Abstract
Introduction
The aim of this article was to comprehensively review the relationship between light exposure and myopia with a focus on the effects of the light wavelength, illuminance, and contrast on the occurrence and progression of myopia.
Methods
This review was performed by searching PubMed data sets including research articles and reviews utilizing the terms “light”, “myopia”, “refractive error”, and “illuminance”, and the review was concluded in November 2021. Myopia onset and progression were closely linked with emmetropization and hyperopia. To better elucidate the mechanism of myopia, some of the articles that focused on this topic were included. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
The pathogenesis and prevention of myopia are not completely clear. Studies have provided evidence supporting the idea that light could affect eye growth in three ways. Changing the corresponding conditions will cause changes in the growth rate and mode of the eyes, and preliminary results have shown that FR/NIR (far red/near-infrared) light is effective for myopia in juveniles.
Conclusion
This review discusses the results of studies on the effects of light exposure on myopia with the aims of providing clues and a theoretical basis for the use of light to control the development of myopia and offering new ideas for subsequent studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Myopia is a significant challenge for global health because of its high prevalence. |
Substantial progress in treatment options and their effects has been made, but the mechanism of myopia remains incompletely understood. |
Elucidating the relationship between light exposure and myopia may contribute to the development of new therapeutic modalities for myopia. |
The effect of red light on myopia has become popular recently. |
FR/NIR (far red/near-infrared) light has the potential to control myopia. |
Introduction
Myopia is a common disorder that occurs in childhood and early adulthood and has become a major cause of blindness. Myopia is defined as parallel light passing through the refractive system of the eye and focusing in front of the retina when the eye is in its relaxed state, which leads to the formation of blurred images [1]. Myopia is mainly related to axial growth (axial myopia), but a small proportion of myopia is caused by excessive curvature of the cornea or lens (refractive myopia), such as keratoconus [2]. In general, spherical equivalent (SE) ≤ − 0.5 diopters (D) is the standard for the diagnosis of myopia, and an SE ≤ − 5.0 D or − 6.0 D or an axial length of greater than 26 mm is considered indicative of high myopia [3, 4]. Myopia is a multifactorial disorder that is complex, and it is difficult to explain its mechanism, which is regulated by both environmental and genetic factors [5,6,7]. Myopia is closely related to complications such as cataracts, retinal detachment, macular degeneration, and even vision loss [8,9,10,11]. Some epidemiological and animal studies over the past few decades have investigated the potential causes of myopia. According to some researchers, near-work activity [12], little time outdoors [13], and high educational pressure [14] play important roles in the progression of myopia. Despite extensive research, some molecular mechanisms of myopia are still being debated, which hinders the search for therapeutic targets. Thus, controlling the onset of myopia and stopping or reversing its progression are the greatest challenges for researchers. Fortunately, the identification of some effective measures for controlling the progression of myopia, such as increasing the time spent outdoors [15, 16], and decreasing the duration of near work [17], and a new finding involving improving scleral hypoxia [18], indicate that our understanding of myopia has progressed substantially. In recent years, more attention has been given to controlling myopia, particularly the relationship between light exposure and the progression of myopia. The main conclusion drawn by researchers is that both the light wavelength and the light intensity can affect myopia because retinal dopamine (DA) secretion, which can affect the progression of myopia, is affected by the light intensity and the effect of different light wavelengths on myopia is related to the longitudinal chromatic aberration (LCA) theory. Understanding the relationship between light exposure and myopia allows refinement of the specific pathogenesis of myopia from a new dimension and provides new ideas for the control of myopia.
Relationship Between Light Luminance/Illuminance and Myopia
The pathogenesis of myopia is unclear; however, studies have demonstrated that both gene–gene and gene–environment interactions are involved in the pathogenesis of myopia, and multifactorial involvement needs to be considered [6]. A reproducible and repeatedly reported conclusion from a few cross-sectional studies and longitudinal epidemiological studies conducted over the past decade suggests that adequate outdoor activity time among adolescents is considered an effective factor for myopia prevention [19, 20], and this conclusion is also supported by several observational studies on seasonal changes and myopia development. The mechanism of myopia prevention through outdoor activity is most likely related to outdoor light exposure because studies have found that both axial growth and myopia progression are slower during summer months [21]. This phenomenon provides a theoretical basis for the hypothesis that light exposure affects the occurrence and development of myopia; indeed, some investigators who performed a series of experiments on the association of light environment with myopia in the early years found a correlation.
A potential link exists between light luminance and myopia. Two models of myopia are used for research: form-deprived myopia (FDM), which refers to myopia that can be induced by depriving the eye of form vision during a period of susceptibility, such as lid-suture [22]; and defocus-induced myopia (DIM), which is also called lens-induced myopia (LIM) and refers to myopia induced by concave (negative) lenses, as observed in chickens with lenses in front of their eyes [23]. Outdoor light illuminance is typically 10–1000 times higher than indoor light illuminance [24, 25]. Ashby and Schaeffel [26] used a DIM model and found that chicks exposed to high-intensity light (15,000 lx) had stronger resistance to the development of myopia and exhibited a slower progression of myopia than chicks exposed to low-intensity light (500 lx). Similarly, an FDM model has been extensively studied by Ashby et al. [27], Stone et al. [28], and Karouta and Ashby [29], who utilized chick models of FDM to demonstrate that exposure to intense light can suppress myopia development. The relationship between light exposure and the control of myopia was subsequently validated in FDM models in monkeys and mice by Smith et al. [30] and Chen et al. [31]. An assessment of the relationship between near work, outdoor activity, and myopia in school-aged children was conducted by Rose et al. [15], who found that outdoor activity can significantly reduce the incidence of myopia despite high levels of near-vision activity, which indicates that light in the outdoor environment may play a key role. Longitudinal studies and randomized controlled trials have demonstrated that the risk of myopia onset and its progression are decreased in children and adolescents who spend more time outdoors [32, 33]. A school-based cluster randomized trial conducted by Wu et al. [34] concluded that a longer duration of exposure to moderate outdoor light intensities, such as 1000 lx or more or 3000 lx or more, also exerted myopia-prevention effects, and few side effects were observed after exposure to intense light.
