Abstract
Introduction
This study reports the outcomes of fluocinolone acetonide intravitreal implant (FAc, Iluvien®, SIFI, Italy) in patients affected by macular edema secondary to chronic non-infectious uveitis of the posterior segment (NIU-PS).
Methods
This was a retrospective study of patients with NIU-PS and macular thickening undergoing FAc implant at San Raffaele Hospital (Milan, Italy). Clinical data, including best-corrected visual acuity (BCVA), intraocular pressure (IOP), and central macular thickness (CMT), were collected at the time of FAc administration (baseline) and at 1, 6, and 12 months. The area under the curve (AUC) of the BCVA (AUCBCVA) and CMT (AUCCMT) was correlated with baseline factors; β estimates and 95% confidence interval (CI) are provided.
Results
Ten eyes of seven patients (60 ± 12 years; 4 male, 57%) were included. The BCVA significantly improved from month 6 (p = 0.03). The CMT improved from month 1 and was persistently lower than baseline until month 12 (p < 0.001). The AUCBCVA correlated with baseline BCVA (β = 2.5 logMAR; 95% CI 1.59–3.41; p < 0.001), while the mean AUCCMT positively correlated with the baseline CMT (β = 2.1 μm; 95% CI 0.41–3.80; p = 0.02). No adverse events were recorded over 1 year.
Conclusions
Better visual acuity at the time of FAc administration was associated with better visual function after FAc. Less severe macular edema correlated with better anatomic response. The FAc implant was a safe option for resolving macular edema secondary to NIU-PS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Macular edema in patients with posterior non-infectious uveitis (NIU-PS) may lead to irreversible visual loss if undertreated. |
Fluocinolone acetonide (FAc) intravitreal implant is effective in sustained control of macular edema in NIU-PS. |
The area under the curve (AUC) quantifies the global visual and morphologic improvement over time. |
What was learned from this study? |
The AUC of the best-corrected visual acuity (BCVA) after FAc correlates with the BCVA at the time of the injection but not with the macular thickness during the follow-up. |
The AUC of the central macular thickness (CMT) positively correlates with the baseline CMT and is perhaps negatively associated with the duration of uveitis. |
Introduction
Non-infectious uveitis of the posterior segment (NIU-PS) may lead to irreversible visual loss if undertreated. Complications such as macular edema, vitreous opacities, cataract, and choroidal or epiretinal neovascularization are major risk factors for poor visual prognosis [1].
Immunosuppressive therapy is the mainstay treatment of NIU-PS and allows long-term control of inflammation and prevention of local complications. Systemic corticosteroids are highly effective but bear an unacceptable rate of side effects on prolonged usage. Systemic immunomodulatory therapies are steroid-sparing options [2]. However, these drugs are not free from systemic toxicity and are contraindicated in specific categories of patients. Furthermore, complete control of intraocular inflammation and prevention of its recurrence are not always accomplished with systemic treatments [2]. In keeping with this, the uveitis specialists’ interest has been progressively heading toward local routes of treatment delivery, alone or in combination with systemic medications.
Depot corticosteroid implants allow sustained release of steroids to the posterior segment [3], with a favorable burden of side effects [4]. The fluocinolone acetonide intravitreal implant (FAc, Iluvien® SIFI, Italy) is a 0.19-mg intravitreal non-bioerodable implant that releases the drug steadily and continuously into the vitreous cavity for up to 3 years. FAc implant is approved in Europe for the treatment of diabetic macular edema (DME) and NIU-PS [5]. The efficacy of the implant has been recently evaluated with an area under the curve (AUC) approach in eyes with DME [6]. No such analysis has been employed in the uveitis field.
Here, we report the outcomes of FAc implant in patients affected by chronic non-infectious posterior uveitis, quantifying the functional and morphologic response in terms of AUC; furthermore, we provide the analysis of the clinical factors associated with the AUC up to 1 year.
Methods
This is a single-center observational study of patients with NIU-PS undergoing FAc implant at the Uveitis Service of the Department of Ophthalmology, San Raffaele Hospital (Milan, Italy) between June 2018 and May 2020. This study adhered to the Helsinki declaration and its later amendments. The local institutional review board approved the retrospective review of data. The FAc implant was administered off-label, and individual participants gave their written informed consent when receiving the implant.
