Abstract
Introduction
The risk of thromboembolic events or death in patients treated with intravitreal anti-vascular endothelial growth factor (IVT anti-VEGF) is poorly described on a large scale and by molecule. This study aimed to assess the risk of myocardial infarction (MI), stroke, or death in new users of IVT aflibercept versus ranibizumab in real-world practice.
Methods
A nationwide cohort study using the French National Health Insurance databases covering 99% of the French population was conducted in patients aged 18 years or older who initiated IVT therapy with ranibizumab or aflibercept between 2014 and 2018. Patients were followed for up to 6 years until December 31, 2019. The risks of MI, stroke, and death were compared in new aflibercept versus ranibizumab users using Kaplan–Meier and multivariate Cox proportional hazards models adjusted on sociodemographic characteristics and cardiovascular disease or risk factors. Subgroup analyses were performed according to history of ischemic heart disease or stroke, diabetes, indication for treatment, sex, age, and number of IVT anti-VEGF injections.
Results
When compared to new users of ranibizumab (n = 174,794, mean age 76.0 ± 11.9 years, 59.2% female), new users of aflibercept (n = 76,242, mean age 76.6 ± 11.2 years, 59.2% female) did not have an increased risk of MI (n = 1523 incident MI, adjusted hazard ratio [aHR] 1.00; 95% CI 0.89–1.11), stroke (n = 2306 incident strokes, aHR 1.03; 95% CI 0.95–1.13), or death (n = 4135 deaths, aHR 0.98; 95% CI 0.92–1.05). However, a small but non-statistically significant increase in the risk of stroke was observed in new users of aflibercept versus ranibizumab among patients with diabetes (aHR 1.15; 95% CI 0.98–1.35), particularly those with diabetic macular edema (aHR 1.20; 95% CI 1.00–1.44). The remaining subgroup analyses did not change the results.
Conclusion
Aflibercept and ranibizumab appear to have similar safety profiles with respect to the risk of MI, stroke, or death under real-world conditions of use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Uncertainties remain regarding the risk of thromboembolic events or death in patients treated with intravitreal anti-vascular endothelial growth factor, which is poorly described on a large scale and by molecule. |
These potential risks may be more likely to occur with aflibercept due to possible systemic accumulation than with ranibizumab. Assessing this potential risk could help improve patient management. |
What was learned from the study? |
This French population-based retrospective cohort study showed that initiation of intravitreal aflibercept was not associated with an increased risk of myocardial infarction, stroke, or death compared to initiation of intravitreal ranibizumab under real-life conditions of use. |
The safety profiles appear to be similar for aflibercept and ranibizumab intravitreal injections with respect to the risk of myocardial infarction, stroke, or death under real-world conditions of use. |
Introduction
Intravitreal (IVT) anti-vascular endothelial growth factor (anti-VEGF) agents have radically changed the management of neovascular retinal diseases [1]. Ranibizumab and aflibercept were approved in Europe in 2007 and 2012, respectively, for IVT treatment of neovascular age-related macular degeneration (nAMD). Bevacizumab, which was approved in oncology, received a recommendation for IVT use in nAMD in France in 2015 because of its beneficial cost-effectiveness ratio [2]. Since 2011, ranibizumab and aflibercept have been approved in Europe for the treatment of other ocular diseases associated with retinal edema and/or neovascularization, such as diabetic macular edema (DME), macular edema secondary to retinal vein occlusion (RVO), and myopic choroidal neovascularization (CNV). In France, IVT anti-VEGF treatments are completely covered by the national health insurance, subject to an ophthalmological prescription attesting that the indication for treatment is justified, and the number of reimbursed injections is not limited.
VEGF is a potent vasodilator and also plays an important role in the development of collateral circulation in peripheral arterial ischemia or myocardial ischemia. Therefore, prolonged systemic use of anti-VEGF agents in oncology has been associated with an increased risk of hypertension and embolism [3]. Although the doses used for IVT injections are much lower than those in oncology, the systemic passage (even small) of these agents makes cardiovascular risks plausible, especially in high-risk patients (e.g., elderly patients, diabetics, patients with a recent cardiovascular event) [4]. The risk of thromboembolic events has not been established in randomized clinical trials, which are generally based on selected populations and are not sufficiently powered to detect rare side effects [4]. The vast majority of observational studies conducted on the topic have found that IVT anti-VEGF treatment is safe [5,6,7,8,9,10,11,12,13,14,15]. However, most of these studies lacked the statistical power to assess the risk in subgroups of patients with risk factors and may have been subject to bias, particularly related to the choice of a comparative group not exposed to IVT anti-VEGF.