Artificially increases in light exposure may also reduce the progression of myopia, as was demonstrated in an experiment using light therapy glasses for young adults, which revealed that this therapy resulted in a slightly thickened choroid [35]. A study conducted by Wang [36] considered that exposure to natural light was beneficial for reducing hyperopic defocus-induced myopia and that exposure to sunlight early in life would promote normal emmetropization later in life. To further intervene in myopia progression, Landis et al. [37] found that dim light exposure may be another important strategy for preventing myopia by rod pathways other than cone cells and that a broad range of light levels are essential in refractive development [38]. Thus, light intensity could decrease the progression of myopia and protect our eyes, but the underlying mechanism is unclear. Among the mechanisms linking myopia and light intensity, the most widely considered hypothesis is that bright light increases the synthesis and release of DA in the retina [39], and thus potentially affects ocular growth. DA is an important neurotransmitter in the retina and mediates diverse functions, including refractive development, visual signal transduction, and β receptor activation. Data from several experiments among different species indicate that DA acts as a “stop” signal in refractive ocular development [40] and is involved in myopia development by activating its receptor. The DA receptors are G-protein-coupled receptors that are present in almost all neuronal classes within the retina. The retina expresses four DA receptor subtypes: D1R, D2R, D4R, and D5R [41]. A large number of studies have been conducted, and the results showed that D2-like receptors are more important in controlling the progression of myopia than D1-like receptors in chicks. Data from mouse models support the homeostatic regulation of myopia hypothesis, which states that the activation of D2-like receptors leads to myopia, whereas the activation of D1-like receptors leads to hyperopia [42]. The release of DA is amplified in a linear manner by light stimulation over four log units of intensity [39], and the DA of the retina may induce choroidal thickening and ocular growth inhibition via the release of NO from the retina or choroid [43,44,45] and thus slowing the development of myopia. According to Wang et al. [46], red, blue, and UV light all stimulated the release of retinal DA, but there were wavelength-dependent differences in DA release. For example, UV and blue light produced less deprivation myopia than red and white light. Circadian rhythms may be another mechanism of light exposure in myopia, which overlaps with eye growth, and DA is an important regulator of these rhythms [47, 48]; additionally, melatonin may play an important role in the link between light and circadian functions [49]. The axial length of eyes and choroidal thickness experience opposite and subtle changes with circadian rhythms and can be disrupted in DIM modes, and this result adds to the evidence indicating that optical defocus could play an important role in defining the axial length and choroidal thickness [50,51,52]. In general, light intensity, as a protective factor, is negatively correlated with the development of myopia.
Relationship Between Light Wavelength and Myopia
In addition to the effects of light intensity on myopia, the effects of different wavelengths of light on myopia have recently attracted increasing attention. Light waves are electromagnetic waves with a wide range of frequencies and include radio waves, visible light, infrared rays, ultraviolet rays, etc. Data from our previous studies demonstrated that extremely low frequency electromagnetic fields (ELF-EMFs), a form of electromagnetic waves with a long wavelength, could inhibit the expression of type I collagen in human fetal scleral fibroblasts (HFSFs) and play an important role in scleral remodeling, which may accelerate the development of myopia [53]. Light, as an electromagnetic wave, also has an impact on the progression of myopia. There are differences in the wavelength of light sensed by different people and thus the range of visible light wavelengths is not entirely consistent [54].
In 2002, Seidemann and Schaeffel [55] found that chickens reared under blue light had a tendency toward hyperopia, whereas those exposed to red light had a tendency toward myopia, and these findings have attracted extensive attention. A similar conclusion was drawn by Rucker and Wallman [56] in 2009 on the basis of the results from a study using chickens as an experimental animal model: blue light inhibited the development of myopia, whereas red light promoted the progression of myopia. Interestingly, Torii et al. [57] found that violet light (360–400 nm) could suppress the axial elongation in both chick models and humans, and this suppression increases the expression of the EGR1 gene known to prevent myopia. In addition, a recent study by Jiang [58] revealed that the violet light–neuropsin (OPN5)–retinal pathway played an important role in preventing myopia progression and could regulate choroidal thickness in mouse models. Using mouse models, Yang [59] found that red light induced a hyperopic shift and that emmetropization was not affected by blue light. However, in the same year, the opposite result was obtained by Ryan [60], who found that short-wavelength light (400 ± 20 nm) could slow eye growth, produce a hyperopic shift, and inhibit lens-induced myopia, which was consistent with the results reported by Torii and Jiang. Guinea pigs, as classical myopia animal models, were evaluated by Liu et al. [61] in 2011 and reared under two monochromatic exposures, green and blue light, and their refractive and axial growth changes were examined. This experiment revealed that exposure to blue light exerted more hyperopic effects and suppressed axial growth, whereas exposure to green light was associated with tendency toward myopia [61]. A positive lens enables light to focus on the anterior part of the retina and thus induces defocus-induced hyperopia, whereas a negative lens causes DIM. Jiang et al. [62] found that blue light suppressed axial myopia caused by negative lenses and slow axial growth, whereas red light and positive lenses no longer caused hyperopia in guinea pigs. These experiments fully illustrate that light wavelengths play an important role in refractive development. Although the effects of light intensity and the differential effects of different wavelengths on the progression of myopia have been observed, the specific mechanism has not been elucidated. To further illustrate the relationship between light wavelength and myopia, rhesus monkeys, which are considered more advanced animals, were used by Smith et al., who obtained completely different results. These researchers found no evidence that an environment dominated by red light promoted the development of myopia; conversely, red light might promote hyperopia [63]. Moreover, using the tree shrew as an experimental model, Gawne et al. [64] found that stable red light inhibited the progression of myopia, whereas blinking blue light promoted the progression of myopia; this finding indirectly supports the conclusion drawn by Smith. The results from various experiments suggest that wavelength differences among different lights can produce refractive and axial changes, and this effect can exist independently of the light intensity. These different phenomena can be explained by complex and diverse mechanisms.