In our clinical practice, FAc implant was prescribed by the treating physician (G.M. or E.M.) on the basis of each single case. Overall, inclusion criteria were NIU-PS complicated by macular edema; persistence of macular edema and/or posterior-segment inflammation despite systemic immunosuppressive therapy; previous clinical response to intravitreous dexamethasone implant. Patients with infectious uveitis and those with a history of uncontrolled glaucoma or steroid response were not eligible for receiving the FAc implant. In the case of intraocular pressure (IOP) elevation judged as potentially harmful by the treating physician, IOP-lowering measures were adopted. In patients with bilateral active disease, both eyes received the FAc implant.
The diagnosis of NIU-PS was made through a detailed medical and ocular history, specific laboratory investigations, and non-invasive imaging tests, such as optical coherence tomography (OCT), fundus fluorescein angiography (FFA), or indocyanine green angiography (ICGA). The implant was injected through the pars plana into the vitreous cavity using a 25-gauge applicator in a sterile setting by a single physician (F.B.). The patients were instructed to return every 2 months to monitor treatment response and potential side effects.
Data Collection
Medical and ocular history and previous ocular and systemic treatments were collected from the electronic patients’ charts. All the patients underwent a complete ocular examination with measurement of best-corrected visual acuity (BCVA), slit-lamp examination, Goldmann applanation tonometry, and ocular fundus exam through a dilated pupil. Spectral-domain OCT (Spectralis Heidelberg Retinal Angiograph+, Heidelberg, Germany) was performed at each visit; other imaging modalities, such as ultra-widefield fundus photography (UWF-FP; California; Optos, Dunfermline, Scotland, UK), infrared reflectance (IR), fundus autofluorescence (FAF), FFA, or ICGA were undertaken on the physicians’ discretion.
The clinical data, including the BCVA and the IOP, the central macular thickness (CMT), and the central choroidal thickness (CCT), were collected at the time of FAc administration (baseline) and 1 month, 6 months, and 12 months after the injection. The CMT was automatically extracted from the central 1-mm-diameter circle of ETDRS thickness map using the Spectralis Software (Heidelberg Eye Explorer 1.9.11.0, Heidelberg, Germany). The CCT was measured as the space from the outer section of the retinal pigment epithelium to the hyporeflective line of the sclero-choroidal interface through the Heidelberg caliper by a trained resident (V.S.). These measurements were performed at the center of the fovea on a horizontal enhanced depth imaging-OCT scan.
Adverse events, such as the need of IOP-lowering medications or procedures, were recorded.
Statistical Analysis
Statistical calculations were performed with the open-source programming language R.
The BCVA was transformed into logMAR and used as a continuous variable. Descriptive statistics were summarized as mean ± standard deviation (SD) or median and interquartile range (IQR) for quantitative variables and absolute and relative frequencies for categorical variables.
The primary outcome of the study was the measurement of the BCVA and CMT over a 12-month follow-up. The BCVA and CMT changes over time were investigated with linear mixed models with a repeated-measures design. In this analysis, BCVA and CMT were considered as the dependent variables. The follow-up visit was the fixed factor; the patients’ and eyes’ identification numbers were the random effect terms, with a nested structure to account for “within-subject” and “within-eye” correlations. Pairwise differences between time points were assessed with Tukey’s correction for multiple comparisons. The IOP values were analyzed over time with a similar method.
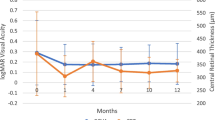
The secondary outcome was to quantify the AUCBCVA (expressed as logMAR) and the AUCCMT (expressed as micrometers) from baseline to month 12 and their clinical associations. The AUC was measured with the trapezoidal rule, including all the BCVA and CMT observations available for each eye, respectively (Fig. 1). As the BCVA was expressed in logMAR, higher AUC indicated worse visual acuity over time and vice versa. Associations between the AUCBCVA and the AUCCMT were sought with univariable linear regression models with each patient’s identification number included as a random factor. Beta (β) regression estimates and 95% confidence interval (CI) are presented for significant associations.
Graphical display of the best-corrected visual acuity (BCVA) and central macular thickness (CMT) during the time of observation after the fluocinolone acetonide (FAc) injection and corresponding area under the curve (AUC, dark gray). The x-axis displays the number of observations (baseline, 1 month, 6 months, and 12 months) after the FAc injection. The y-axis of the BCVA is expressed as logMAR; the y-axis of the CMT is defined as micrometers
Statistical significance was set at p < 0.05.