Comparative data on systemic risk between the molecules under actual conditions of use are limited, although their distinct pharmacokinetic and pharmacodynamic profiles may be associated with differences in the occurrence of such risks. Ranibizumab appears transiently in the systemic circulation, is cleared rapidly, and has little effect on plasma-free VEGF levels, whereas bevacizumab and aflibercept have greater systemic exposure, appear to accumulate with repeated dosing, and produce a marked reduction in plasma-free VEGF, making the occurrence of systemic side effects possible, particularly with the latter two molecules [4, 16]. Observational studies comparing the systemic safety of IVT anti-VEGF are scarce and generally found no difference between the molecules [5, 11,12,13]. However, they rarely included patients using aflibercept, because of its more recent approval. This longitudinal study aimed to evaluate the risk of myocardial infarction (MI), stroke, and death in patients initiating treatment with aflibercept versus ranibizumab in the general French population.
Methods
Data Source
Patients with a first injection of IVT anti-VEGF between January 1, 2014, and December 31, 2018, were identified from the French National Health Insurance Databases, i.e., Système National des Données de Santé (SNDS), covering 99% of the French population (i.e., more than 66 million inhabitants). The SNDS contains anonymized individual data on all reimbursed health expenditures, including drugs (coded according to the Anatomical Therapeutic Classification [ATC]), ambulatory health care, and outpatient laboratory tests. The SNDS does not contain the medical indications of the reimbursements recorded, but the existence of 100% coverage of the patient for care related to a serious and costly long-term illness (cancer, diabetes, ischemic heart disease, etc.) is indicated and coded according to the International Classification of Diseases, 10th Revision (ICD-10), as well as the date of the onset of the illness. The SNDS also contains sociodemographic data, such as age, sex, area of residence (zip code), and deprivation index, corresponding to an index of the participant’s area of residence calculated from socioeconomic data [17]. This information is linked, via a unique and anonymous individual identifier, to the national hospital database, which contains data on all stays in public and private hospitals (i.e., admission and discharge dates, ICD-10 coded diagnoses, care and surgical procedures performed, costly drugs, or medical devices provided). The SNDS has been described and used in other pharmacoepidemiological studies [18,19,20,21,22]. This research adheres to the Declaration of Helsinki of 1964 and its later amendments. Institutional review board approval was not required because the data in the SNDS are fully anonymous.
Study Population
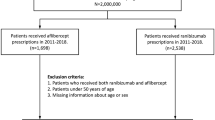
Individuals 18 years of age or older with at least one IVT injection of aflibercept (ATC code S01LA05) or ranibizumab (S01LA04) between 2014 and 2018 registered in the SNDS databases were initially identified. IVT bevacizumab users were not included because in France this drug is only prescribed in hospitals and its use is rare (n = 180 patients who initiated treatment between 2014 and 2018). Patients with a first injection of IVT ranibizumab or aflibercept (i.e., new users) were included. New use was defined as the absence of any IVT anti-VEGF (i.e., ranibizumab, aflibercept, pegaptanib-S01LA03, and bevacizumab-L01XC07) injection in the last 2 years. The index date corresponds to the first IVT anti-VEGF dispensing date recorded during the inclusion period.
Exposure Definition
Patients who initiated aflibercept therapy were compared to those who initiated ranibizumab therapy.
In the main analyses, we hypothesized that only sufficient anti-VEGF accumulation in the blood could be consistent with the occurrence of a systemic adverse outcome [23]. Thus, patients who received a single IVT anti-VEGF injection, those who received different molecules, or those who were lost to follow-up or died within 3 months of the index date were secondarily excluded. These patients were re-included in a secondary analysis described below.