Conflicting conclusions from numerous experiments with different species demonstrate that the relationship between light exposure and myopia is extremely complex, and the current findings indicated that during the period after birth in humans [65,66,67] and other animals, such as tree shrews [68], guinea pigs [69], rhesus monkeys [70], marmosets [71], and chickens [72], marked refractive errors are usually hyperopic, whereas in Falco sparverius, these refractive errors are usually myopic [73]. The eye has an active emmetropization mechanism using optics as a visual cue to dynamically regulate the elongation of the ocular axis to match the retina and focal plane, and the emmetropization state is maintained for a short period after birth through micromodulation of the refractive state [74,75,76,77,78,79]. At present, most scholars believe that other mechanisms of emmetropization in addition to optical defocusing exist and that LCA may be the underlying mechanism. LCA is a physical phenomenon in which the lens edge has a larger refractive index to light of shorter wavelengths and focus occurs closer to the lens, whereas long-wavelength light has a smaller refractive index and is focused further away from the lens, which could promote emmetropization (Fig. 1) [55, 56, 80,81,82,83,84,85,86]. LCA in myopia may act in two ways: one possibility is that the LCA mechanism is used as a target, matching the focal plane of the dominant wavelength by increasing the speed of growth when the dominant wavelength is long and decreasing the speed of growth when the dominant wavelength is short; a result supported by some studies have supported under monochromatic light exposure [55, 81, 87]. The other possibility is that LCA is used as a cue. If the long-wavelength light focuses better than short-wavelength light, the eye will stop growing in response to a signal. The eye is sufficiently long; thus, it has to decrease the speed of growth to achieve emmetropization and vice versa, as has been demonstrated by Smith et al. [63], who studied infant monkeys that wore filters that transmitted only long wavelengths. This condition is for white and polychromatic light. In general, the eye can derive signs of defocus by comparing the relative cone contrast, which can reflect the color contrast of the eyes to promote emmetropization; changes in color contrast are not essential because the eye can emmetropize in monochromatic light [84].
Whether light luminance and LCA are interrelated and whether greater protection could be gained through a combination of intense and chromatically adjusted light are issues that need to be addressed [88]. Early research suggested that the myopia sign originated from light intensity guidance, which could be associated with the magnitude of the blurring of retinal images [89], but the blur hypothesis did not explain some unusual cases: the eye could still achieve emmetropization in the models of positive and negative lens induction when the amount of blur is similar but the sign of defocus is different compared with increasing blur [90, 91]. The eye could use the light intensity only as guidance for emmetropization, as has been demonstrated with monochromatic light. However, the eye could use color cues by reducing the proportion of effectiveness of luminance by using astigmatism or reducing the light intensity [86, 92,93,94]. For example, in dim light (0.67 lx), the compensation of eye growth for equal amounts of defocus was reduced in blue and red light compared with white light, and compensation was impaired in monochromatic light with loss of the choroidal response in white light [93].
Color and Luminance Contrast of Space–Time and Myopia
The normal color vision of individuals needs three different types of color vision: long-wavelength-sensitive cones, middle-wavelength-sensitive cones, and short-wavelength-sensitive cones, which correspond to three color perception channels. Accurate information on light dark, color, and saturation is required for at least two contrast of cone (cone contrast)/color vision channels [95], and these signals will enter different pathways, including a brightness pathway and two color vision alignment pathways [96,97,98]. According to LCA theory, long wavelengths are focused farther back in the eye than shorter wavelengths; however, short-wavelength-sensitive (SWS “blue”) cones are only sparsely distributed on the retina [99, 100], which makes the assessment of degree of short wavelengths focusing on the retina difficult. How is emmetropization achieved by using LCA as a cue? Previous studies have demonstrated that flickering light can reduce myopia produced by form deprivation [101], and DA metabolism affected by flickering light can be restored [102]. Rucker and Wallman [103] found that chicks that were exposed for 3 days to 2-Hz sinusoidal-flickering illumination in response to temporal changes in color and luminance contrast exhibit notable changes and concluded that the emmetropization mechanism is sensitive to 2-Hz temporal changes in color and luminance contrast. Moreover, temporal changes in color contrast are recognized as hyperopic defocus, whereas temporal changes in luminance contrast are distinguished as myopic defocus in the absence of color change. Many studies have examined the effects of modulated ambient light on emmetropization [94, 102, 104,105,106,107,108,109,110,111,112,113,114]; however, as a result of species variability or differences in experimental conditions, different experiments have yielded diverse results. For example, Di et al. found that chronic exposure to 0.5 and 5 Hz temporally modulated illumination promoted myopia progression in guinea pigs [111] instead of hyperopia, whereas Gawne et al. [64] found that steady and flickering red light both produced strong hyperopia, flickering blue light produced myopia, and steady blue light had no effect. Gawne et al. [64] argued that although the number of blue cones is small, the visual system can utilize the sensitivity of longer-wavelength cones to blue light to optimize focus; therefore, blue light can induce emmetropization, and the absolute amount of flicker detected in the SWS channel will be greater than that in the long-wavelength-sensitive (LWS) channel. Spatial contrast also plays an important role in myopia. Rucker et al. [94, 96] argued that the eye can use a single sample of retinal images and compare the contrast of two different cone types, which can determine the state of hyperopia or myopia, rather than contrasting the images in two different planes. These researchers also found that in addition to the contrast of a single retinal image, the eye could acquire the information from a comparison of the change in luminance and color contrast with the eye’s defocus change [103], which means that as a result of LCA, cone contrast of the L- and M-cone decreases quite similarly when the eye is in a myopic or hyperopic defocus state, whereas cone contrast of the S-cone decreases differently. S-cone contrast shows asymmetric changes on two sides of the in-focus plane, and the ratio of S-cone contrast to L- or M-cone contrast produces a chromatic change with the degree of defocus when in the state of hyperopic defocus; the ratio is relatively constant with myopic defocus, which mainly causes a change in luminance contrast (Fig. 2). In general, although both myopic defocus and hyperopic defocus could cause changes in luminance contrast, studies have revealed predominant changes in color with hyperopia and in luminance with myopia.