Results
The study included ten eyes of seven patients (mean age 60 ± 12 years; 4 male, 57%) with NIU-PS. Four patients were affected by bilateral idiopathic non-infectious uveitis (in one patient, only one eye was treated with FAc), one patient had ocular involvement from sarcoidosis, one patient had Behçet disease with asymmetric eye involvement, and one patient had serpiginous choroiditis.
At the inclusion visit, five eyes demonstrated macular hyperfluorescence in the late phases of FFA; in six eyes, peripheral vascular leakage (in at least one or more retinal quadrants) was observed in the late angiographic phase. Areas of focal capillary non-perfusion (persistent hypofluorescence during the entire dye exam) were noticed in three eyes. On ICGA, three eyes (one affected by serpiginous choroiditis, one by sarcoidosis, one by idiopathic uveitis) showed multiple scattered areas of hypofluorescence, stable through all the angiographic phases.
Mean duration of posterior uveitis was 8 ± 5 years (range 3–20); all eyes had received at least one dexamethasone implant (range 1–7 injections) before FAc implant administration. The demographic and clinical features are summarized in Table 1.
Functional and Morphologic Changes over Follow-Up
The BCVA improved from 0.67 ± 0.41 logMAR at baseline to 0.45 ± 0.37 logMAR at month 12 (p = 0.004); the improvement in BCVA was significant starting from month 6 through month 12. The mean CMT decreased from 449 ± 105 μm at baseline to 336 ± 118 μm at month 12 (p < 0.001). The choroidal thickness reduced from 251 ± 133 to 190 ± 115 μm (p < 0.001). The FAc implant efficacy in resolving macular thickening can be seen in the case reported in Fig. 2. The improvements in CMT and CCT occurred earlier, starting from month 1, and were sustained up to month 12 (Table 2).
A case of non-infectious uveitis of the posterior segment (NIU-PS) treated with fluocinolone acetonide implant (FAc). The left eye of a patient affected by chronic idiopathic posterior uveitis and relapsing cystoid macular edema (a). After 2 months from FAc injection, the macular edema appears resolved with a slight defect in the subfoveal ellipsoid band (b) and no relapse was observed through 6-month (c) and 12-month (d) follow-up
AUC of BCVA and CMT
The mean AUCBCVA was 1.61 ± 1.12 logMAR, ranging from 0.13 logMAR (best vision) to 3.25 logMAR (worst vision). The AUCBCVA positively correlated with baseline BCVA (β = 2.5 logMAR; 95% CI 1.59–3.41; p < 0.001; Fig. 3a), but not with the presence of macular edema during the follow-up, expressed as the AUCCMT.
The mean AUCCMT was 1111 ± 312 μm, ranging from 730 μm (thinner retina) to 1669 μm (thickest retina). The AUCCMT was positively correlated with the baseline CMT (β = 2.1 μm; 95% CI 0.41–3.80; p = 0.02) and negatively correlated with the duration of uveitis (β = − 37.6 μm; 95% CI − 76.7 to 1.54; p = 0.06; Fig. 3b), albeit this association was non-significant.
Safety
Macular edema resolved in all eyes, with no recurrences observed over the follow-up; no rescue or adjunctive local therapy was needed.
The IOP did not significantly change over 12 months after FAc injection (p = 0.2). One patient was under IOP-lowering treatment before FAc injection. One patient experienced an IOP upsurge up to 35 mmHg at month 6 and was treated with one IOP-lowering agent; the IOP normalized thereafter. No other local or systemic side effects were recorded over the observation period.
Discussion
In the present study, we analyzed the effects of FAc implant on the visual function and the macular thickness, expressed as their AUC over 1 year. We found that the visual response to FAc was associated with the baseline visual function; better visual acuities on follow-up followed better visual acuity at the time of FAc administration. On the contrary, higher CMT at baseline was associated with persistent retinal thickening over time. Patients with long-standing uveitis had, perhaps, a more favorable anatomic response to FAc, but the small size of our cohort did not allow us to reach definitive conclusions.