Follow-Up
Patients who initiated treatment were followed from the index date plus 3 months until one of the following events, whichever came first: occurrence of an outcome of interest or cancer, end of study (12-31-2019), loss to follow-up, change of IVT anti-VEGF molecule, or treatment interruption. A treatment interruption was considered to have occurred when a period of more than 6 months was observed between two IVT anti-VEGF injections or between the last IVT anti-VEGF injection and the end of follow-up. The interruption date corresponded to the most recent date in these intervals, and patients were censored 9 months after the interruption date.
Outcome Definition
The outcomes were defined as the occurrence of MI or stroke resulting in hospitalization during the follow-up or death. MI and stroke were identified by using the main or related diagnoses of incident hospital stays registered in the national hospital database (ICD-10 codes for MI: I21, I22; ICD-10 codes for stroke: I60, I61, I62, I63, I64). In the main analyses, MI and stroke that occurred between the index date and the start of follow-up (i.e., 3 months after the index date) were considered as medical history.
Covariates: Sociodemographic Characteristics and Medical History of Intravitreal Anti-VEGF Users
Age, sex, and deprivation index were measured at the index date. History of ischemic heart disease (including MI), arrhythmia or conduction disorders (including non-valvular atrial fibrillation), heart failure, stroke, chronic smoking index, morbid obesity index, and chronic alcoholism index were measured within 6 years before the index date. Dispensing of antihypertensive, lipid-lowering, antiplatelet, antidiabetic, anticoagulant, and antiarrhythmic treatments was measured during the year preceding the index date. Likely and possible indications for IVT anti-VEGF were inferred on the basis of medical information recorded in the 2 years before and after the index date. Medical history was identified on the basis of ICD-10 codes recorded on the day of a hospitalization or diagnosis of a long-term illness, or reimbursements for medications or disease-specific care. Medical history and indications for IVT anti-VEGF therapy were identified using the algorithms provided in the electronic Supplementary Material, Table S1.
Statistical Analysis
New users of aflibercept and ranibizumab were described according to the distribution of covariates mentioned previously. Incidence of MI, stroke, and death was calculated for each group. Kaplan–Meier analyses were used to assess the 6-year risk of MI, stroke, or death in new users of aflibercept and ranibizumab.
The risks of MI, stroke, or death in new users of aflibercept compared to new users of ranibizumab were assessed using univariate and multivariate Cox proportional hazards regression models. Multivariate models were adjusted on year of inclusion, sociodemographic variables (i.e., age, sex, deprivation index, and region of residence), cardiovascular diseases or risk factors (i.e., history of ischemic heart disease including MI, heart failure, stroke, arrhythmia diagnosis or antiarrhythmic treatment, history of treatment by antihypertensive, lipid-lowering, antiplatelet, oral anticoagulant, and antidiabetic drugs, history of chronic smoking, morbid obesity, and chronic alcoholism), and origin of the prescription (i.e., private or hospital practitioner).
The risks of MI, stroke, or death in patients initiating treatment with aflibercept versus ranibizumab were also assessed using multivariate models weighted by the inverse propensity score to validate the findings of the main multivariate models. Weights were derived from the reciprocal of the propensity scores containing the same covariates as those described in the main multivariate models. To reduce instability induced by large weights, the stabilized weights were computed and then were truncated at the first and 99th percentiles. The standardized differences method for assessing balance in observed baseline covariates between new users of aflibercept and new users of ranibizumab was applied to compare the prevalence of covariates in the stabilized weighted sample. Imbalances below 10% were considered negligible. Adjustments on age, sex, and region of residence were added in multivariate models weighted by the inverse propensity score.
Subgroup analyses were conducted according to history of ischemic heart disease (including MI) or stroke, treatment indications (AMD or DME), history of diabetes, age groups (≤ 70, 71–80 or > 80 years), sex, and number of IVT anti-VEGF injections received during the follow-up (2–3, 4–8, or > 8).
Secondary Analyses
Secondary analyses were conducted to assess potential variations in outcomes by (1) changing the definition of censoring (i.e., 1, 3, or 6 months after treatment interruption versus 9 months in the main analyses), (2) considering only ischemic stroke as an event (ICD-10 code: I63) instead of all strokes (i.e., ischemic and hemorrhagic), and (3) reintroducing patients who received a single injection of IVT anti-VEGF. In the latter analysis, patients were followed from the index date until the occurrence of one of the censoring events.