(Modified from Rucker et al. [103] with permission from ARVO)
As a result of LCA, for myopic defocus, the ratio of S-cone contrast to L-or M-cone contrast is relatively constant with the increase in myopic defocus, creating a dominant change in luminance contrast and slow growth of the eye. For hyperopic defocus, the ratio of S-cone contrast to L- or M-cone contrast is nonlinear with the increase in hyperopic defocus, creating a change in the chroma and promoting the growth of the eye, which leads to myopia
Controversies and Considerations
The effects of light on myopia appear contradictory. Interestingly, red light, which is a type of long-wavelength light, should induce hyperopia defocus [62, 115, 116] and cause myopia but this is not the case. Even in different species, the effect of red light on myopia is different; however, the main effect of red light on myopia is to cause hyperopia rather than myopia [63, 64]. Notably, Liu’s experiment revealed little difference in refractive error performance in rhesus monkeys raised in quasi-monochromatic red light [114], whereas Smith found that rhesus monkeys wearing long-wavelength-pass (red) filters exhibited apparent hyperopia [63], and this difference was multifactorial. First, chromatic cues are not essential, as demonstrated by Liu’s experiment. Second, decreases in the luminance contrast results in chromatic contrast predominating in emmetropization [86, 94], which may cause an imbalance between the strengths of long- and short-wavelength signals according to Smith’s research [63]. Third, we considered not only that the spectral wavelength would affect the development of myopia but also that the specific wavelength ranges would be equally important. The 630 nm light used by Hung et al. [117] and the 624 ± 10 nm light used by Gawne et al. [118] showed significant effects on myopia control in rhesus monkeys and tree shrews. Recently, He et al. found that repeated low-level red light (RLRL) therapy (650 nm, 1600 lx) could effectively improve the progression of myopia in children aged 8–13 years [119], and low-intensity, long-wavelength red light therapy (LLRT, 635 nm) inhibited myopia progression in children in an Eastern China-based cohort [120]. The effect of red light on myopia has recently gained more attention (Table 1).
Far Red/Near-Infrared Light
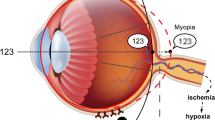
We hypothesized that this phenomenon may be associated with photobiomodulation (PBM) of far red/near-infrared light (FR/NIR), which includes the wavelength range of 630–1000 nm. FR/NIR light is produced from a laser or a light-emitting diode (LED), and its therapeutic qualities have been confirmed [121]. FR/NIR light therapy has been widely used over the years, including for increasing cerebral blood flow (CBF) [122, 123], augmenting brain energy metabolism [124, 125], improving the antioxidant capacity [126], promoting cell growth [127], and improving the reparative ability of cells [128]. In the ophthalmic field, FR/NIR also had uneventful grades. Studies have demonstrated that NIR/FR has a protective effect on optical nerves, promotes their functional recovery [129, 130], and benefits retinal diseases. According to previous studies, DA stimulates the synthesis and release of NO (nitric oxide) and NO works with NOS (nitric oxide synthase) to regulate eye growth. Atropine and NO inhibit form-deprived myopia in a dose-dependent manner, and NOS inhibitors block inhibition of myopia mediated by atropine [131, 132]. Significantly, Wu et al. found upregulation activity of NOS and increased concentrations of cGMP in form-deprivation myopia in guinea pigs [133]. One of the mechanisms of PBM was promoting the release of NO from intracellular stores [134, 135] and this process may be not dependent of NOS activation [136]. Kosaka considered that NO suppresses oxidative agents and improves oxygen transport, and hemoglobin (Hb) combined with oxygen would release oxygen by NO competition [137]. Zhang et al. [138] found protection against hypoxia and reoxygenation injury in cardiomyocytes by 670 nm light, which is dependent on NO derived from NOS and non-NOS sources. Quirk [139] considered that PBM may increase production of NO by reducing nitrite to NO by cytochrome c oxidase (CCO) or myoglobin (Mb)/Hb. Recent studies have also found that PBM had an effect on the activation of the TGFβ/Smad pathway in the process of wound healing, which was one of the key pathways in myopia progression [140, 141]. At a wavelength of 670 nm, the levels of collagen I and vascular endothelial growth factor (VEGF) increased during the wound healing process [142]. In addition, studies have demonstrated that collagen type I alpha 1 chain (COL1A1) was decreased and a-SMA was increased in myopia models and may be associated with sclera hypoxia [18], which is a major factor in myopia control. Thus, we supposed that FR/NIR treatment could thicken the choroid and increase blood flow by releasing NO, which acts as a vasodilator, leading to relative displacement of the retina to improve myopia. Then NO may have various downstream antihypoxia effects and cause amelioration of scleral hypoxia. The effect of activating the TGFβ/Smad pathway could improve scleral remodeling by increasing production of COL1A1 and, together with NO, the transdifferentiation of sclera fibroblasts could be reversed (Fig. 3); however, the specific mechanism remains to be further investigated.