The functional and anatomic effectiveness of the FAc implant in NIU-PS has been reported in randomized clinical trials and real-world studies. Specific disease data are available only for birdshot retinochoroiditis [7]. Ajamil-Rodanes et al. reported the outcomes of FAc implant in 15 eyes with birdshot retinochoroiditis at 31 months [7]: all eyes achieved resolution of the macular edema and functional improvement of photoreceptors on electro-functional tests. Data about the use of 0.19 mg FAc implant other NIU-PS, such as rheumatoid arthritis-associated panuveitis, acute zonal outer occult retinopathy, multifocal choroiditis and panuveitis, multiple sclerosis-related uveitis, idiopathic uveitis, and sarcoid posterior uveitis, are reported in a few studies or case reports [8, 9]. Overall, all confirm the favorable clinical and safety profile in a heterogeneous group of posterior-segment inflammatory conditions [8, 9].
In our cohort, we observed a steady BCVA increase within the first months from the injection; the functional improvement was sustained up to 12 months. The timing of the visual response is in line with the clinical trial results from Jaffe et al., in which a rapid and persistent BCVA gain was observed during the 36 months follow-up [10]. These data reflect the pharmacokinetics of the FAc implant, in which the aqueous drug levels peak at 3 months (greater than 2 ng/mL) and reach a steady state from the 6th to the 36th month (0.5–1.0 ng/mL) [11]. Our and others’ data support that a sustained control of inflammation is beneficial to the functional outcome. In this context, our results suggest a better anatomic effect in long-standing NIU-PS [12]. We speculate that patients with long-standing uveitis have a lower grade of inflammation than eyes with more recent-onset uveitis. The lower degree of intraocular inflammation would be better tackled by extended-release, continuous dosage of steroids, rather than pulse administration of the drug.
The macular thickening markedly improved over the follow-up, with an early peak at the second month. Our results are in accordance with the 36-month FAc trial, in which macular edema showed a prompt and sustained response as early as 28 days [10]. The choroidal thickness is a quantitative OCT biomarker of ocular inflammation [13]. We observed a progressive thinning of the choroid, which paralleled the morphologic retinal response.
Contrary to what we experienced in patients with DME [14], we found no edema recurrence or inflammatory flare-up over the follow-up. In previous studies, the recurrence rate of macular edema due to NIU-PS varied from 28% at 6 months to 65% at 36 months after FAc injection [9, 10, 15]. We hypothesize that the purely inflammatory nature of uveitic macular edema responds better to intravitreal corticosteroids than DME, where other molecular pathways unrelated to inflammatory cytokines are involved. This study may come in support of cost-effectiveness analyses of FAc implant in NIU-PS [16]. No concerning IOP-related adverse event was observed through the follow-up period. These results fit with the 36-month trial and support the overall safety of FAc implant.
The strength of the paper is the use of the AUC method for quantifying the functional and the morphologic response to FAc. AUC gives an informative longitudinal evaluation of treatment efficacy, especially with sustained-release medications. While time-point measurements provide only a snapshot of the visual function or the macular thickness at each visit, the AUC offers a clearer view of the average drug effect over time. The follow-up was set at 12 months; although it is relatively short compared to the registration trials, it helps to understand the short- and medium-term clinical responses to the FAc implant in patients with NIU-PS. Limitations of the study include its retrospective nature and the small sample size, which precluded more advanced statistics. We did not analyze other potentially informative biomarkers associated with the clinical response, such as the state of the photoreceptors on OCT and the specific features on dye angiography of the underlying uveitis (e.g., the degree of capillary non-perfusion or vasculitis).
Conclusions
The 0.19-mg intravitreal non-bioerodable FAc implant was effective in improving the visual function and the macular thickening in eyes affected by NIU-PS; the visual acuity and the severity of macular edema at the time of FAc administration were the main determinants of the drug response. Our findings may help the clinical decision and may influence the clinical expectation of both physicians and patients at the time of FAc prescription.
References
Singh RB, Sinha S, Saini C, Elbasiony E, Thakur S, Agarwal A. Recent advances in the management of non-infectious posterior uveitis. Int Ophthalmol. 2020;40:3187–207.
Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: fundamentals of care for uveitis (FOCUS) initiative. Ophthalmology. 2018;125:757–73.
Ong DN, Lim LL. The efficacy of intravitreal dexamethasone implants for non-infectious posterior segment uveitis: increasing the uveitis armamentarium. Clin Exp Ophthalmol. 2019;47:1119–21.
Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the periocular vs. intravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126:283–95.