All statistical tests were two-tailed, and we considered p values less than 0.01 to be statistically significant. Statistical analyses were performed using SAS Enterprise Guide software, version 7.15.
Results
Description of the Study Population
A total of 76,242 new users of aflibercept (mean age 76.6 ± 11.2 years, 59.2% female) and 174,794 new users of ranibizumab (mean age 76.0 ± 11.9 years, 59.2% female) who received at least two injections in the first 3 months were included. The characteristics of these patients are described in Table 1. New users of aflibercept and ranibizumab presented similar sociodemographic and medical characteristics. In particular, cardiovascular comorbidities or risk factors (i.e., history of ischemic heart disease, arrhythmia or conduction disorders, heart failure, stroke, chronic smoking, morbid obesity, and chronic alcoholism) and the use of systemic cardiovascular (i.e., antihypertensives, lipid-lowering drugs, antiplatelet drugs, oral anticoagulants, and antiarrhythmics) and antidiabetic drugs were common, with similar prevalence in the comparison groups. The main treatment indications were AMD (74.3% for aflibercept and 73.0% for ranibizumab new users) and DME (22.0% for aflibercept and 22.7% for ranibizumab new users). For patients who initiated aflibercept therapy, the primary prescription was more often hospital-based than for patients who initiated ranibizumab therapy (31.4 versus 27.1%).
Risk of Myocardial Infarction, Stroke, or Death
During a maximum follow-up of 6 years (mean 12.1 ± 9.2 months; 13.5 ± 10.3 months for aflibercept and 11.5 ± 8.7 months for ranibizumab), 1523 MI, 2306 strokes, and 4135 deaths occurred after a mean of 9.0, 9.6, and 9.8 months, respectively. Other censoring reasons were treatment interruption (62.8%), change of IVT anti-VEGF molecule (18.0%), end of the study period (12.5%), cancer (3.0%), and loss to follow-up (0.5%), as shown in the electronic Supplementary Material, Table S2.
In Kaplan–Meier analyses, new users of aflibercept and ranibizumab had similar cumulative risk of MI, stroke, or death (log-rank test p = 0.76 for MI; p = 0.80 for stroke; p = 0.18 for death) (Fig. 1). Among patients who initiated aflibercept therapy, incidence rates per 1000 person-years were 5.9 for MI, 9.2 for stroke, and 15.9 for death. These rates were 6.1 for MI, 9.1 for stroke, and 16.6 for death among patients who initiated ranibizumab therapy. Compared with new use of ranibizumab, new use of aflibercept was not associated with an increased risk of MI (adjusted hazard ratio [aHR] 1.00; 95% CI 0.89–1.11), stroke (aHR 1.03; 95%CI 0.95–1.13), or death (aHR 0.98; 95% CI 0.92–1.05) (Table 2, and Table S3 in the electronic Supplementary Material presenting the full models).
Subgroup analyses by history of ischemic heart disease (including MI) or stroke (Table 3), indications for treatment (Table 4), history of diabetes (see Table S4 in the electronic Supplementary Material), age or sex (see Table S5 in the electronic Supplementary Material), and number of IVT anti-VEGF injections received during the follow-up (see Table S6 in the electronic Supplementary Material) did not alter the conclusions. However, a small but non-statistically significant increase in the risk of stroke was found for new users of aflibercept compared to new users of ranibizumab among individuals with DME (aHR 1.20; 95% CI 1.00–1.44) or diabetes (aHR 1.15; 95% CI 0.98–1.35) (see Table 4 and Table S4 in the electronic Supplementary Material).
After weighting by the inverse propensity score, the prevalence of categorical covariates was very similar between the comparison groups (see Figure S1 in the electronic Supplementary Material). Similar estimates were found in the multivariate models weighted by the inverse propensity score, which validated our main multivariate approach (Table 2).