Conclusion and Future Perspectives
Myopia has recently become increasingly widespread, particularly in East Asia. Close relationships exist between light and myopia. Light, including its luminance and wavelength, directs the direction of eye growth, and light exposure is a practical strategy for preventing myopia until ideal pharmacological targets have been found. The available research evidence suggests that increasing the time spent outdoors is an effective measure for preventing myopia because bright light could increase the synthesis and release of DA in the retina. Similarly, the wavelength of light can guide the emmetropization of eyes, which could become a new powerful potential measure of myopia prevention (Fig. 4).
In recent years, blue and violet light exposure has been considered as a means of controlling myopia, and red light appears to have the potential to improve choroidal blood perfusion and could be a new tool for controlling myopia in the future. In conclusion, the application of light for myopia control offers a promising avenue for the development of improved public health strategies for the prevention of myopia and the development of more effective therapeutic interventions to slow and prevent myopia progression. Light, as a useful tool, may have high potential for myopia control in the future.
References
Baird PN, Saw SM, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6:99. https://doi.org/10.1038/s41572-020-00231-4.
Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39:263–70. https://doi.org/10.1097/ico.0000000000002150.
Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet. 2012;379:1739–48. https://doi.org/10.1016/s0140-6736(12)60272-4.
Bremond-Gignac D. Myopia in children. Med Sci (Paris). 2020;36:763–8. https://doi.org/10.1051/medsci/2020131.
Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–44. https://doi.org/10.1167/iovs.05-1081.
Cai XB, Shen SR, Chen DF, Zhang Q, Jin ZB. An overview of myopia genetics. Exp Eye Res. 2019;188: 107778. https://doi.org/10.1016/j.exer.2019.107778.
Saw S-M, Hong C-Y, Chia K-S, Stone RA, Tan D. Nearwork and myopia in young children. Lancet. 2001. https://doi.org/10.1016/s0140-6736(05)71520-8.
Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157:9-25.e12. https://doi.org/10.1016/j.ajo.2013.08.010.
Lakawicz JM, Bottega WJ, Fine HF, Prenner JL. On the mechanics of myopia and its influence on retinal detachment. Biomech Model Mechanobiol. 2020;19:603–20. https://doi.org/10.1007/s10237-019-01234-1.
Pan CW, Cheng CY, Saw SM, Wang JJ, Wong TY. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol. 2013;156(1021–1033): e1021. https://doi.org/10.1016/j.ajo.2013.06.005.
Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61:49. https://doi.org/10.1167/iovs.61.4.49.
Saw SM, Zhang MZ, Hong RZ, Fu ZF, Pang MH, Tan DT. Near-work activity, night-lights, and myopia in the Singapore–China study. Arch Ophthalmol. 2002;120:620–7. https://doi.org/10.1001/archopht.120.5.620.
Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–5. https://doi.org/10.1016/j.ophtha.2012.11.009.
Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. https://doi.org/10.1016/j.preteyeres.2004.06.004.
Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–85. https://doi.org/10.1016/j.ophtha.2007.12.019.
Zhang J, Deng G. Protective effects of increased outdoor time against myopia: a review. J Int Med Res. 2019;48:0300060519893866. https://doi.org/10.1177/0300060519893866.
Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila). 2016;5:386–93. https://doi.org/10.1097/APO.0000000000000236.
Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci. 2018;115:E7091–100. https://doi.org/10.1073/pnas.1721443115.
Guo Y, Liu LJ, Xu L, et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology. 2013;120:277–83. https://doi.org/10.1016/j.ophtha.2012.07.086.
Jones-Jordan LA, Sinnott LT, Cotter SA, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012;53:7169–75. https://doi.org/10.1167/iovs.11-8336.
Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002;79:46–51. https://doi.org/10.1097/00006324-200201000-00012.
Yinon U. Myopia induction in animals following alteration of the visual input during development: a review. Curr Eye Res. 1984;3:677–90. https://doi.org/10.3109/02713688409003072.
Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vis Res. 1988;28:639–57. https://doi.org/10.1016/0042-6989(88)90113-7.
Dharani R, Lee CF, Theng ZX, et al. Comparison of measurements of time outdoors and light levels as risk factors for myopia in young Singapore children. Eye (Lond). 2012;26:911–8. https://doi.org/10.1038/eye.2012.49.
Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104:593. https://doi.org/10.1136/bjophthalmol-2019-314675.
Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–53. https://doi.org/10.1167/iovs.09-4689.
Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–54. https://doi.org/10.1167/iovs.09-3419.
Stone RA, Cohen Y, McGlinn AM, et al. Development of experimental myopia in chicks in a natural environment. Invest Ophthalmol Vis Sci. 2016;57:4779–89. https://doi.org/10.1167/iovs.16-19310.
Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014;56:299–309. https://doi.org/10.1167/iovs.14-15499.
Smith EL, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–8. https://doi.org/10.1167/iovs.11-8652.
Chen S, Zhi Z, Ruan Q, et al. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017;58:2306–16. https://doi.org/10.1167/iovs.16-20402.
He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–8. https://doi.org/10.1001/jama.2015.10803.
French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013;120:2100–8. https://doi.org/10.1016/j.ophtha.2013.02.035.
Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125:1239–50. https://doi.org/10.1016/j.ophtha.2017.12.011.
Read SA, Pieterse EC, Alonso-Caneiro D, et al. Daily morning light therapy is associated with an increase in choroidal thickness in healthy young adults. Sci Rep. 2018;8:8200. https://doi.org/10.1038/s41598-018-26635-7.
