC, Ma DH. Photorefractive keratectomy for correcting residual refractive error following cataract surgery with premium intraocular lens implantation. Taiwan J Ophthalmol. 2018;8:149–58.
Acknowledgements
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector. The journal’s Rapid Service Fees were funded by the authors.
Medical Writing, Editorial, and Other Assistance
We would like to thank Ismail Lutfi Leventoglu and Metin Fettahli for providing administrative, technical, referential, and material support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study concept and design (Faruk Semiz, Anita Syla Lokaj); data collection (Faruk Semiz, Anita Syla Lokaj, Njomza Hima Musa, Ceren Ece Semiz, Olcay Semiz, Zekeriya Alp Demirsoy); analysis and interpretation of data (Faruk Semiz, Anita Syla Lokaj); writing the manuscript (Faruk Semiz, Anita Syla Lokaj, Njomza Hima Musa); critical revision of manuscript (Faruk Semiz, Anita Syla Lokaj); administrative, technical, or material support (Njomza Hima Musa, Ceren Ece Semiz, Olcay Semiz, Ismail Lutfi Leventoglu, Metin Fettahli); and supervision (Faruk Semiz, Anita Syla Lokaj, Njomza Hima Musa, Olcay Semiz).
Disclosures
Faruk Semiz, Anita Syla Lokaj, Njomza Hima Musa, Ceren Ece Semiz, Zekeriya Alp Demirsoy and Olcay Semiz declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Approval was obtained from the national research ethics committee of the Ministry of Health, Kosovo. Our study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients were aware of the collection of their data for this study and signed a consent form at the time of enrollment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Semiz, F., Lokaj, A.S., Musa, N.H. et al. SMILE for the Treatment of Residual Refractive Error After Cataract Surgery. Ophthalmol Ther 11, 1539–1550 (2022). https://doi.org/10.1007/s40123-022-00526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00526-7