Secondary Analyses
Changing the definition of censoring (i.e., 1, 3, 6 months after treatment interruption or withdrawal instead of 9 months in the main analyses) did not alter the conclusions (see Table S7 in the electronic Supplementary Material). Considering ischemic stroke as the event instead of all strokes did not modify the conclusions (n = 1880 ischemic strokes, aHR 1.03; 95% CI 0.94–1.14). Moreover, reintroducing patients with a single IVT anti-VEGF injection within the 3 three months (i.e., 17,010 new users of aflibercept and 47,786 new users of ranibizumab) did not change the results (see Table S8 in the electronic Supplementary Material).
Discussion
Main Results
This observational cohort study, conducted on the scale of the entire French population using national health insurance databases, showed that there was no increased risk of myocardial infarction, stroke, or death in patients initiating treatment with aflibercept (n = 76,242) compared to patients initiating treatment with ranibizumab (n = 174,794). Similar findings were obtained when assessing these risks by sex, age, history of ischemic heart disease (including MI) or stroke, and number of injections received during the follow-up (2–3, 4–8, or > 8). However, a small but non-statistically significant increase in the risk of stroke was observed in patients initiating aflibercept therapy compared with those initiating ranibizumab therapy in patients with diabetes, and particularly in those also being managed for DME.
Comparison with Other Studies
Few studies have compared the systemic safety of IVT anti-VEGF agents. Our results are supported by safety data from the two pivotal phase III clinical trials, VIEW 1 and VIEW 2, which enrolled 2457 patients to assess the non-inferiority and clinical equivalence of aflibercept versus ranibizumab and showed no differences in the incidence of systemic adverse events between the molecules [24, 25]. A meta-analysis of 24 randomized controlled trials involving 6000 patients treated for DME also concluded that there were no signals of difference in overall safety between ranibizumab, bevacizumab, and aflibercept, but reported that estimates were imprecise for cardiovascular events and death [26]. Nevertheless, the clinical trials and meta-analyses of these trials may have limitations including a lack of power to detect these rare adverse events and poor generalizability to patients receiving the treatment in routine clinical practice [4, 13, 27, 28]. Therefore, large-scale post-marketing observational studies, such as the one presented here, provide important safety information that complements the clinical trial data. Our results are supported by a retrospective cohort study comparing patients initiating treatment for nAMD, diabetic retinal disease, or retinal venous occlusive disease, with bevacizumab (n = 69,007), ranibizumab (n = 10,895), or aflibercept (n = 7942), registered in a large US administrative claims database of commercially insured and Medicare Advantage enrollees. The study showed no difference between the molecules in the risk of acute MI, acute cerebrovascular diseases, major bleeding or stroke, or all-cause hospitalization within 180 days of starting treatment. As in our study, no statistically significant difference in risk was found in the subgroups of patients with risk factors [5]. However, this study did not assess the potential risks beyond 6 months after initiation of treatment, unlike ours. Other observational studies have compared the risk of systemic adverse events between bevacizumab and ranibizumab. It is interesting to compare the results of these studies with our own, as for both bevacizumab and aflibercept, the risks of systemic accumulation and subsequent adverse events after IVT injections appear to be greater than for ranibizumab. A retrospective cohort study of 146,942 Medicare beneficiaries treated for AMD between 2005 and 2006 showed significantly lower hazards of all-cause mortality, incident MI, and stroke with ranibizumab injection compared to bevacizumab injection after a maximum of 1 year of follow-up [12]. However, significant differences in study outcomes were no longer observed when taking potential indication bias into account. A nested case–control study conducted among 91,378 patients with retinal disease registered in the Ontario Health Insurance Plan database between 2006 and 2011 reported no increased risk of MI, stroke, venous thromboembolism, or heart failure in patients treated with bevacizumab compared to those treated with ranibizumab [13]. Other observational studies have been conducted to assess the safety of IVT anti-VEGF agents with respect to cardiovascular risk or mortality, but they were not designed to compare potential risk by molecule and are therefore not directly comparable to our results [6,7,8, 10, 29,30,31].