Wang Y, Ding H, Stell WK, et al. Exposure to sunlight reduces the risk of myopia in rhesus monkeys. PLoS ONE. 2015;10:e0127863–e0127863. https://doi.org/10.1371/journal.pone.0127863.
Landis EG, Yang V, Brown DM, Pardue MT, Read SA. Dim light exposure and myopia in children. Invest Ophthalmol Vis Sci. 2018;59:4804–11. https://doi.org/10.1167/iovs.18-24415.
Landis EG, Park HN, Chrenek M, et al. Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci. 2021;62:28. https://doi.org/10.1167/iovs.62.1.28.
Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. https://doi.org/10.1016/j.exer.2012.08.004.
Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–19. https://doi.org/10.1016/j.exer.2013.02.007.
Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Progr Retin Eye Res. 2017;61:60–71. https://doi.org/10.1016/j.preteyeres.2017.06.003.
Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017;61:60–71. https://doi.org/10.1016/j.preteyeres.2017.06.003.
Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88:1092–9. https://doi.org/10.1016/j.exer.2009.01.012.
Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004;81:111–8. https://doi.org/10.1097/00006324-200402000-00009.
Sekaran S, Cunningham J, Neal MJ, Hartell NA, Djamgoz MB. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci. 2005;21:2199–208. https://doi.org/10.1111/j.1460-9568.2005.04051.x.
Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci. 2018;59:4413–24. https://doi.org/10.1167/iovs.18-23880.
Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. https://doi.org/10.1016/j.exer.2013.01.001.
Burfield HJ, Patel NB, Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2018;59:5176–87. https://doi.org/10.1167/iovs.18-25389.
Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom. 2019;102:99–108. https://doi.org/10.1111/cxo.12824.
Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–9. https://doi.org/10.1167/iovs.11-7364.
Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399–407. https://doi.org/10.1007/s00359-005-0077-2.
Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. https://doi.org/10.1016/j.exer.2012.08.002.
Wang J, Cui J, Zhu H. Suppression of type I collagen in human scleral fibroblasts treated with extremely low-frequency electromagnetic fields. Mol Vis. 2013;19:885–93.
Sliney DH. What is light? The visible spectrum and beyond. Eye (Lond). 2016;30:222–9. https://doi.org/10.1038/eye.2015.252.
Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vis Res. 2002;42:2409–17. https://doi.org/10.1016/s0042-6989(02)00262-6.
Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vis Res. 2009;49:1775–83. https://doi.org/10.1016/j.visres.2009.04.014.
Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–9. https://doi.org/10.1016/j.ebiom.2016.12.007.
Jiang X, Pardue MT, Mori K, et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci USA. 2021;118: e2018840118. https://doi.org/10.1073/pnas.2018840118.
Yang J, Yang L, Chen R, et al. A role of color vision in emmetropization in C57BL/6J mice. Sci Rep. 2020;10:14895. https://doi.org/10.1038/s41598-020-71806-0.
Strickland R, Landis EG, Pardue MT. Short-wavelength (violet) light protects mice from myopia through cone signaling. Invest Ophthalmol Vis Sci. 2020;61:13. https://doi.org/10.1167/iovs.61.2.13.
Liu R, Qian YF, He JC, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92:447–53. https://doi.org/10.1016/j.exer.2011.03.003.
Jiang L, Zhang S, Schaeffel F, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vis Res. 2014;94:24–32. https://doi.org/10.1016/j.visres.2013.10.020.
Smith EL III, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investig Ophthalmol Vis Sci. 2015;56:6490–500. https://doi.org/10.1167/iovs.15-17025.
Gawne TJ, Siegwart JT, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017;155:75–84. https://doi.org/10.1016/j.exer.2016.12.004.
Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–13. https://doi.org/10.1016/0002-9394(51)90481-3.
Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. https://doi.org/10.1001/archopht.119.11.1625.
Mutti DO, Mitchell GL, Jones LA, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. https://doi.org/10.1167/iovs.04-1040.
Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vis Res. 1992;32:833–42. https://doi.org/10.1016/0042-6989(92)90026-f.
Zhou X, Qu J, Xie R, et al. Normal development of refractive state and ocular dimensions in guinea pigs. Vis Res. 2006;46:2815–23. https://doi.org/10.1016/j.visres.2006.01.027.
Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–29.
Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vis Res. 1993;33:1311–24. https://doi.org/10.1016/0042-6989(93)90039-y.
Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vis Res. 1988;28:323–8. https://doi.org/10.1016/0042-6989(88)90160-5.
Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol A. 1992;170:565–74. https://doi.org/10.1007/BF00199333.
Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. https://doi.org/10.1093/ilar.40.2.59.
Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98:507–17. https://doi.org/10.1111/cxo.12312.
Schaeffel F, Howland HC. Mathematical model of emmetropization in the chicken. J Opt Soc Am A. 1988;5:2080–6. https://doi.org/10.1364/josaa.5.002080.
Smith EL, Hung LF, Arumugam B. Visual regulation of refractive development: insights from animal studies. Eye (Lond). 2014;28:180–8. https://doi.org/10.1038/eye.2013.277.
Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. https://doi.org/10.1016/j.neuron.2004.08.008.
Wildsoet CF. Active emmetropization–evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. https://doi.org/10.1111/j.1475-1313.1997.tb00059.x.
Kroger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996;179:837–42. https://doi.org/10.1007/BF00207362.
Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992;449:363–76. https://doi.org/10.1113/jphysiol.1992.sp019090.
Rucker FJ, Kruger PB. Cone contributions to signals for accommodation and the relationship to refractive error. Vis Res. 2006;46:3079–89. https://doi.org/10.1016/j.visres.2006.04.009.
Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vis Res. 1991;31:717–34. https://doi.org/10.1016/0042-6989(91)90011-s.
Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vis Res. 1993;33:1593–603. https://doi.org/10.1016/0042-6989(93)90026-s.