Steeples LR, Pockar S, Jones NP, Leal I. Evaluating the safety, efficacy and patient acceptability of intravitreal fluocinolone acetonide (0.2 mcg/day) implant in the treatment of non-infectious uveitis affecting the posterior segment. Clin Ophthalmol. 2021. https://doi.org/10.2147/opth.s216912.
Zarranz-Ventura J, Mali JO. Effectiveness of 190 µg fluocinolone acetonide and 700 µg dexamethasone intravitreal implants in diabetic macular edema using the area-under-the-curve method: the CONSTANT analysis. Clin Ophthalmol. 2020. https://doi.org/10.2147/OPTH.S253370.
Ajamil-Rodanes S, Testi I, Luis J, Robson AG, Westcott M, Pavesio C. Evaluation of fluocinolone acetonide 0.19 mg intravitreal implant in the management of birdshot retinochoroiditis. Br J Ophthalmol. 2020. https://doi.org/10.1136/bjophthalmol-2020-317372.
Weber LF, Marx S, Auffarth GU, et al. Injectable 0.19-mg fluocinolone acetonide intravitreal implant for the treatment of non-infectious uveitic macular edema. J Ophthalmic Inflamm Infect. 2019. https://doi.org/10.1186/s12348-019-0168-9.
Cai CX, Skalak C, Keenan RT, Grewal DS, Jaffe GJ. Time to disease recurrence in non-infectious uveitis following long-acting injectable fluocinolone acetonide implant. Graefes Arch Clin Exp Ophthalmol. 2020. https://doi.org/10.1007/s00417-020-04614-x.
Jaffe GJ, Pavesio CE. Effect of a fluocinolone acetonide insert on recurrence rates in noninfectious intermediate, posterior, or panuveitis: three-year results. Ophthalmology. 2020. https://doi.org/10.1016/j.ophtha.2020.04.001.
Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013. https://doi.org/10.1016/j.ophtha.2012.09.014.
Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005. https://doi.org/10.1016/j.ophtha.2005.06.009.
Bittencourt MG, Kherani S, Ferraz DA, et al. Variation of choroidal thickness and vessel diameter in patients with posterior non-infectious uveitis. J Ophthalmic Inflamm Infect. 2014. https://doi.org/10.1186/s12348-014-0014-z.
Saedon H, Anand A, Yang YC. Clinical utility of intravitreal fluocinolone acetonide (Iluvien®) implant in the management of patients with chronic diabetic macular edema: a review of the current literature. Clin Ophthalmol. 2017. https://doi.org/10.2147/OPTH.S131165.
Jaffe GJ, Foster CS, Pavesio CE, Paggiarino DA, Riedel GE. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology. 2019;126:601–10.
Pouwels XGLV, Petersohn S, Carrera VH, et al. Fluocinolone acetonide intravitreal implant for treating recurrent non-infectious uveitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2020. https://doi.org/10.1007/s40273-019-00851-z.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole. All the authors have given their approval for this version to be published.
Author Contributions
Elisabetta Miserocchi, Marco Battista and Maria Vittoria Cicinelli conceived the concept and design of the paper. Maria Vittoria Cicinelli contributed to the statistical analysis and Marco Battista, Vincenzo Starace, Luigi Capone and Maria Vittoria Cicinelli to drafting the manuscript. Elisabetta Miserocchi, Alessandro Marchese, Giulio Modorati and Francesco Bandello supervised and made the final revision.
Disclosures
Marco Battista, Vincenzo Starace, Maria Vittoria Cicinelli, Luigi Capone, Alessandro Marchese, Giulio Modorati, Elisabetta Miserocchi all have nothing to disclose. Francesco Bandello is a consultant for: Alcon (Fort Worth, Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee,Belgium), Zeiss (Dublin, USA).
Compliance with Ethics Guidelines
This single-center observational study of patients with NIU-PS undergoing FAc implant, conducted in the Ophthalmology Department of San Raffaele Hospital, Milan, Italy, adhered to the Helsinki declaration and its later amendments. The local institutional review board was informed and approved the retrospective review of data.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Battista, M., Starace, V., Cicinelli, M.V. et al. Efficacy of 0.19 mg Fluocinolone Acetonide Implant in Non-infectious Posterior Uveitis Evaluated as Area Under the Curve. Ophthalmol Ther 11, 215–224 (2022). https://doi.org/10.1007/s40123-021-00426-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00426-2