Strengths and Limitations
This observational cohort study conducted on the scale of the entire French population has several strengths. First, the real-world approach and the large sample size made it possible to assess the risk of rare systemic side effects. This allowed assessment of these risks in different subgroups of patients based on cardiovascular risk factors, and maximized the generalizability of the results. Second, our study had a maximum follow-up of 6 years, which enabled the evaluation of long-term adverse effects. Third, our study assessed systemic adverse effects in patients treated with IVT aflibercept versus ranibizumab. Most observational studies conducted on the subject have not compared the risk of systemic adverse events between IVT anti-VEGF molecules [6,7,8, 10, 29,30,31], whereas at least from a theoretical point of view, these risks could be higher with aflibercept and bevacizumab than with ranibizumab, due to pharmacokinetic differences [16]. Fourth, this study included a large sample of patients initiating aflibercept therapy, whereas most previous observational studies have been conducted in patients treated with ranibizumab or bevacizumab due to their earlier approval. Finally, the introduction of an active comparator (i.e., patients treated with ranibizumab) minimized residual confounding [32]. In routine practice, there does not appear to be a strong argument for prescribing a particular molecule, and patients who initiated treatment with aflibercept or ranibizumab were highly comparable in terms of sociodemographic characteristics, cardiovascular comorbidities potentially related to increased risk of cardiovascular outcome, and indications for treatment.
This study also has several limitations. First, information about treatment indication was not available directly in the SNDS databases. Although significant efforts were made to develop algorithms to identify indications for IVT anti-VEGF therapy, some misclassification cannot be ruled out. Because patients with nAMD and DME do not necessarily have the same cardiovascular risk profile, an imbalance between the comparison groups regarding treatment indications would compromise the validity of the results. Nevertheless, the distribution of treatment indications was similar between the groups, and differential misclassification of treatment indications between the comparison groups is unlikely. Second, some confounding factors, such as smoking, alcoholism, or obesity, are not measured perfectly from the SNDS databases. Nevertheless, selection of an IVT anti-VEGF agent based on these potential confounders is unlikely. Third, the purpose of this study was not to determine the systemic risk of all or any of the IVT anti-VEGF agents compared with a control group not treated with IVT anti-VEGF. Previous observational studies that have compared users of any IVT anti-VEGF to non-users (e.g., patients with dry AMD or other ocular disease, treated with intravitreal implants or photocoagulation, or with other chronic disease) may have introduced confounding due to associations of ocular disease with systemic cardiovascular risk in patients who require anti-VEGF therapy.
Conclusions
This study suggests that aflibercept and ranibizumab have similar risk profiles for myocardial infarction, stroke, or all-cause death after intravitreal injection in the management of retinal disease in routine clinical practice. The small but non-statistically significant increase in the risk of stroke observed in diabetic patients using aflibercept versus ranibizumab may warrant further investigations or precautions in these high cardiovascular risk patients.
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
CATT Research Group, Martin DF, Maguire MG, Ying G, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Ferroni P, Formica V, Roselli M, Guadagni F. Thromboembolic events in patients treated with anti-angiogenic drugs. Curr Vasc Pharmacol. 2010;8:102–13.
Porta M, Striglia E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med. 2020;15:199–210.
Maloney MH, Payne SR, Herrin J, Sangaralingham LR, Shah ND, Barkmeier AJ. Risk of systemic adverse events after intravitreal bevacizumab, ranibizumab, and aflibercept in routine clinical practice. Ophthalmology. 2021;128:417–24.
Maloney MH, Schilz SR, Herrin J, Sangaralingham LR, Shah ND, Barkmeier AJ. Risk of systemic adverse events associated with intravitreal anti-VEGF therapy for diabetic macular edema in routine clinical practice. Ophthalmology. 2019;126:1007–15.
Dalvin LA, Starr MR, AbouChehade JE, Damento GM, Garcia M, Shah SM, et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol. 2019;137:483–90.
Rim TH, Lee CS, Lee SC, Kim DW, Kim SS. Intravitreal ranibizumab therapy for neovascular age-related macular degeneration and the risk of stroke: a National Sample Cohort Study. Retina (Philadelphia, PA). 2016;36:2166–74.
Yashkin AP, Hahn P, Sloan FA. Introducing anti-vascular endothelial growth factor therapies for AMD did not raise risk of myocardial infarction, stroke, and death. Ophthalmology. 2016;123:2225–31.
Etminan M, Maberley DA, Babiuk DW, Carleton BC. Risk of myocardial infarction and stroke with single or repeated doses of intravitreal bevacizumab in age-related macular degeneration. Am J Ophthalmol. 2016;163:53–8.