Rucker F. Monochromatic and white light and the regulation of eye growth. Exp Eye Res. 2019;184:172–82. https://doi.org/10.1016/j.exer.2019.04.020.
Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vis Res. 2008;48:1980–91. https://doi.org/10.1016/j.visres.2008.06.003.
Summers JA, Schaeffel F, Marcos S, Wu H, Tkatchenko AV. Functional integration of eye tissues and refractive eye development: mechanisms and pathways. Exp Eye Res. 2021;209: 108693. https://doi.org/10.1016/j.exer.2021.108693.
Ashby R. Animal studies and the mechanism of myopia—protection by light? Optometry Vis Sci. 2016;93:2.
Norton TT, Siegwart JT Jr. Animal models of emmetropization: matching axial length to the focal plane. J Am Optom Assoc. 1995;66:405–14.
Schaeffel F, Diether S. The growing eye: an autofocus system that works on very poor images. Vis Res. 1999;39:1585–9. https://doi.org/10.1016/s0042-6989(98)00304-6.
Park TW, Winawer J, Wallman J. Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vis Res. 2003;43:1519–31. https://doi.org/10.1016/s0042-6989(03)00180-9.
Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis Res. 1997;37:2183–94. https://doi.org/10.1016/s0042-6989(97)00026-6.
Cernota N, Rucker FJ, Wallman J. When severe astigmatic blur reduces visual cues, monochromatic light impairs the choroidal component of lens compensation. Investig Ophthalmol Vis Sci. 2012;53:3443–3443.
Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalm Physiol Opt. 2013;33:196–214. https://doi.org/10.1111/opo.12050.
Swanson WH, Cohen JM. Color vision. Ophthalmol Clin. 2003;16:179–203. https://doi.org/10.1016/S0896-1549(03)00004-X.
Rucker FJ, Kruger PB. Accommodation responses to stimuli in cone contrast space. Vis Res. 2004;44:2931–44. https://doi.org/10.1016/j.visres.2004.07.005.
Kelly DH, van Norren D. Two-band model of heterochromatic flicker. J Opt Soc Am. 1977;67:1081–91. https://doi.org/10.1364/JOSA.67.001081.
Hendry SHC, Reid RC. The koniocellular pathway in primate vision. Ann Rev Neurosci. 2000;23:127–53. https://doi.org/10.1146/annurev.neuro.23.1.127.
Muller B, Peichl L. Topography of cones and rods in the tree shrew retina. J Comp Neurol. 1989;282:581–94. https://doi.org/10.1002/cne.902820409.
Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–2. https://doi.org/10.1038/17383.
Wallman J. Myopia and the control of eye growth. Introduction. Ciba Found Symp. 1990;155:1–4. https://doi.org/10.1002/9780470514023.ch1.
Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686:169–81. https://doi.org/10.1016/0006-8993(95)00370-6.
Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12:123–9. https://doi.org/10.1167/12.6.23.
Britton S, Fellows M, Rucker F. Temporal frequency sensitivity of the emmetropization mechanism in chicks to color and luminance flicker without blue light. Invest Ophthalmol Vis Sci. 2013;54:5178.
Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. NeuroReport. 2002;13:1029–32. https://doi.org/10.1097/00001756-200206120-00010.
Crewther SG, Barutchu A, Murphy MJ, Crewther DP. Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Exp Eye Res. 2006;83:322–8. https://doi.org/10.1016/j.exer.2005.12.016.
Kee CS, Hung LF, Qiao-Grider Y, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–62. https://doi.org/10.1167/iovs.06-0743.
Rucker F, Britton S, Spatcher M, Hanowsky S. Blue light protects against temporal frequency sensitive refractive changes. Invest Ophthalmol Vis Sci. 2015;56:6121–31. https://doi.org/10.1167/iovs.15-17238.
Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vis Res. 1997;37:2661–73. https://doi.org/10.1016/s0042-6989(97)00114-4.
Di Y, Liu R, Chu RY, Zhou XT, Zhou XD. Myopia induced by flickering light in guinea pigs: a detailed assessment on susceptibility of different frequencies. Int J Ophthalmol. 2013;6:115–9. https://doi.org/10.3980/j.issn.2222-3959.2013.02.01.
Di Y, Lu N, Li B, et al. Effects of chronic exposure to 0.5 Hz and 5 Hz flickering illumination on the eye growth of guinea pigs. Curr Eye Res. 2013;38:1182–90. https://doi.org/10.3109/02713683.2013.807931.
Yu Y, Chen H, Tuo J, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalm Res. 2011;46:80–7. https://doi.org/10.1159/000323179.
Luo X, Li B, Li T, et al. Myopia induced by flickering light in guinea pig eyes is associated with increased rather than decreased dopamine release. Mol Vis. 2017;23:666–79.
Liu R, Hu M, He JC, et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Investig Ophthalmol Vis Sci. 2014;55:1901–9. https://doi.org/10.1167/iovs.13-12276.
Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013;54:8004–12. https://doi.org/10.1167/iovs.13-12476.
Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Investig Ophthalmol Vis Sci. 2018;59:4413–24. https://doi.org/10.1167/iovs.18-23880.
Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018;176:147–60. https://doi.org/10.1016/j.exer.2018.07.004.
Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vis Res. 2017;140:55–65. https://doi.org/10.1016/j.visres.2017.07.011.
Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy in myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2021. https://doi.org/10.1016/j.ophtha.2021.11.023.
Zhou L, Xing C, Qiang W, Hua C, Tong L. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic Physiolo Opt. 2022. https://doi.org/10.1111/opo.12939.
Geneva II. Photobiomodulation for the treatment of retinal diseases: a review. Int J Ophthalmol. 2016;9:145–52. https://doi.org/10.18240/ijo.2016.01.24.
Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. 2016;48:343–9. https://doi.org/10.1002/lsm.22471.
Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg. 2011;30:231–3. https://doi.org/10.1089/pho.2011.3044.
Lu Y, Wang R, Dong Y, et al. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165–82. https://doi.org/10.1016/j.neurobiolaging.2016.10.003.
Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex—evidence from two transgenic mouse models. Alzheimers Res Therapy. 2014;6:2. https://doi.org/10.1186/alzrt232.
Leung MCP, Lo SCL, Siu FKW, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med. 2002;31:283–8. https://doi.org/10.1002/lsm.10096.
Whelan HT, Smits RL, Buchmann EV, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–14. https://doi.org/10.1089/104454701753342758.
Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–71. https://doi.org/10.1074/jbc.M409650200.
Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye and brain. 2011;3:49–67. https://doi.org/10.2147/EB.S21391.
Assia E, Rosner M, Belkin M, Solomon A, Schwartz M. Temporal parameters of low energy laser irradiation for optimal delay of post-traumatic degeneration of rat optic nerve. Brain Res. 1989;476:205–12. https://doi.org/10.1016/0006-8993(89)91240-7.
Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep. 2016;6:9. https://doi.org/10.1038/s41598-016-0002-7.
Mathis U, Feldkaemper M, Wang M, Schaeffel F. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefes Arch Clin Exp Ophthalmol. 2020;258:319–33. https://doi.org/10.1007/s00417-019-04573-y.
Wu J, Liu Q, Yang X, Yang H, Wang X-m, Zeng J-w. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res. 2007;1186:155–63. https://doi.org/10.1016/j.brainres.2007.09.077.
Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol. 2009;47:256–63. https://doi.org/10.1016/j.yjmcc.2009.03.009.
Shiva S, Gladwin MT. Shining a light on tissue NO stores: near infrared release of NO from nitrite and nitrosylated hemes. J Mol Cell Cardiol. 2009;46:1–3. https://doi.org/10.1016/j.yjmcc.2008.10.005.
Oishi JC, De Moraes TF, Buzinari TC, Cárnio EC, Parizotto NA, Rodrigues GJ. Hypotensive acute effect of photobiomodulation therapy on hypertensive rats. Life Sci. 2017;178:56–60. https://doi.org/10.1016/j.lfs.2017.04.011.
Kosaka H. Nitric oxide and hemoglobin interactions in the vasculature. Biochim Biophys Acta. 1999;1411:370–7. https://doi.org/10.1016/s0005-2728(99)00026-2.
Zhang R, Mio Y, Pratt PF, et al. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol. 2009;46:4–14. https://doi.org/10.1016/j.yjmcc.2008.09.707.
Quirk BJ, Whelan HT. What lies at the heart of photobiomodulation: light, cytochrome c oxidase, and nitric oxide-review of the evidence. Photobiomodul Photomed Laser Surg. 2020;38:527–30. https://doi.org/10.1089/photob.2020.4905.
Mokoena D, Dhilip Kumar SS, Houreld NN, Abrahamse H. Role of photobiomodulation on the activation of the Smad pathway via TGF-β in wound healing. J Photochem Photobiol B Biol. 2018;189:138–44. https://doi.org/10.1016/j.jphotobiol.2018.10.011.
Mokoena DR, Houreld NN, Dhilip Kumar SS, Abrahamse H. Photobiomodulation at 660 nm stimulates fibroblast differentiation. Lasers Surg Med. 2020;52:671–81. https://doi.org/10.1002/lsm.23204.
Otterço AN, Andrade AL, Brassolatti P, Pinto KNZ, Araújo HSS, Parizotto NA. Photobiomodulation mechanisms in the kinetics of the wound healing process in rats. J Photochem Photobiol B. 2018;183:22–9. https://doi.org/10.1016/j.jphotobiol.2018.04.010.
Lin G, Taylor C, Rucker F. Effect of duration, and temporal modulation, of monochromatic light on emmetropization in chicks. Vis Res. 2020;166:12–9. https://doi.org/10.1016/j.visres.2019.11.002.
Wang H, Zhuang K, Gao L, Zhang L, Yang H. Increased expression of CCN2 in the red flashing light-induced myopia in guinea pigs. BioMed Res Int. 2013;2013: 761823. https://doi.org/10.1155/2013/761823.
Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investig Ophthalmol Vis Sci. 2013;54:8004–12. https://doi.org/10.1167/iovs.13-12476.
Thakur S, Dhakal R, Verkicharla PK. Short-term exposure to blue light shows an inhibitory effect on axial elongation in human eyes independent of defocus. Investig Ophthalmol Vis Sci. 2021;62:22–22. https://doi.org/10.1167/iovs.62.15.22.
Ward AH, Norton TT, Huisingh CE, Gawne TJ. The hyperopic effect of narrow-band long-wavelength light in tree shrews increases non-linearly with duration. Vis Res. 2018;146–147:9–17. https://doi.org/10.1016/j.visres.2018.03.006.
Gisbert S, Feldkaemper M, Wahl S, Schaeffel F. Interactions of cone abundancies, opsin expression, and environmental lighting with emmetropization in chickens. Exp Eye Res. 2020;200: 108205. https://doi.org/10.1016/j.exer.2020.108205.
Acknowledgements
Funding
No funding or sponsorship was received for this study. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Huang Zhu MD contributed to the study conception and design. Literature review, analysis, and chart making were performed by Pengbo Zhang. The draft of the manuscript was written by Pengbo Zhang. All authors read and approved the final manuscript.
Disclosures
Huang Zhu and Pengbo Zhang have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, P., Zhu, H. Light Signaling and Myopia Development: A Review. Ophthalmol Ther 11, 939–957 (2022). https://doi.org/10.1007/s40123-022-00490-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00490-2