Hwang DJ, Kim YW, Woo SJ, Park KH. Comparison of systemic adverse events associated with intravitreal anti-VEGF injection: ranibizumab versus bevacizumab. J Korean Med Sci. 2012;27:1580–5.
Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–9.
Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ. 2012;345:e4203.
Kim J, Kim DW, Kim DH, Ryu SY, Chung EJ. Risk of stroke associated with intravitreal ranibizumab injections in age-related macular degeneration: a nationwide case-crossover study. Eye (Lond). 2021;35:601–607.
Jeon H-L, Byun SJ, Pratt NL, Sultana J, Park SJ, Shin J-Y. Cardiovascular risk in patients receiving ranibizumab for exudative age-related macular degeneration: a nationwide self-controlled case-series study. Br J Ophthalmol. 2021;105:543–8.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37:1847–58.
Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33.
Billioti de Gage S, Bertrand M, Grimaldi S, Zureik M. Intravitreal anti-VEGF use in France: a cross-sectional and longitudinal Nationwide observational study. Acta Ophthalmol. Published online June 14, 2021.
Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:954–62.
Weill A, Dalichampt M, Raguideau F, Ricordeau P, Blotière P-O, Rudant J, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
Bouillon K, Bertrand M, Maura G, Blotière P-O, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2:e150-159.
Bouillon K, Bertrand M, Bader G, Lucot J-P, Dray-Spira R, Zureik M. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319:375–87.
Georgakopoulos CD, Pallikari A, Plotas P, Kagkelaris K, Mastronikolis S, Plota M, et al. Effect of intravitreal injection of aflibercept on cardiovascular risk parameters in patients with neovascular age related macular degeneration. Curr Rev Clin Exp Pharmacol. 2021;16:289–93.
Heier JS, Brown DM, Chong V, Korobelnik J-F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419.
Semeraro F, Morescalchi F, Parmeggiani F, Arcidiacono B, Costagliola C. Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration. Curr Vasc Pharmacol. 2011;9:629–46.
Vandenbroucke JP, Psaty BM. Benefits and risks of drug treatments: how to combine the best evidence on benefits with the best data about adverse effects. JAMA. 2008;300:2417–9.
Hanhart J, Comaneshter DS, Freier Dror Y, Vinker S. Mortality in patients treated with intravitreal bevacizumab for age-related macular degeneration. BMC Ophthalmol. 2017;17:189.
Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol. 2015;160:569-580.e5.
Pratt NL, Ramsay EN, Kemp A, Kalisch-Ellett LM, Shakib S, Caughey GE, et al. Ranibizumab and risk of hospitalisation for ischaemic stroke and myocardial infarction in patients with age-related macular degeneration: a self-controlled case-series analysis. Drug Saf. 2014;37:1021–7.
Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–8.
Acknowledgements
Funding
The journal’s rapid service fee will be covered by the ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: Sophie Billioti de Gage, Mahmoud Zureik. Acquisition, analysis, or interpretation of the data: all the authors. Drafting of the manuscript: Sophie Billioti de Gage. Critical revision of the manuscript for important intellectual content: all the authors. Statistical analyses: Marion Bertrand, Sophie Billioti de Gage. Administrative, technical, or material support: Marion Bertrand. Supervision: Mahmoud Zureik.
Prior Presentation
The manuscript was presented (oral presentation) at the 37th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (Virtual Conference, 24–28 August 2021).
Disclosures
Sophie Billioti de Gage, Marion Bertrand, Sébastien Grimaldi and Mahmoud Zureik have nothing to disclose.
Compliance with Ethics Guidelines
This research adheres to the Declaration of Helsinki of 1964, and its later amendments. Institutional review board approval was not required because the data in the French National Health Insurance Databases (SNDS) are fully anonymous.
Data Availability
Marion Bertrand and Sophie Billioti de Gage have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Billioti de Gage, S., Bertrand, M., Grimaldi, S. et al. Risk of Myocardial Infarction, Stroke, or Death in New Users of Intravitreal Aflibercept Versus Ranibizumab: A Nationwide Cohort Study. Ophthalmol Ther 11, 587–602 (2022). https://doi.org/10.1007/s40123-021-00451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00451-1